Abstract
\(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films were deposited onto soda-lime glass substrates held at \(360\,^{\circ }\)C using spray pyrolysis technique. The influence of thiourea concentration on the growth and properties of these films is studied by varying the thiourea concentration (0.04–0.09 M) and keeping cupric chloride (0.015 M) and stannic chloride (0.005 M) concentrations as constant in solution. These films were analyzed by studying their elemental composition, structural, microstructural, optical and electrical properties using appropriate characterization techniques. X-ray diffraction and Raman spectroscopy analyses revealed that the films deposited from solutions with 0.04 and 0.05 M thiourea concentrations contain CuS and \(\hbox {Cu}_{2}\hbox {SnS}_{3}\) (CTS) phases, respectively. Nanocrystalline \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films with cubic structure could be obtained by increasing the thiourea concentration from 0.07 to 0.09 M in the starting solution. The lattice parameter and crystallite size of these films are found to be 0.540 and 6 nm, respectively. The direct optical band gap of these films is found to decrease from 1.75 to 1.70 eV with increasing the thiourea concentration from 0.07 to 0.09 M in the solution. The room temperature electrical resistivity of these films is found to lie in the range \(1.9\times 10^{-3}\)–\(0.9\times 10^{-3}\Omega\) cm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, thin film solar cells based on Cu(In,Ga)\(\hbox {Se}_{2}\) (CIGS) have reached a maximum conversion efficiency of 21.7 % [1] at the laboratory level. However, the scarcity of In, Ga and the toxicity of Se pose problems for the large scale production of these cells. In order to explore the low-cost and non-toxic materials for the fabrication of thin film solar cells, studies on the new materials and approaches are required. In recent years, the focus is mainly on \(\hbox {Cu}_{2}\hbox {ZnSnS}_{4}\) (CZTS) owing to its direct optical band gap of 1.45 eV, high optical absorption co-efficient and p-type electrical conductivity. The CZTS based thin film solar cells have exhibited an efficiency of 8.4 % [2] at laboratory level. In addition to these developments, ternary compounds such as \(\hbox {Cu}_{2}\hbox {SnS}_{3}\), \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) and \(\hbox {Cu}_{4}\hbox {SnS}_{4}\) semiconductor thin films have also drawn attention of the investigators due to their potential applications in the fields of photovoltaics, optoelectronics and microbatteries [3–6]. Among them, \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) with a direct optical band gap of 1.60 eV [7], high optical absorption coefficient (\(10^{4}~\hbox {cm}^{-1}\)), p-type electrical conductivity and the presence of abundant constituent elements, is a promising material as an absorber layer in thin film heterojunction solar cells. A thorough understanding of the growth and properties of this compound semiconductor thin films is very much essential to use them in the device fabrication.
The work done on the growth and properties of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films is quite meagre [7–13]. \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films were first prepared by Bouaziz et al. [7, 8] using a spray pyrolysis technique. Fernandes et al. [9, 10] grown \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films by sulfurizing sputter deposited Cu-Sn mettalic precurors. Su et al. [11] prepared \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films by a modified successive ionic layer adsorption and reaction (SILAR) method. Guan et al. [12] prepared \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films by annealing SILAR deposited films. In our previous report [13], we also made an attempt to grow \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films using spray pyrolysis technique. Spray pyrolysis is a simple and low cost technique generally used for the deposition of sulfide, selenide and oxide thin films [14]. The properties of thin films prepared by spray pyrolysis are mostly depend on the starting materials proportions, substrate temperature, spray rate etc. These process parameters need to be carefully adjusted to obtain phase pure and uniform films. Bouaziz et al. [7, 8] studied the effect of annealing temperature [7] and Cu/Sn ratio [8] on the growth and properties of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films. They have used a higher amount of thiourea (20 M) in the starting solution to overcome the precipitation problem and observed a change in the \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) crystal structure from tetragonal to orthorhombic with increasing the Cu/Sn ratio from 1.8 to 4.8. In our previous report [13], we have used cupric chloride (0.03 M), stannic chloride (0.01 M) and thiourea (0.14 M) as the source materials to deposit \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films. In the present investigation, we have attempted to study the influence of thiourea concentration on the growth and properties of spray deposited \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films. The starting materials proportions were reduced to half than our previous work [13] to overcome the precipitation problem.
2 Experimental details
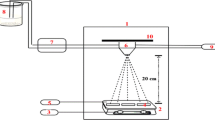
The schematic diagram of the spray system used in the present study is presented in Fig. 1. The starting solution for the growth of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films consists of analar grade cupric chloride, stannic chloride and thiourea dissolved in distilled water. The concentrations of these chemicals and the deposition conditions are presented in Table 1. The concentrations of cupric chloride (0.015 M) and stannic chloride (0.005 M) were kept constant in the solution and the concentration of thiourea was varied from 0.04 to 0.09 M (2.0–4.5 times than the stoichiometric requirement) in order to study its influence on \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) film growth. Excess thiourea than the stoichiometric requirement was taken to compensate for the loss of sulfur at higher substrate temperature due to its volatile nature and also to study its influence on the film growth. This aqueous solution was sprayed onto soda-lime glass substrates held at \(360\,^{\circ }\)C using a pneumatically controlled air atomizing spray nozzle. Compressed air was used as the carrier gas. The film thickness was determined using a microbalance.
These films were systematically analyzed by studying their compositional, structural, microstructural, optical and electrical properties. X-ray diffraction (XRD) patterns were recorded on BRUKER X-ray diffractometer (Model: D8 Advance) in the \(2\theta\) range \(10^{\circ }{-}60^{\circ }\) with Cu \(\hbox {K}_\alpha\) radiation (\(\lambda = 0.15406\) nm). Raman spectra were recorded on Horiba Jobin Yvon HR 800UV confocal micro-Raman spectrometer with Nd:YAG laser source (\(\lambda = 532\) nm). The micrographs and elemental composition were obtained using Carl Zeiss scanning electron microscope (Model: EVO MA 15) attached to an Oxford Instruments energy dispersive spectrometer (EDS) (Model: INCA250). Spectral transmittance of these films were recorded with Cary 5000 UV-Vis-NIR double beam spectrophotometer. The room temperature electrical resistivity of the films was determined using four probe technique.
3 Results and discussion
3.1 Composition
The elemental compositions of the films deposited from solutions with different thiourea concentrations are shown in the Table 2. The uncertainty in the determination of elemental composition by EDS analysis is \(\pm 5\) at%. The elemental composition of the films deposited from a solution with 0.04 M thiourea concentration is deviated much from the stoichiometry. The films are highly S-poor, Cu-rich and Sn-rich. On increasing the thiourea concentration in the solution, the sulfur at% in the films increases and a corresponding decrease in the at% of Cu and Sn is observed. Cu/Sn ratio is found to increase from 2.32 to 2.91 and S/(Cu+Sn) ratio is found to increase from 0.74 to 1.02 with increase in the thiourea concentration from 0.05 to 0.09 M in the solution. The compositions of the films deposited from solutions with 0.07, 0.08 and 0.09 M thiourea concentrations are found to be near-stoichiometric \(\hbox {Cu}_{3}\hbox {SnS}_{4}\), considering the accuracy limits of EDS analysis (Table 2).
3.2 Structural analysis
3.2.1 X-ray diffraction
Figure 2 shows the XRD patterns of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films deposited from solutions with different thiourea concentrations. The diffraction peaks of these films are seems to be broad and their signal to noise ratio is verly low indicating the nanocrystalline nature of the films. The XRD pattern of films deposited from a solution with 0.04 M thiourea concentration (Fig. 2a) exhibit the diffraction peaks at \(27.75^{\circ }\), \(29.29^{\circ }\), \(31.77^{\circ }\), \(32.90^{\circ }\) and \(47.95^{\circ }\) corresponding to CuS phase (JCPDS Card No. 78-2121). The peaks due to \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase are not observed in this film, which indicates that 0.04 M thiourea concentration in the starting solution is not providing sufficient amount of sulfur to form \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase due to the re-evaporation of sulfur at \(360\,^{\circ }\)C. XRD pattern of films deposited from a solution with 0.05 M of thiourea concentration (Fig. 2b) shows the peaks at \(28.56^{\circ }\) and \(47.55^{\circ }\) corresponding to tetragonal \(\hbox {Cu}_{2}\hbox {SnS}_{3}\) phase (JCPDS Card No. 89-4714). A peak at \(31.8^{\circ }\) corresponding to CuS phase is also present in these films. XRD patterns of the films deposited with further increase in the thiourea concentration from 0.06 to 0.09 M (Fig. 2c–f) exhibit the diffraction peaks at \(28.75^{\circ }\), \(47.70^{\circ }\) and \(56.90^{\circ }\) due to \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase. The observed diffraction peaks at \(28.75^{\circ }\), \(47.70^{\circ }\) and \(56.90^{\circ }\) are quite close to the diffraction peaks of cubic (Sphalerite) \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase reported by Benedetto et al. [15], who did Rietveld analysis for the XRD patterns recorded with a collection time of 30 s for each step. Thus, the diffraction peaks observed in the films deposited from the solutions with increase in the thiourea concentration from 0.06 to 0.09 M are attributed to cubic \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase. The lattice parameter of these \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films is found to be a = 0.540 nm and is close to the reported lattice parameter value of a = 0.542 nm [15]. The average crystallite size, calculated from Scherrer’s formula [16], for the films deposited from solutions with increasing thiourea concentration from 0.06 to 0.09 M is found to be 6 nm.
3.2.2 Raman spectroscopy
We have used Raman spectroscopy as a complementary tool for phase analysis. The Raman spectra of the films deposited from solutions with different thiourea concentrations are shown in Fig. 3. The Raman spectrum of the films deposited from a solution with 0.04 M thiourea concentration (Fig. 3a) exhibit an intense mode at 475 \(\hbox {cm}^{-1}\) followed by two weak modes at 141 and 269 \(\hbox {cm}^{-1}\) corresponding to CuS phase [17–19]. The diffraction pattern of this film also revealed CuS as the major phase. The Raman spectrum of the films deposited from a solution with 0.05 M thiourea concentration (Fig. 3b) exhibit the modes at 269, 292, 320, 333, 354 and 475 \(\hbox {cm}^{-1}\). The intense mode observed at 333 \(\hbox {cm}^{-1}\) in this film is attributed to tetragonal CTS phase [9, 10]. The modes observed at 292 and 354 \(\hbox {cm}^{-1}\) are attributed to monoclinic CTS phase [20, 21]. The modes observed at 269 and 475 \(\hbox {cm}^{-1}\) are due to CuS phase [17–19] present in this film. XRD pattern of this film revealed the presence of dominant tetragonal CTS phase and a minor CuS phase. The presence of monoclinic CTS modes might be due to the polymorphic nature of CTS [20, 21]. A small mode observed at 320 \(\hbox {cm}^{-1}\) in this film is close to the reported intense Raman mode of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase [9, 10, 13] and hence attributed to \(\hbox {Cu}_{3}\hbox {SnS}_{4}\). The diffraction peaks due to \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase are not appeared in the XRD pattern of this film might be due their presence in minute quantity. But, the presence of a low intensity mode at 320 \(\hbox {cm}^{-1}\) corresponding to \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase in this film suggests that this phase formation starts in the films deposited from a solution with 0.05 M thiourea concentration. The Raman spectrum of the films deposited from a solution with 0.06 M thiourea concentration (Fig. 3c) exhibits an intense mode at 331 \(\hbox {cm}^{-1}\) corresponding to tetragonal CTS phase, two low intensity modes at 292 and 351 \(\hbox {cm}^{-1}\) due to monoclinic CTS phase, a low intensity mode at 319 \(\hbox {cm}^{-1}\) due to \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase and two modes at 264 and 475 \(\hbox {cm}^{-1}\) due to CuS phase. The XRD analysis of this film revealed \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) as the dominant phase. But the presence of dominant CTS modes in the Raman spectrum of this film might be due to the presence of CTS phase within the Raman probe depth. In the Raman spectra of films deposited from solutions with further increase in the thiourea concentration from 0.07 to 0.09 M (Fig. 3d–f), the intensity of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) mode (317–320 \(\hbox {cm}^{-1}\)) is found to increased. The modes due to tetragonal CTS, monoclinic CTS and CuS phases still remain in these films. The XRD patterns of these films do not revealed the presence of tetragonal CTS, monoclinic CTS and CuS phases might be due to their minute presence below the detection limit. From this analysis, it is clear that \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase formation starts in the films deposited from a solution with 0.05 M thiourea concentration and becomes dominant with a further increase in the thiourea concentration.
3.3 Microstructure
The scanning electron microscopy (SEM) images of the films deposited from solutions with different thiourea concentrations are shown in Fig. 4. The micrograph of the films deposited from a solution with 0.04 M thiourea concentration (Fig. 4a) shows different nut shaped grains, a few brighter grains, a smeary background and some cracked regions. The nut shaped grains might be due to CuS phase since the dominant phase in these films is CuS as revealed by XRD and Raman analyses. These nut shaped grain morphology disappeared to a greater extent and a mesh-like morphology is appeared with a few needle shaped grains on it by depositing the films from a solution with 0.05 M thiourea (Fig. 4b). This mesh-like morphology might be due to tetragonal CTS phase since tetragonal CTS is the dominant phase in this film. A few nut shaped grains observed in this micrograph might be due to the CuS phase present in this film. The micrographs of the films deposited from solutions with increase in the thiourea concentration from 0.06 to 0.07 M, resulted in the disapperance of mesh-like morphology and appearance of flower-like morphology with a smeary background. The microstructure of the films deposited from solutions with further increase in the thiourea concentration from 0.08 to 0.09 M, shows the uniform morphology with fine grains (Fig. 4e, f). The average grain size of these films (Fig. 4e, f) is found to \({\sim }200\) nm. A few bright grains are also seen on the surface of these films.
3.4 Optical absorption
Figure 5 shows the spectral transmittance curves of the films deposited from solutions with different thiourea concentrations. The lower transmittance of the films at higher wavelengths might be due to the degenerate nature of the films. The onset of fundamental absorption edge for the films deposited from a solution with 0.04 M thiourea concentration occurs at \({\sim }750\) nm and ends at \({\sim }500\) nm, which is close to the reported onset of fundamental absorption edge of CuS phase [22, 23]. The onset of absorption of the films deposited from a solution with 0.05 M thiourea concentration occurs at \(\sim\)1150 nm and ends at \({\sim }550\) nm and is attributed to tetragonal CTS phase since the onset of fundamental absorption of this phase was reported to occur at \({\sim }1200\) nm [9]. The onset of absorption edge for the films deposited from solutions with further increase in the thiourea concentration (0.06–0.09 M) occurs at \({\sim }1100\) nm and ends at \({\sim }550\) nm. Raman analysis revealed the presence of CTS and CuS as the secondary phases in these films. The onset of fundamental absorption of tetragonal CTS phase was reported to occur at \({\sim }1200\) nm while that of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase was reported to occur at \({\sim }800\) nm [9, 13]. This clearly suggests that the fundamental absorption edge of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase in these films could not be distinctly seen due to the close overlap of the strong absorption region of CTS phase with the onset of absorption edge of \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) phase.
The optical absorption coefficient (\(\alpha\)) of these films is calculated from their spectral transmittance (T) data using \(\alpha\) = ln(1/T)/t, where ’t’ is the film thickness. Figure 6 shows the optical absorption coefficient (\(\alpha\)) versus h\(\nu\) curves of these films deposited from solutions with different thiourea concentration. It is seen that the optical absorption coefficient of these films is larger than \(4\times 10^{4}~ \hbox {cm}^{-1}\) in the visible region indicating that the films are suitable for solar cell absorber layer. The nature of the optical transition can be estimated from, \(\alpha \hbox {h}\nu = \hbox {A}(\hbox {h}\nu -\hbox {E}_{g})^{n}\), where ’A’ is a constant and ’n’ can take values 1/2, 2, 3/2 or 3 based on the transition is direct-allowed, indirect-allowed, direct-forbidden or indirect-forbidden, respectively. From the linear fit region of \((\alpha \hbox {h}\nu )^{1/n}\) versus h\(\nu\) plot, the band gap is obtained by extending the linear portion onto h\(\nu\)-axis and taking the intercept. In the present study, the above equation is found to be satisfied for n = 1/2 indicating the optical transitions are direct-allowed.
Figure 7 shows the \((\alpha \hbox {h}\nu )^{2}\) versus h\(\nu\) plots of the films deposited from solutions with different thiourea concentrations. The direct band gap of the films deposited from a solution with 0.04 M thiourea concentration (Fig. 7a) is found to be 1.95 eV and is close to the reported band gap of CuS phase [22, 23]. The direct band of the films deposited from a solution with 0.05 M thiourea concentration is found to be 1.65 eV. XRD and Raman analyses of this film revealed tetragonal CTS as the major phase. Fernandes et al. [9, 10] reported direct optical band gap value of 1.35 eV for tetragonal CTS phase. The obtained slightly higher band gap value in the present case might be due to the nanocrystalline nature of the films [21] or the presence of seconday phases in this film. The direct band gap of the films deposited from solutions by increasing the thiourea concentration from 0.06 to 0.09 M is found to decrease from 1.75 to 1.70 eV (Fig. 7c–f). The slight decrease in the band gap value from 1.75 to 1.70 eV with increase in the thiourea concentration from 0.06 to 0.09 M might be due to the slight changes in the elemental composition as a result of incorporation of sulfur in the films. Fernandes et al. [9, 10] reported 1.55–1.60 eV band gap values for \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films. The observed slightly higher band gap values than the reported value might be due to a slight variation in the elemental composition or crystallinity of these films. The band gap values of these films are presented in Table 3 (Fig. 7).
3.5 Electrical resistivity
The room temperature electrical resistivity of the films deposited from solutions with different thiourea concentrations is determined using four probe technique. The electrical resistivity of these films are presented in Table 3. It is seen from the table that the electrical resistivity of these films is found to decrease from \(5.0 \times 10^{-3}\) to \(0.9 \times 10^{-3}\,\Omega\) cm. The decrease in the electrical resistivity of the films deposited from solutions with increasing thiourea concentration might be due to the slight changes in the elemental composition. Fernandes et al. [9] observed \(7.40\times 10^{-4}\Omega\) cm for the films prepared by sulfurization of sputter deposited metallic precursors. Su et al. [11] reported \(1.5\times 10^{-4}\Omega\) cm resistivity value for the films prepared by modified SILAR techniques. The observed resistivity values in the present study are slightly higher than the reported values.
4 Conclusions
In conclusion, the influence of thiourea concentration on the growth and properties of spray deposited \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films is investigated. \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) thin films with cubic structure could be deposited from solutions by increasing the thiourea concentration from 0.07 to 0.09 M. Minor CTS and CuS secondary phases are present in these films. The lattice parameter of these films is found to be a = 0.540 nm and the crystallite size is found to be 6 nm. The direct optical band gap value of these films is found to decrease from 1.75 to 1.70 eV with increasing the thiourea concentration from 0.07 to 0.09M. The room temperature electrical resistivity of these films is found to decrease from \(1.9\times 10^{-3}\) to \(0.9\times 10^{-3}\Omega\) cm with increasing thiourea concentration. Further studies are necessary to reduce the secondary phases and to obtain device quality \(\hbox {Cu}_{3}\hbox {SnS}_{4}\) films.
References
P. Jackson, D. Hariskos, R. Wuerz, O. Kiowski, A. Bauer, T.M. Friedlmeier, M. Powalla, Phys. Status Solidi (RRL) 9999, 28 (2014)
B. Shin, O. Gunawan, Y. Zhu, N.A. Bojarczuk, S.Jay Chey, S. Guha, Prog. Photovolt. Res. Appl. 21(1–3), 72 (2013)
L.K. Samanta, D.K. Ghosh, G.C. Bhar, Phys. Status Solidi A 93(1), K51 (1986)
T.A. Kuku, O.A. Fakolujo, Sol. Energy Mater. 16(1–3), 199 (1987)
Y. Xiong, Y. Xie, G. Du, H. Su, J. Inorg. Chem. 41(11), 2953 (2002)
D. Avellaneda, M.T.S. Nair, P.K. Nair, J. Electrochem. Soc. 157, D346 (2010)
M. Bouaziz, J. Ouerfelli, M. Amlouk, S. Belgacem, Phys. Status Solidi A 204(10), 3354 (2007)
M. Bouaziz, K. Boubaker, M. Amlouk, S. Belgacem, J. Phase Equilibria Diffus. 31(6), 498 (2010)
P.A. Fernandes, P.M.P. Salomé, A.F. da Cunha, J. Phys. D Appl. Phys. 43, 215403 (2010)
P.A. Fernandes, P.M.P. Salomé, A.F. da Cunha, Phys. Status Solidi (c) 7(3–4), 901 (2010)
Z. Su, K. Sun, Z. Han, F. Liu, Y. Lai, J. Li, Y. Liu, J. Mater. Chem. 22(32), 16346 (2012)
H. Guan, H. Shen, C. Gao, X. He, J. Mater. Sci. Mater. Electron. 24(5), 1490 (2013)
U. Chalapathi, Y.B. Kishore Kumar, S. Uthanna, V. Sundara Raja, Thin Solid Films 556, 61 (2014)
R. Chamberlin, J. Skarman, J. Electrochem. Soc. 113(1), 86 (1966)
F. Di Benedetto, D. Borrini, A. Caneschi, G. Fornaciai, M. Innocenti, A. Lavacchi, C. Massa, G. Montegrossi, W. Oberhauser, L.A. Pardi, M. Romanelli, Phys. Chem. Miner. 38(6), 483 (2011)
B.D. Cullity, Elements of X-ray Diffraction (Addison Wesley, London, 1956)
M. Ishii, K. Shibata, H. Nozaki, J. Solid State Chem. 105(2), 504 (1993)
B. Minceva-Sukarova, M. Najdoski, I. Grozdanov, C. Chunnilall, J. Mol. Struct. 410, 267 (1997)
C.G. Munce, G.K. Parker, S.A. Holt, G.A. Hope, Colloids Surf. A Physicochem. Eng. Asp. 295(1), 152 (2007)
D.M. Berg, R. Djemour, L. Gütay, S. Siebentritt, P.J. Dale, X. Fontane, V. Izquierdo-Roca, A. Pérez-Rodriguez, Appl. Phys. Lett. 100, 192103 (2012)
U. Chalapathi, Y. Jayasree, S. Uthanna, V. Sundara Raja, Phys. Status Solidi A 210(11), 2384 (2013)
S. Bagul, S. Chavhan, R. Sharma, J. Phys. Chem. Solids 68(9), 1623 (2007)
A. Bollero, M. Grossberg, B. Asenjo, M. Gutiérrez, Surf. Coat. Technol. 204(5), 593 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chalapathi, U., Poornaprakash, B. & Park, SH. Effect of thiourea concentration on the growth and properties of Cu\(_{3}\)SnS\(_{4}\) thin films prepared by spray pyrolysis. J Mater Sci: Mater Electron 28, 2954–2961 (2017). https://doi.org/10.1007/s10854-016-5880-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5880-8