Abstract
Aluminum doped zinc oxide (AZO) thin films have become technologically important materials due to their application potential for thermoelectric devices. In this paper, AZO thin films are deposited on the glass substrates using the solution derived by sol–gel route for dip-coating cycles and the radio-frequency sputtering process. The crystalline structure of two types of AZO films is characterized by X-ray diffraction exhibiting the typical hexagonal wurzite structure of ZnO. The rough surface of the heat treated films are observed by the SEM image. The average grain size of obtained films is evaluated based on these investigations showing they are consistent with each other. The sheet resistivity and Seebeck coefficient are measured between room temperature and 400 °C and used to determine the electrical conductivity and power factor of all films. The obtained results provide the advantages and disadvantages of the prepared films by each method for aiming at thermoelectrical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Thermoelectric (TE) effects are related to the ability of the materials that can convert heat directly into electricity or vice versa. The efficiency of a TE converter is proportional to the material electrical conductivity (σ), Seebeck coefficient (S), and inversely thermal conductivity (κ) [1, 2]. Recently, the oxide compounds used for TE applications have stimulated a great interest of researchers in the field of material science because they are stable at high temperatures or under oxidizing conditions and environmental friendly. These features make them to be advantage compared to the traditional TE materials. Among those materials the transparent zinc oxide ZnO was considered the most popular semiconductor having high electron mobility as favorable properties for TE application. Particularly, recent studies of aluminum-doped (ZnO:Al or AZO) as the non toxic and low cost TE materials showed the relatively good properties at high temperature [3–11].
From the summary of oxide thin films to broaden the application of these materials, we need a cheap and up-scalable fabrication method. Herein, we report a comparative study of the structural and thermoelectric properties of Al-doped ZnO films that sol–gel is chosen as the main focus and as compared with RF sputtering. It is because the interests of the AZO films derived by sol–gel method were mostly for the optical and electrical properties [12–16] but only one work of investigating the TE property reported [5]. The typical experiments including X-ray diffractometry (XRD), scanning electron microscope (SEM), electron diffraction spectrometry (EDS), four-point probe, and the Seebeck measurement technique were used to study the crystal structure, surface morphology, electric conductance, and Seebeck coefficient of obtained AZO films.
2 Materials and experimental methods
The preparation procedures of AZO thin films by the sol–gel technique consist of three major steps including the preparation of solution, the film coating, and the heat treatment. The precursor solution was synthesized by mixing, in appropriate proportions, zinc acetate dihydrate (ZnAc) and aluminum nitrates 9-hydrate as dopant source. The Zn/Al ratios in the solution were chosen 98/2 at.% weight relying on the reported works [3–11] that showed the best TE property. In our work, ethylene glycol, isopropanol, glyerin, and triethylenamin (TEA) were used as solvent and catalyst. A homemade dip-coating apparatus was used to accomplish the coating procedure. The withdrawing speed of the glass substrate (Corning 1737F) was set at 20 cm/min for uniform deposition. After the multiple coatings, the AZO coated samples were finally post-heated at 550 °C in a furnace with N2/H2 mixing ambient for 1 h to set the crystal structures. The detail procedure of the method is shown in Fig. 1.
For the preparation of AZO thin films by the RF sputtering, the chamber pressure was pumped up to 10−6 Torr at first using an AZO (ZnO 98 wt.%: Al2O3 2 wt.%) ceramic target. The Corning 1737F glass substrates were heated to 300 °C. The ambient composition is adjusted with the relative gas inlet flow rates of Ar and N2 corresponding to a value of 10 sccm. The power is kept a constant at 60 W and sputtering pressure of 10−2 Torr for 30 min. All films fabricated by both methods have the thickness around 300 nm.
X-ray diffraction (Cu-Kα, Siemen D5005 Brucker, λ = 1.54056 A) was employed to identify the crystal structure of the samples at room temperature. SEM Hitachi S-4800 system was used to examine the grain size and morphology. The sheet resistivity of the AZO thin films was analyzed by DC four-terminal method, and its TE characterization was investigated by using a home-made Seebeck measurement setup. Due to the lack of equipment for the measurement of thermal conductivity, the figure of merit of fabricated AZO films is not dealt with this work.
3 Results and discussion
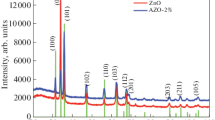
Figure 2 is X-ray diffraction spectra for analysis of crystal structure of Al-doped ZnO films deposited onto glass substrates by using sol–gel solution and sputtering method. In general, two spectra show a dominant peak at 34.5° reflecting the preferential orientation of the prepared films along the crystallographic axis [002] and another common peak at 63.5° which is from the diffraction of the (103) planes. For films derived by sputtering method no other peaks can be seen in the patterns, indicating the preferred orientation with the c-axis normal to the substrate. However, for films chemically fabricated the two other diffraction peaks can be found at 31.8° and 36.2°, corresponding to (100) and (101) planes. It can be seen that the (002) peak for this type of films has a lower intensity compared with that physically deposited, indicating a degradation of the crystalline structure due to the instability of chemical method. In fact, there is a small shift in the (002) plane between two types of samples. This result may be due to a compressive residual stress in the films chemically fabricated. Based on the measured XRD patterns, the average grain size can be calculated using famous Scherrer’s formula. The grain size of AZO crystallite calculated using that equation is in range of 12–14 nm for sol–gel derived films and 35–40 nm for sputtering derived films.
The chemical composition of AZO films derived by using sol–gel solution was verified by EDS method as shown in Fig. 3. It is possible to see that the intensities of the peaks of the elements zinc and oxygen are consistent with the concentrations in the film. The presence of silicon and calcium which are elements of the substrate can be explained by film thickness which is smaller than 1 μm that the electron beam can easily reach the substrate during scanning. A small peak of aluminum is clearly observed. The semi-quantitative calculations based on the EDS spectra show that the aluminum concentration in the obtained AZO films is about 1.9 at.%, less than 0.1 at.% as desired. This result also affirms that there was an incorporation of dopant ions in the correct locations of ZnO crystal structure with uniform and homogeneous distribution to provide the free electrons as main carriers. It therefore can be said that our sol–gel procedure to make AZO films is very stable.
Figure 4 shows the film morphology obtained by the scanning electron microscopy (SEM) images. The typical clusters of the coherent grains can be easily observed in these pictures. It can be found that the surface topography of both types of films is different from each other. Visually, the films chemically fabricated have the wormlike grains with length of about 30–50 nm and size of about 15–20 nm including many pores inside. Inversely, the films physically have the circular-shaped isometric and uniform grains with larger average dimension of about 40–50 nm. These results correspond with the shape of XRD spectra and predicted calculation relying on that structural analysis. We can also easily see that this type of films is more connected and dense compared to that chemically fabricated.
Both the electrical and TE property of AZO thin films were investigated at the range between room temperature and 400 °C or equivalently 300 and 773 K. The Seebeck coefficient of films is determined by measuring voltage at two ends one of which is at hot temperature (T H ) and the remainder is at cold one (T C ) with the difference between them of about 10 K.
As well known, the electrical conductivity (σ) of thin films can be determined by using the measurement results of sheet resistance as σ = 1/ρ, where resistivity (ρ) is calculated by multiplication of the films sheet resistance and thickness. The plot showing this property of both types of AZO films in our study is demonstrated in Fig. 5. Since the glass substrate is an insulator, its contribution to conductivity of AZO films can be neglected. We can see that the electrical conductivity σ increases with temperature exhibiting n-type semiconductor characterization for obtained AZO films. The lower electrical conductivity of the chemical samples compared to physical ones may be due to the existence of pores in films chemically produced resulting in the electron scattering at grain-boundaries. Beside, as can be seen in SEM image, the films deposited by sputtering method have better density with the large grains and consequently small amount of grain boundaries accordingly resulting in the higher values of the electrical conductivity.
The Seebeck coefficient (S) is an important parameter characterizing the thermoelectric properties of the AZO films. The temperature dependence of this parameter for two types of films in our study is shown in Fig. 6. For both types of films, the values of S linearly increase with the ambient temperatures. These linear variations are related to free electron model which are temperature dependent phenomena. The typically lowest and highest values of Seebeck coefficient are about 80–130 μV/K for sol–gel samples while only about 45–85 μV/K for sputtering ones at 300 and 700 K, respectively. The particle size of sol–gel samples shown in Fig. 5 is small that effected on the continuity of moving carriers causing the charge accumulation at two ends of measured sample and consequently larger thermoelectric voltages and larger Seebeck coefficient. The lower Seebeck coefficient of the sputtering sample is consistent with its higher electrical conductivity that results in the smaller change in carrier concentration at two ends leading to the smaller thermoelectric voltage. It should be noted that these obtained results of Seebeck coefficient for sputtering samples are relatively good and promising when evaluated to other thin films with the same n-type conductivity and high temperature heatproof that were reported before. Generally, it is much better than that of Sb-doped FTO, Fe- and Cu-doped tin [17], and equivalent to Ag2S [18] as well as CoSb3 [19] thin films. However, one point should be again emphasized that the advantages of AZO thin films are more convenient to fabricate and less harmful to environment than those ones.
In general, the absolute values of both electrical conductivity and Seebeck coefficient for chemical samples in our study are better than that reported in previously work [5]. Probably, using ethanol base solution makes better soluble for zinc acetate and implementation of solvents like glycerin and TEA improved the viscosity of sol–gel solution. As a result, these chemicals result in the improvement of the film quality leading to the better material properties compared to the reported ones before.
Another typical TE property is power factor (PF) which determine the performance of the thermoelectric energy converter. It is calculated by the values of Seebeck coefficient and electrical conductivity, as PF = σS 2. The graph of this quantity versus temperature is represented in Fig. 7 showing the similar trend of Seebeck coefficient characteristics that its values increase with the measuring temperatures. Both types of films show the relatively good efficiency of thermoelectricity. Namely, the best value of pF for chemically produced films is about half of that for physically produced films that is 0.16 mW/mK2 compared to 0.33 mW/mK2. Once again, this TE characterization of chemical samples using sol–gel solution showed the impressional result when compared with that obtained by physical method. It should be noted that the calculated values of PF for sputtering films in our study are better than those reported in [7, 8], as same as in [9] and comparably with the results obtained by PLD [10, 11] which is considered a best method for fabrication of TE AZO films.
4 Conclusion
In this work, the AZO films deposited on glass substrates with 2 at.% Al doping were produced by both of chemical and physical methods. The material characterizations of crystal structure, morphology and electrical and TE properties were investigated. AZO thin films physically produced show a relatively stronger texture and better crystalline for high electrical conductivity, but smaller Seebeck coefficient compared to those of films chemically fabricated. The increasing trend of Seebeck coefficient and the power factor of the obtained AZO films along with increased temperature is expected result for the better TE property at higher temperature. The TE property of sputtering in our study was also compared to some other thin films made of other materials showing the equivalent results as those reported before. The films fabricated by chemical method exhibit the lower power factor than that of films physically produced. At present, the energy efficiency of TE devices is hard to have multiple times increased even employed by the best fabrication method. Therefore, in this situation, the sol–gel method is still acceptable for the aspect of TE applications. Economically, if we can provide the TE devices using the materials prepared by simple method with the price is twice lower than other ones fabricated by using materials produced by the expensive equipment. It is a reason why our study has been performed in this situation.
References
D.M. Rowe, Handbook of Thermoelectrics—Macro to Nano (CRC/Taylor & Francis, Boca Raton, 2006)
H. Julian Goldsmid, Introduction to Thermoelectricity, e-ISBN 978-3-642-00716-3, (Springer, Heidelberg Dordrecht London New York, 2010)
L. Li, L. Fang, X.J. Zhou, Z.Y. Liu, L. Zhao, S. Jiang, X-ray photoelectron spectroscopy study and thermoelectric properties of Al-doped ZnO thin films. J. Electron Spectrosc. Relat. Phenom. 173, 7–11 (2009)
M. Ruoho, V. Pale, M. Erdmanis, I. Tittonen, Influence of aluminium doping on thermoelectric performance of atomic layer deposited ZnO thin films. Appl. Phys. Lett. 103, 203903 (2013). doi:10.1063/1.4831980
M.H. Hong, C.S. Park, W.S. Seo, Y.S. Lim, J.K. Lee, H.H. Park, Thermoelectric properties of Al-doped mesoporous ZnO thin films. J. Nanomater. 2013, 1 (2013). doi:10.1155/2013/131537
A.I. Abutaha, S.R. Sarath Kumar, H.N. Alshareef, Crystal orientation dependent thermoelectric properties of highly oriented aluminum-doped zinc oxide thin films. Appl. Phys. Lett. 102(5), 053507 (2013)
Z. H. Zheng, P. Fan, G. X. Liang, P. J. Liu, P. J. Cao, D. P. Zhang, Z. K. Cai1, X. Ou, C. Y. Lai, Thermoelectric Properties of Al Doped ZnO Thin Films, in Materials Science Forum, vol. 743–744 (Trans Tech Publications, Switzerland, 2013), pp. 138–143
P. Fan, Y.-Z. Li, Z.-H. Zhenga, Q.-Y. Lin, J.-T. Luo, G.-X. Liang, M.-Q. Zhang, M.-C. Chen, Thermoelectric properties optimization of Al-doped ZnO thin films prepared by reactive sputtering Zn–Al alloy target. Appl. Surf. Sci. 284, 145–149 (2013)
J. Loureiro, N. Neves, R. Barros, T. Mateus, R. Santos, S. Filonovich, S. Reparaz, C.M. Sotomayor-Torres, F. Wyczisk, L. Divay, R. Martins, I. Ferreira, Transparent aluminium zinc oxide thin films with enhanced thermoelectric properties. J. Mater. Chem. A 2, 6649–6655 (2014)
S. Saini, P. Mele, H. Honda, K. Matsumoto, K. Miyazaki, A. Ichinose, Thermoelectric properties of Al-doped ZnO thin films. J. Electron. Mater. 43(6), 2145–2150 (2014)
P. Mele, S. Saini, H. Honda, K. Matsumoto, K. Miyazaki et al., Effect of substrate on thermoelectric properties of Al-doped ZnO thin films. Appl. Phys. Lett. 102, 253903 (2013). doi:10.1063/1.4812401
G.G. Valle, P. Hammer, S.H. Pulcinelli, C.V. Santilli, Transparent and conductive ZnO: Al thin films prepared by sol-gel dip-coating. J. Eur. Ceram. Soc. 24, 1009–1013 (2004)
H.-M. Zhou, D.-Q. Yi, Z.-M. Yu, L.-R. Xiao, J. Li, Preparation of aluminum doped zinc oxide films and the study of their microstructure, electrical and optical properties. Thin Solid Films 515, 6909–6914 (2007)
T. Kemmitt, B. Ingham, R. Linklater, Optimization of SolGel-formed ZnO: Al processing parameters by observation of dopant Ion location using solid-state Al NMR spectrometry. J. Phys. Chem. C 115, 15031–15039 (2011)
M.-C. Jun, S.-U. Park, J.-H. Koh, Comparative studies of Al-doped ZnO and Ga-doped ZnO transparent conducting oxide thin films. Nanoscale res. lett. 7, 639 (2012)
M.F. Malek, M.H. Mamat, Z. Khusaimi, M.Z. Sahdan, M.Z. Musa, A.R. Zainun, A.B. Suriani, N.D. Md Sin, S.B. Abd Hamid, M. Rusop, Sonicated sol–gel preparation of nanoparticulate ZnO thin films with various deposition speeds: the highly preferred c-axis (002) orientation enhances the final properties. J. Alloy. Compd. 582, 12–21 (2014)
E. Mokaripoor, M.-M. Bagheri-Mohagheghi, Effect of very low to high Sb-doping on the structural, electrical, photo-conductive and thermoelectric properties of fluorine-doped SnO2 (FTO) thin films prepared by spray pyrolysis technique. J. Mater. Sci.: Mater. Electron. (2015). doi:10.1007/s10854-015-4026-8
M. Adelifard, R. Torkamani, Influence of growth temperature and silver to sulfur molar ratios on optical, electrical and thermoelectrical properties of nanostructured Ag2S thin films. J. Mater. Sci.: Mater. Electron. (2015). doi:10.1007/s10854-015-3392-6
P. Fan, Y. Zhang, Z.-H. Zheng, W.-F. Fan, J.-T. Luo, G.-X. Liang, D.-P. Zhang, Thermoelectric properties of cobalt antimony thin films deposited on flexible substrates by radio frequency magnetron sputtering. J. Electron. Mater. 44(2), 630–635 (2015). doi:10.1007/s11664-014-3546-y
Acknowledgments
This work has been supported by the Vietnam’s National Foundation for Science and Technology Development (NAFOSTED) grant funded by Ministry of Science and Technology (MOST) under Grant Number 103.02-2013.52.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trinh, T.Q., Nguyen, T.T., Vu, D.V. et al. Structural and thermoelectric properties of Al-doped ZnO thin films grown by chemical and physical methods. J Mater Sci: Mater Electron 28, 236–240 (2017). https://doi.org/10.1007/s10854-016-5516-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5516-z