Abstract
The multiferroic Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3, Bi0.9Ca0.1Fe0.9Cu0.1O3 samples were prepared by a simple sol–gel method. Rietveld refinement of X-ray diffraction data and Raman spectra reflect a structural phase transition from single phase (rhombohedral, pure BiFeO3) to two phase coexistence (rhombohedral R3c and cubic Fm-3 m). The structural distortion of Bi0.9Ca0.1Fe0.9Ni0.1O3 is very marked. SEM images show the co-doped nanoparticles having an average size of 50 nm. A contribution from the leakage current have been observed in the P–E loops. XPS results reveal that the concentration of Fe2+ and oxygen vacancy decreased after transition metal elements (Co, Ni, Cu) doped into Bi0.9Ca0.1FeO3. Moreover Co, Ni doping can enhance the saturation magnetization, while Cu doping can enhance the coercive field in Bi0.9Ca0.1FeO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiferroic materials are currently attracting intensive studies interests due to the potential applications for developing novel storage media and spintronics devices [1–4]. The multiferroic materials could coupled with electric, magnetic, and structural order parameters which result in simultaneous ferroelectricity, ferromagnetism, and/or ferroelasticity [5]. BiFeO3 (BFO) is the most outstanding multiferroic inorganic compound among the currently research, because its ferroelectric Curie point (T C = 1103 K) and antiferromagnetic Néel point (T N = 643 K) are both above room temperature (RT) [6]. However, the net spins are canceled and the macroscopic magnetization is averaged to zero due to the cycloid spin modulation with a period of about 62 nm in BFO, which restrict multiferroic properties [7].
Recently, numerous investigations have focused on substitution of rare-earth, alkaline earth or transition metal elements at A-site (Ba [8], Ca [9], Eu [10], Nd [11], La [12]), B-site (Mn [13], Co [14], Ni [15], Cu [16], Zn [17]) or A–B-site [18–21] of BFO to improve its multiferroic. Additionally the overall properties of BFO depend on numerous factors including structure, defect, size, and morphology, which can also be promoted by this strategy. Our previous works focus on alkali metal element Ca. It is worthy noticing that the Ca-doped structure has half-metallic characteristic and enhanced ferromagnetism [22]. The study of Ca co-doped with rare earth element Nd in BFO have been published recently, in which we gain a relatively low current leakage density and an enhanced ferromagnetic properties [23]. As we known Fe-site substitution by 3d transition metal ions might induce structure modifications to suppress the cycloidal spin structure and the ferromagnetic interaction between Fe and 3d transition metal ions which might further enhance the room temperature ferromagnetism [24]. We would further investigate on Ca co-doped with 3d transition metal elements to recover the influence on the properties of BFO.
In this paper, BFO, Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 were prepared via a facile sol–gel method. Detailed studies on structure, electrical and magnetic properties of the samples were investigated.
2 Experimental details
Samples of BFO, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 were prepared by the sol–gel method. High purity Bi2O3 (analytical pure), CaO (≥98.5 %), Co2O3 (≥98.5 %), NiO (≥98.5 %), CuO (≥98.5 %), Fe(NO3)3·9H2O (≥98.5 %) powders were carefully weighed in stoichiometric proportions and thoroughly dissolved in nitric acid and deionized water under constant magnetic stirring. Tartaric acid was added as a chelating agent. All the chemicals in this work were purchased from the Shanghai Chemical Factory of China and were used without further purification. The mixed solution was dry at 110 °C for 4 h to form a homogeneous gel. The gel was then dried at 80 °C for 48 h and calcined at 600 °C for 2 h.
The phase identification was examined for the samples by X-ray diffractometer (XRD) (Bruker D8 Advance X-ray diffractometer) with Cu Ka radiation. Structure simulation based on XRD patterns were obtained by using Rietveld crystal structure refinement software (FullProf). The surface morphology was examined by scanning electron microscope (SEM: S4800). Raman measurements were carried out on Labram HR800 Raman microscope (λ = 514 nm) at room temperature. In order to measure the ferroelectric hysteresis loops and leakage current, the nanoparticles were pressed to pellets and sintered under 500 °C. The dielectric properties were measured by using Radiant precision materials analyzer. The combination states of Fe 2p electron in the samples were examined by X-ray photoelectron spectroscopy (XPS) (ESCALAB 250Xi). The magnetic hysteresis loops were measured at room temperature by Vibrating sample magnetometer (SQUID).
3 Results and discussion
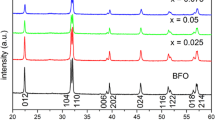
Figure 1 shows XRD patterns of Co, Ni, Cu-substituted Bi0.9Ca0.1FeO3 nanoparticles. The symbol “*” indicates the impurity phases of Bi2O3. Impurity peak is restrained after doping. The occurrence of impurity peaks is due to the volatile nature of Bi in the pure BFO sample, which can be weakened after Ca2+ substitute of Bi3+. For pure BFO all diffraction peaks are well matched with the rhombohedrally distorted perovskite structure with R3c space group (JCPDS no. 71-2494). It is obvious that the (104) and (110) peaks merge into one broad peak in Bi0.9Ca0.1FeO3 Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 compared to the pure BFO sample, which indicate structural phase transition. The merged peaks were shifted to a lower 2θ angle direction, which may not affected by ionic radius (RCa2+ < RBi3+). For more detailed study of structural transformation, Rietveld’s whole-profile fitting were performed using FullProf software. Fitting parameters are listed in Table 1 and Table 2. It is noticed that the pure BFO confirms a single-phase (rhombohedral structure), while the doped samples show the state of two-phase coexistence. In detail, the rate of rhombohedral phase (R3c:H space group), in Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles, dominates about 91.77, 18.21, 17.8 and 92.17 % respectively. The second phase is cubic Fm-3 m for all the doped samples. Unit cell volume of Bi0.9Ca0.1Fe0.9Ni0.1O3 changed apparently compared with other samples, as can be seen in Table 1, which means the structural distortion of Bi0.9Ca0.1Fe0.9Ni0.1O3 is very seriously. From Table 2 we can see that the estimated bond length of Fe–O bond decreased after doping, which implies the distortion in the magnetic Fe3+ position decreases with dopant and the opening/straightening of the canted spin.
In order to show the structural distortion intuitively, we draw the unit cell structures of all samples by Diamond software, as shown in Fig. 2. The Fe atom has slightly drifted from the center of the FeO6 octahedral for Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3. However the structural transformation evident than the other samples.
Figure 3 shows the SEM images of the co-doped samples. The average size of the co-doped samples are about 50 nm, smaller than 62 nm, which may lead to the destroy of cycloidal spiral structure. Hence, the magnetism can be enhanced. A certain degree of agglomeration of crystal particle can be observed in the co-doped samples.
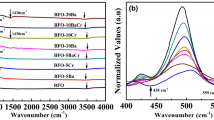
Figure 4 shows the Raman scattering spectra of BFO, Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 samples. According to the group theoretical calculation, there are 27 (13A′ + 14A″), 13 (4A1 + 9E), and 8 (3A1 + B1 + 4E) distinct Raman active modes for BFO with monoclinic (M), rhombohedral (R), and tetragonal (T) structure, respectively [25]. All of the major active modes that appeared in the pure BFO are matched well with those of the rhombohedrally distorted (R3c) perovskite structure. The 13 Raman active modes peaks are located at 141, 167, 215, 267, 275, 302, 343, 369, 467, 520 and 609 cm−1 respectively. The low frequency modes have been assigned to the vibration of Bi–O, high frequency Raman modes are related to the stretching and bending of Fe–O [26]. The intensity of A1-2 phonon mode decreased and A1-1 phonon mode shifts to higher frequency side for doped samples, which could be attributed to the Ca doping, as atomic mass of Ca is less than that of Bi [27]. The phonon modes at high frequency area disappeared or shift to other position which indicate structural distortion after doping. The detailed change of Bi–O and Fe–O bond were shown in Table 2.
Figure 5 shows the polarization hysteresis loops of the Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles under an applied electric field of 10 kV/cm. The values of 2Pr of the Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles are 2.414, 2.537 and 1.535 μC/cm2 respectively. The rounded drop-down tips in the P–E loops of Bi0.9Ca0.1Fe0.9Co0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 is the contribution of the leakage current [28]. The values of 2Ec of the Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles were 0.915, 0.522 and 0.880 kV/mm respectively. Overall, among the transition metal (Co, Ni, Cu) doped Bi0.9Ca0.1FeO3 samples Bi0.9Ca0.1Fe0.9Co0.1O3 showed relative good ferroelectric. However, the leakage current is exist in both of them.
The oxidation state of Fe in Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles was investigated by X-ray photoemission spectroscopy (XPS) as shown in Fig. 6. It should be noted that XPS can only analyze surface structure and composition of the materials, due to the limited depth of the beam penetration. The exposed surface might be oxidized by oxygen in the air. However, for BFO and some metallic oxide the effect of oxidation can be neglected [29]. The XPS spectra shows the Fe 2p region of the samples, where the core level binding energies were aligned with respect to C 1 s peak (284.6 eV). There are two main photoemission peaks around 710 and 724 eV are Fe 2p 3/2 and Fe 2p 1/2, respectively. Fe 2p 3/2 peaks correspond to Fe2+ and Fe3+, they show the satellite peak at 6 or 8 eV above their 2p 3/2 principal peaks. The experimental Fe 2p 3/2 band of all the samples were fitted well by the Gaussian shape, using two peaks corresponding to Fe3+ at 710.8 eV and Fe2+ at 709.4 eV as shown in Fig. 7.
The ratios of Fe3+/Fe2+ determined from the peak areas in the Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles are calculated as 1.7:1, 10.1:1, 7.5:1 and 36.4:1 respectively. The results indicate the decrease of Fe2+ ions with rare earth element doping into Bi0.9Ca0.1FeO3 nanoparticles. Numerous studies point out that less Fe2+ ions imply less oxygen vacancies, which suggest that earth element doping decrease the concentration of oxygen vacancies. In order to prove this, narrow-scan spectra of the O 1 s core levels of Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 samples were fitted by Gaussian curve as shown in Fig. 8a–d [30]. All the samples can be fitted by two Gaussians whose peaks positions are 529.05–529.14 eV and 530.79–531.99 eV. The one with lower binding energy can be attributed to the main peak of oxygen atoms, while the other is related to the presence of oxygen vacancy (VO). The concentration ratio of VO and O in Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 are 0.94:1, 0.78:1, 0.78:1 and 0.59:1 respectively. It obvious that the concentration of oxygen vacancy in Bi0.9Ca0.1FeO3 is higher than the other three samples, which is in accordance with previous studies.
Figure 9a shows room temperature magnetic hysteresis (M–H) loops of the BFO, Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3. The saturation magnetization (Ms) of Bi0.9Ca0.1Fe0.9Ni0.1O3 is 99.87 emu/cm3, which is much larger than the other samples. The big Ms may be attributed to the serious structural distortion. To get better look at the figure, M–H loops without Bi0.9Ca0.1Fe0.9Ni0.1O3 was shown in Fig. 9b. The existence of Fe2+ ions may cause a double exchange interaction between Fe2+ and Fe3+ ions through oxygen, which can result in the enhancement of ferromagnetism. The XPS spectra analysis shows that the concentration of Fe2+ in transition metal (Co, Ni, Cu) doped BFO are smaller than that in Ca-doped BFO. However, the saturation magnetization of Bi0.9Ca0.1Fe0.9Ni0.1O and Bi0.9Ca0.1Fe0.9Cu0.1O3 and the coercive field of Bi0.9Ca0.1Fe0.9Cu0.1O3 is larger than Bi0.9Ca0.1FeO3, which means Fe2+–O–Fe3+ interaction is not the main factor that affect the magnetism. The decrease of oxygen vacancies due to doping can result in significant enhancement of saturation magnetization [31]. Structural transformation and size effect may lead to the collapse of the space-modulated spin structure in BFO, which can also enhance the magnetism. Combined action of the reasons mentioned above result in the M–H loops show in Fig. 9.
4 Conclusions
In summary, we have studied the effect of transition metal (Co, Ni, Cu) substitution on Bi0.9Ca0.1FeO3 nanoparticles on its structure, ferroelectricity, leakage current and magnetic property. BFO, Bi0.9Ca0.1FeO3, Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles were successfully synthesized by sol–gel method. Structural phase transition from rhombohedral to two phase coexistence was observed in all the doped BFO. The values of 2Ec of the Bi0.9Ca0.1Fe0.9Co0.1O3, Bi0.9Ca0.1Fe0.9Ni0.1O3 and Bi0.9Ca0.1Fe0.9Cu0.1O3 nanoparticles were 0.915, 0.522 and 0.880 kV/mm respectively. The leakage current exist in transition metal (Co, Ni, Cu) doped Bi0.9Ca0.1FeO3, which is show in the P–E loops. The decrease of oxygen vacancies can increased the saturation magnetization of all the samples. The obvious structural transformation may play an important role in the enhance of saturation magnetization of Bi0.9Ca0.1Fe0.9Ni0.1O3. Fe2+–O–Fe3+ interaction and size effect also have an effect on ferromagnetism.
References
A.S. Priya, I.B.S. Banu, S. Anwar, Mater. Sci. Eng., B 401, 333–338 (2016)
J.S. Park, Y.J. Yoo, J.S. Hwang, J.H. Kang, B.W. Lee, Y.P. Lee, J. Appl. Phys. 115, 013904 (2014)
G. Cheng, Z.-G. Wang, Y.-L. Liu, J.-L. Zhang, D.-H. Sun, J.-Z. Ni, A.C.S. Appl, Mater. Interfaces 5, 3182–3190 (2013)
G. Cheng, Z.-G. Wang, Y.-L. Liu, J.-L. Zhang, D.-H. Sun, J.-Z. Ni, Chem. Commun. 48, 10240–10242 (2012)
Y.T. Li, Y.W. Fan, H.G. Zhang, X.X. Teng, X.G. Dong, H. Liu, X.P. Ge, Q. Li, W. Chen, X.A. Li, Z.Y. Ge, J. Supercond. Nov. Magn. 27, 1239–1243 (2014)
J.S. Hwang, J.Y. Cho, S.Y. Park, Y.J. Yoo, P.S. Yoo, B.W. Lee, Y.P. Lee, Appl. Phys. Lett. 106, 062902 (2015)
X.J. Xi, S.Y. Wang, W.F. Liu, H.J. Wang, F. Guo, X. Wang, J. Gao, D.J. Li, J. Magn. Magn. Mater. 355, 259–264 (2014)
P. Godara, A. Agarwal, N. Ahlawat, S. Sanghi, K. Kaswan, J. Magn. Magn. Mater. 406, 76–82 (2016)
H.W. Chang, C.Y. Shen, F.T. Yuan, S.H. Tien, S.Y. Lin, W.A. Chen, C.R. Wang, C.S. Tu, S.U. Jen, J. Magn. Magn. Mater. 401, 673–676 (2016)
B.T. El, M. Taibi, A.M. El-Naggar, T.T. Slimani, A.A. Albassam, M. Abd-Lefdil, I.V. Kityk, N.S. AlZayed, A.O. Fedorchuk, J. Mater. Sci.: Mater. Electron. 26, 9949–9954 (2015)
S.M. Lee, A. Cho, Y.S. Cho, RSC Adv. 6, 16602–16607 (2016)
R. Dhanalakshmi, M. Muneeswaran, K. Shalini, N.V. Giridharan, Mater. Lett. 165, 205–209 (2016)
S.K. Singh, H. Ishiwara, K. Sato, K. Maruyama, J. Appl. Phys. 102, 094109 (2007)
J. Wang, M.Y. Li, X.L. Liu, W.Q. Liu, Z.Q. Hu, S.X. Wang, J. Polym. Mater. 20, 727–731 (2013)
S.M. Lee, A. Cho, Y.S. Cho, RSC Adv. 6, 16602–16607 (2016)
Y.T. Li, Y.W. Fan, H.G. Zhang, X.X. Teng, X.G. Dong, H. Liu, X.P. Ge, Q. Li, W. Chen, X.A. Li, J. Supercond Nov. Magn. 27, 1239–1243 (2014)
J.J. Xie, C.D. Feng, X.H. Pan, Y. Liu, Ceram. Int. 40, 703–706 (2014)
J.Y. Chen, Y. Wang, Y. Deng, J. Appl. Phys. 116, 174102 (2014)
X.L. Xu, W.F. Liu, H. Zhang, M.C. Guo, P. Wu, S.Y. Wang, J. Gao, G.H. Rao, J. Appl. Phys. 117, 174106 (2015)
D. Do, J.W. Kim, T.K. Song, S.S. Kim, J. Electroceram. 30, 55–59 (2013)
W.W. Mao, F. Wang, L. Chu, Y.Y. Zhu, Q. Wang, J. Zhang, J.P. Yang, X.A. Li, W. Huang, Phys. Chem. Chem. Phys. 18, 6399–6405 (2016)
C.Y. Quan, Y.M. Han, N. Gao, W.W. Mao, J. Zhang, J.P. Yang, X.A. Li, W. Huang, Ceram. Int. 42, 537–544 (2016)
C.Y. Quan, Y.H. Ma, Y.M. Han, X.X. Tang, M.J. Lu, W.W. Mao, J. Zhang, J.P. Yang, X.A. Li, W. Huang, J. Alloys Comp. 635, 272–277 (2015)
W.W. Mao, X.F. Wang, Y.M. Han, X.A. Li, Y.T. Li, Y.F. Wang, Y.W. Ma, X.M. Feng, T. Yang, J.P. Yang, W. Huang, J. Alloys Comp. 584, 520–523 (2014)
J.-Z. Huang, Y. Shen, M. Li, C.-W. Nan, J. Appl. Phys. 110, 094106 (2011)
J. Zhao, X. Zhang, S. Liu, W. Zhang, Z. Liu, J. Alloys Comp. 557, 120–123 (2013)
P. Kumar, M. Kar, J. Alloys Comp. 584, 566–572 (2014)
Z.Q. Hu, M.Y. Li, Y. Yu, J. Liu, L. Pei, J. Wang, X.L. Liu, B.F. Yu, X.Z. Zhao, Solid State Commun. 150, 1088–1091 (2010)
F. Huang, X. Lu, W. Lin, X. Wu, Y. Kan, J. Zhu, Appl. Phys. Lett. 89, 242914 (2006)
X. Xi, S. Wang, W. Liu, H. Wang, F. Guo, X. Wang, J. Gao, D. Li, J. Magn. Magn. Mater. 355, 259–264 (2014)
G.S. Arya, R.K. Kotnala, N.S. Negi, J. Nanopart. Res. 16, 2155 (2013)
Acknowledgments
We acknowledge the financial support from the Ministry of Education of China (No. IRT1148), Jiangsu Synergistic Innovation Center for Advanced Materials (SICAM), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, YX03001), the National Natural Science Foundation of China (51372119, 61377019, 61136003, 51173081), College Postgraduate Research and Innovation Project of Jiangsu Province (KYLX_0794, KYLX15_0848), the National Natural Science Foundation of Jiangsu (KZ0070715050), the Seed Project Funded by Introducing Talent of NJUPT (XK0070915022) and the Natural Science Foundation of NJUPT (NY214129, NY214130, NY214181).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chuye Quan and Zhengfei Qin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Quan, C., Qin, Z., Zhu, Y. et al. Influence of transition metal element (Co, Ni, Cu) doping on structural, electrical and magnetic properties of Bi0.9Ca0.1FeO3 nanoparticles. J Mater Sci: Mater Electron 28, 3278–3284 (2017). https://doi.org/10.1007/s10854-016-5920-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5920-4