Abstract
In the area of water purification, nanotechnology offers the possibility of an efficient removal of pollutants and germs. Nowadays, nanostructures used for detection and removal of chemical and biological substances include metals, azo dyes, nutrients, cyanide, organics, algae, bacteria, parasites, and etc. In the current study, an attempt is made to synthesize and characterization of NiAl2O4 nanostructures in an aqueous environment through the simple sol–gel method. Besides, three capping agents as glycine, asparagine, and alanine were used to investigate their effects on the morphology and particle size of NiAl2O4 nanostructures. This method starts from of the precursor complex, and involves the formation of homogeneous solid intermediates, reducing atomic diffusion processes during thermal treatment. The formation of pure crystallized NiAl2O4 nanocrystals occurred when the precursor was heat-treated at 800 °C in air for 150 min. The stages of the formation of NiAl2O4, as well as the characterization of the resulting compounds were done made using UV–Vis diffuse reflectance spectroscopy, field emission scanning electron microscopy, energy dispersive X-ray microanalysis, and X-ray diffraction. The magnetic properties of as-prepared NiAl2O4 nanostructures were also investigated with vibrating sample magnetometer. Furthermore, the photocatalytic properties of as synthesized NiAl2O4 were evaluated by degradation of methyl orange as water contaminant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Physical properties and potential applications of nanostructures and nanomaterial have been studied intensively [1–12]. This interest results from the special properties of materials at the nanoscale, such as a large surface-to-volume ratio and increased surface activity, as compared with that of the bulk material. The properties of bulk materials usually depend on the size of the primary particles. Thus, the control of particle size and morphology plays a crucial role in the manufacturing process [13–15]. Transition metal-oxide spinels are important in many application fields because of their high thermal resistance and catalytic, electronic and optical properties. They are commonly used in semiconductor and sensor technology as well as in heterogeneous catalysis [16–24]. Transition metal aluminates are commonly prepared by a solid state reaction [25], co-precipitation method [26], hydrothermal [27], combustion [28], and sol–gel [29, 30]. The disadvantages of solid-state routes, such as inhomogeneity, lack of stoichiometry control, high temperature and low surface area, are improved when the material is synthesized using a solution-based method. Compared with other techniques, the sol–gel method is a useful and attractive technique for the preparation of aluminate spinels because of its advantage of producing pure and ultrafine powders at low temperatures. In this study, we have synthesized nickel aluminate nanocrystals using simple sol–gel method. Furthermore, to investigate the effect of different capping agents such as glycine, asparagine, and alanine on the morphology, particle size, and crystal structure of the products several experiments were performed. To evaluate the catalytic properties of nanocrystalline nickel aluminate, the photocatalytic degradation of methyl orange under UV light irradiation was carried out.
2 Experimental
2.1 Characterization
All the chemicals used in this method were purchased from Merck Company and used without further purification. X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10 < 2θ < 80. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. Spectroscopy analysis (UV–Vis) was carried out using Shimadzu UV–Vis scanning UV–Vis diffuse reflectance spectrometer. The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope. The magnetic measurements of samples were carried out in a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) at room temperature in an applied magnetic field sweeping between ±10,000 Oe.
2.2 Synthesis of NiAl2O4 nanostructures
At first, 1 mmol of Ni(NO3)2·6H2O and 2 mmol of Al(NO3)3·9H2O were dissolved separately in 40 ml distilled water and stirred for 10 min. Then, a solution containing 1 mmol of amino acid was added drop wise into a solution involving 1 mmol of Ni(NO3)2·6H2O. Subsequently, above mention solution was mixed with solution containing 2 mmol of Al(NO3)3·9H2O. Afterwards, the final mixed solution was kept stirring to form a gel at 100 °C. Finally, the obtained product was placed in a conventional furnace in air atmosphere for 150 min and calcine at 800 °C. After thermal treatment, the system was allowed to cool to room temperature naturally, and the obtained precipitate was collected. Reaction conditions are listed in Table 1.
2.3 Photocatalysis experiments
The photocatalytic activities of NiAl2O4 nanocrystal were determined by the degradation of aqueous methyl orange (MO) under UV light. About 0.1 g of the sample was first inserted into a reactor that included 50 ppm of aqueous MO. The suspension was transferred into a self-designed glass reactor, and stirred in darkness to attain the adsorption equilibrium. In the research of photo degradation by UV light, a 400 W mercury lamp with a water cooling cylindrical jacket was utilized. The photocatalytic activity of NiAl2O4 nanostructure was tested by using methyl orange (MO) solution. The degradation reaction was carried out in a quartz photocatalytic reactor. The photocatalytic degradation was carried out with 50 ppm of MO solution containing 0.1 g of nanostructures. This mixture was aerated for 30 min to reach adsorption equilibrium. Then, the mixture was placed inside the photoreactor in which the vessel was 20 cm away from the UV. The quartz vessel and light sources were placed inside a black box equipped with a fan to prevent UV leakage. Aliquots of the mixture were taken at periodic intervals during the irradiation, and after centrifugation they were analyzed with the UV–Vis spectrometer. The methyl orange (MO) degradation percentage was calculated as:
where At and A0 are the obtained absorbance value of the methyl orange solution at t and 0 min by a UV–Vis spectrometer, respectively.
3 Results and discussion
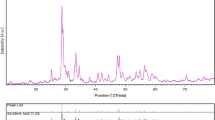
Crystalline structure and phase purity of as-prepared product has been determined using XRD. The XRD pattern of as-prepared NiAl2O4 is shown in Fig. 1. Based on the Fig. 1, the diffraction peaks observed can be indexed to the pure tetragonal phase of NiAl2O4 (a = b = 5.9366 Å, c = 14.5430 Å) with space group of R-3 m and JCPDS no. 01-1299. No diffraction peaks from other species could be detected, which indicates the obtained sample is pure. From XRD data, the crystallite diameter (Dc) of NiAl2O4 nanostructures was calculated to be 13 nm using the Scherer equation:
where β is the breadth of the observed diffraction line at its half intensity maximum (400), K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. Capping agents and surfactants are frequently used in colloidal synthesis to inhibit nanostructures overgrowth and aggregation as well as to control the structural characteristics of the resulted nanostructures in a precise manner [31–41]. In this research glycine, asparagine, and alanine used as capping agent to investigate their effect on the morphology and particle size of the NiAl2O4 nanostructures. Figure 2a–c shows the SEM images of NiAl2O4 nanostructures in the presence glycine, asparagine, and alanine as the capping agent accordance with sample 1–3, respectively. According to the Fig. 2a, product mainly consists of spherical shape nanostructures with average particle size 35–40 nm. Furthermore in the presence of alanine the products mainly consist of nanorod, as shown in Fig. 2c. The EDS analysis measurement was used to investigate the chemical composition and purity of NiAl2O4 nanostructures. According to the Fig. 3, the product consists of Ni, Al, and O elements. Furthermore, neither N nor C signals were detected in the EDS spectrum, which means the product is pure and free of any surfactant or impurity. The VSM magnetic measurements for the nickel aluminate oxide (Fig. 4) show the magnetic properties of nanostructures calcined at 800 °C. The NiAl2O4 nanostructure (sample 1) exhibits paramagnetic behavior at room temperature with a magnetization of 0.12 emu/g. To investigate the optical properties of the NiAl2O4, UV–Vis spectrum was recorded. Figure 5 shows the UV–Vis diffuse reflectance spectrum of NiAl2O4 nanostructures. Using Tauc’s formula, the band gap can be obtained from the absorption data. The energy gap (Eg) of the nanocrystalline NiAl2O4 has been estimated by extrapolating the linear portion of the plot of (αhν)2 against hν to the energy axis. The Eg value of the nanocrystalline NiAl2O4 calculated to be 3.1 eV. According to the obtained Eg value, as-prepared nanostructures NiAl2O4 sample can be employed as the photocatalyst. Photodegradation of methyl orange as water contaminant under UV light illumination was employed to evaluate the properties of the as-synthesized NiAl2O4 nanostructures. Figure 6 exhibits the obtained result. No methyl orange was practically broken down after 80 min without employing UV light illumination or as-prepared NiAl2O4 nanostructures. This observation illustrated that the contribution of self-degradation was insignificant. The proposed mechanism of the photocatalytic degradation of the methyl orange can be assumed as:
Utilizing photocatalytic calculations by Eq. (1), the methyl orange degradation was about 82 % after 80 min illumination of UV light in the presence of NiAl2O4 nanostructures (sample 1). Besides, the whole mechanism is shown in Scheme 1.
4 Conclusions
A facile method is described for synthesizing nanorod and spherical shape nanostructures via a simple sol–gel method in the presence of glycine, asparagine, and alanine as the natural capping agents. Besides, several tests were performed to investigate the effects of natural capping agents on them morphology and particle size of final products. The nickel aluminate oxide nanoparticles exhibit superparamagnetic behaviour at room temperature, with saturation magnetization of 0.12 emu/g. Furthermore, in order to evaluate the photocatalytic properties of nanocrystalline NiAl2O4, the photocatalytic degradations of simulated methyl orange dye wastewater under ultraviolet light irradiation were carried out.
References
V. Arabali, M. Ebrahimi, M. Abbasghorbani, V. KumarGupta, M. Farsi, M.R. Ganjali, F. Karimi, J. Mol. Liq. 213, 312 (2016)
H.R. Naderi, P. Norouzi, M.R. Ganjali, Appl. Surf. Sci. 366, 552 (2016)
S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci.: Mater. Electron. 27, 3240 (2016)
M. Zahraei, A. Monshi, D. Shahbazi-Gahrouei, M. Amirnasr, B. Behdadfar, M. Rostami, J. Nanostruct. 5, 137 (2015)
M. Rahimi-Nasrabadi, J. Nanostruct. 4, 211 (2014)
M. Maddahfar, M. Ramezani, M. Sadeghi, A. Sobhani-Nasab, J. Mater. Sci.: Mater. Electron. 26, 7745 (2015)
S. Khaleghi, J. Nanostruct. 2, 157 (2012)
M. Aliahmad, A. Rahdar, Y. Azizi, J. Nanostruct. 4, 145 (2014)
M. Enhessari, M. Kargar-Razi, P. Moarefi, A. Parviz, J. Nanostruct. 2, 119 (2012)
M. Behpour, M. Mehrzad, S.M. Hosseinpour-Mashkani, J. Nanostruct. 5, 183 (2015)
M. Riazian, J. Nanostruct. 4, 433 (2014)
M. Rahimi-Nasrabadi, M. Behpour, A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, J. Mater. Sci.: Mater. Electron. 26, 9776 (2015)
S.M. Hosseinpour-Mashkani, M. Maddahfar, A. Sobhani-Nasab, J. Mater. Sci.: Mater. Electron. 27, 474 (2016)
S.S. Hosseinpour-Mashkani, S.S. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci.: Mater. Electron. 27, 4351 (2016)
K. Saberyan, N.S. Mazhari, M. Rahiminezhad-Soltani, M.A. Mohsen, J. Nanostruct. 4, 185 (2014)
C.O. Areán, M.P. Mentruit, E.E. Platero, F.X. Xamena, J.B. Parra, Mater. Lett. 39, 22 (1999)
E.E. Platero, C.O. Areán, J.B. Parra, Res. Chem. Intermed. 25, 187 (1999)
J. Merikhi, H.O. Jungk, C. Feldmann, J. Mater. Chem. 10, 1311 (2000)
W. Li, J. Li, J. Guo, J. Eur. Ceram. Soc. 23, 2289 (2003)
D.M.A. Melo, J.D. Cunha, J.D.G. Fernandes, M.I. Bernardi, M.A.F. Melo, A.E. Martinelli, Mater. Res. Bull. 38, 1559 (2003)
P. Thorma-hlen, E. Fridell, N. Cruise, M. Skoglundh, A. Palmqvist, Appl. Catal. B Environ. 31, 1 (2001)
W.M. Shaheen, Thermochim. Acta 385, 105 (2002)
G.A.E. Shobaky, G.A. Fagal, N.H. Amin, Thermochim. Acta 141, 205 (1989)
L. Dussault, J.C. Dupin, C. Guimon, M. Monthioux, N. Latorre, T. Ubieto, E. Romeo, C. Royo, A. Monzon, J. Catal. 251, 223 (2007)
W.S. Hong, L.C. De-Jonghe, X. Yang, M.N. Rahaman, J. Am. Ceram. Soc. 78, 3217 (1995)
T. Mimani, J Alloy Comp 315, 123 (2001)
M. Zawadzki, J. Wrzyszcz, Mater. Res. Bull. 35, 109 (2000)
A.K. Adak, A. Pathak, P. Pramanik, J. Mater. Sci. Lett. 17, 559 (1998)
M. Zayat, D. Levy, Chem. Mater. 12, 2763 (2000)
N. Guilhaume, M. Primet, J. Chem. Soc. Faraday Trans. 90, 1541 (1994)
J. Safaei-Ghomi, S. Zahedi, M. Javid, M.A. Ghasemzadeh, J. Nanostruct. 5, 153 (2015)
M. Rahimi-Nasrabadi, S.M. Pourmortazavi, A.A. Davoudi-Dehaghani, S.S. Hajimirsadeghi, M.M. Zahedi, Cryst. Eng. Comm. 15, 4077 (2013)
M. Behpour, M. Chakeri, J. Nanostruct. 2, 227 (2012)
S.M. Pourmortazavi, M. Taghdiri, V. Makari, M. Rahimi-Nasrabadi, Spectrochim. Acta A Mol. Biomol. Spectrosc. 136, 1249 (2015)
M. Rahimi-Nasrabadi, S.M. Pourmortazavi, M.R. Ganjali, Mater. Manuf. Process. 30, 34 (2015)
S.M. Pourmortazavi, M. Rahimi-Nasrabadi, S.S. Hajimirsadeghi, J. Dispers. Sci. Technol. 33, 254 (2012)
S.M. Hosseinpour-Mashkani, M. Ramezani, A. Sobhani-Nasab, M. Esmaeili-Zare, J. Mater. Sci. Mater. Electron. 26, 6086 (2015)
A. Sobhani-Nasab, M. Maddahfar, S.M. Hosseinpour-Mashkani, J. Mol. Liq. 216, 1 (2016)
S.M. Pourmortazavi, M. Rahimi-Nasrabadi, M. Khalilian-Shalamzari, H.R. Ghaeni, S.S. Hajimirsadeghi, J. Inorg. Organomet. Polym Mater. 24, 333 (2014)
M. Rahimi-Nasrabadi, S.M. Pourmortazavi, M.R. Ganjali, S.S. Hajimirsadeghi, M.M. Zahedi, J. Mol. Struct. 1047, 31 (2013)
S.M. Pourmortazavi, M. Rahimi-Nasrabadi, A.A. Davoudi-Dehaghani, A. Javidan, M.M. Zahedi, S.S. Hajimirsadeghi, Mater. Res. Bull. 47, 1045 (2012)
Acknowledgments
The authors are grateful to council of University of Kashan for providing financial support to undertake this work by Grant No (572801/2).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rahimi-Nasrabadi, M., Ahmadi, F. & Eghbali-Arani, M. Different morphologies fabrication of NiAl2O4 nanostructures with the aid of new template and its photocatalyst application. J Mater Sci: Mater Electron 28, 2415–2420 (2017). https://doi.org/10.1007/s10854-016-5812-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5812-7