Abstract
Copper (Cu) and iron (Fe) doped zinc oxide (ZnO) nanoparticles (NPs) were synthesized by co-precipitation method. The average crystallite sizes of the synthesized NPs were found to be 28 and 30 nm for Cu and Fe doped ZnO NPs respectively. From FESEM images, Cu and Fe doped ZnO NPs showed that the spherical structure morphology. The amount of dopant (Cu2+ and Fe3+) ions incorporated with ZnO NPs was determined by EDAX. FT-IR spectra confirmed the presence of Zn–O stretching bands at 438, 416 and 431 cm−1 in the respective ZnO NPs. Optical absorption spectra revealed the estimated band gap was found to increase for Cu and Fe doped ZnO NPs as compared to the pure ZnO NPs. The photoluminescence measurements revealed the broad emissions were composed seven different bands due to zinc vacancies, oxygen vacancies and surface defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ZnO NPs is an important direct band gap semiconducting material, belongs to II–VI group compound. In room temperature, ZnO has a band gap of 3.37 eV and large exciton binding energy 60 meV. Which have properties such as piezoelectricity, chemical stability, and optical transparency in this visible region [1], optoelectronic devices and gas sensors [2], transparent electrodes [3–5], solar cells, ferroelectric memories and acoustic wave devices [6–8].
ZnO NPs has significant applications in cosmetic pigments, surface acoustic wave devices, varistors, optical materials, catalyst, Ultraviolet absorbers and as window material for displays and solar cell [9]. In future application, next generation of inorganic light emitting diode [10], and laser for example, ZnO NPs appear as a probable substitute for gallium nitrate and its derivatives whose fabrication remains expensive and polluting. The advents of such a creative technology suffer from need of simply obtainable p–n homojunctions. However, efficient and excellent quality of p-type has been the main hectic task which delays the launching of ZnO-based optoelectronics devices [11].
The band gap, optical, electrical and magnetic properties are alter during the substitution of foreign impurities [12–14]. Photoluminescence spectra defect level was reducing in the ZnO NPs, by increasing of Fe concentration in the ZnO lattice sites [15]. In literature, the Cu doped ZnO NPs reduction in the energy gap from 3.49 to 3.43 eV revealed the substitution of Cu2+ ions in the ZnO lattice [16].
A variety of chemical techniques were used to synthesize ZnO NPs, such as solvothermal [17], hydrothermal [18], co-precipitation [19] and sol–gel methods [20], etc. Among these synthetic routes, co-precipitation method has been popularly adopted to synthesize by using ZnO NPs due to its low cost and high yield of nanoparticles with uniform size.
In the present work, Cu and Fe doped ZnO NPs were synthesized by co-precipitation method. We have studied the structural and optical properties of the Cu and Fe doped ZnO NPs for using optoelectronic application.
2 Materials and methods
The experimental procedure for the preparation of Cu and Fe doped ZnO NPs were as follows. The details of the experimental procedure for the preparation of ZnO NPs have been reported in our previous paper [21].
Similarly, Cu and Fe doping on ZnO were done as following and 0.003 M (copper nitrate for cu doping and Iron nitrate for Fe doping) solutions were added into 0.097 M zinc nitrate solution. Then 0.8 M of NaOH solution was added drop-wise in the above mentioned solutions. The solution with the brown precipitate was stirred at room temperature for 6 h. This solution was refluxed for 24 h. Then, a clear solution was obtained, which found to be stable at ambient condition. Thus, Cu and Fe doped ZnO NPs samples were obtained. These samples were annealed at 700 °C for 5 h. The annealed samples were used for further analysis.
The Cu and Fe doped ZnO NPs were characterized by X-ray diffractometer (model: X’PERT PRO PANalytical). The diffraction patterns were recorded in the range of 20°–80° for the ZnO samples used with the monochromatic wavelength of 1.5405 Å. The samples were analyzed by FESEM (model: SUPRA 55) with EDAX (model: ULTRA 55). The FT-IR spectra were recorded in the range of 400–4000 cm−1 by using Shimadzu IR Affinity-1S spectrometer. The UV–Vis spectra were recorded in the range 190–1110 nm by using Lambda 35 UV–Vis spectrophotometer. The photoluminescence (PL) measurement was performed on a (450 W high pressure Xenon lamp as the excitation source, Photomultiplier, range 325–550 nm record for Horiba Jobin–Yvon spectrofluorometer (model: FLUOROMAX-4).
3 Results and discussion
3.1 X-ray diffraction studies
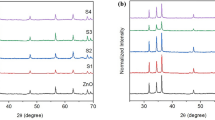
The XRD patterns of Cu and Fe doped ZnO NPs are shown in Fig. 1. The ZnO NPs was reported in early paper [21]. The pronounced diffraction peaks are clearly exhibiting the crystalline nature with peaks corresponding to (100), (002), (101), (102), (110), (103), (200), (112) and (201) planes. The standard diffraction peaks reveal that the crystal structure of Cu and Fe doped ZnO NPs are of hexagonal wurtzite structure (space group p63mc, JCPDS data card no: 79-220).
In XRD result observed that, Fe doped ZnO NPs shows (Fig. 1) no additional peaks, which confirms there is no additional phase formation but there is a slight higher angle shift as compared to ZnO NPs diffraction pattern [21]. This is due to the replacement of Zn2+ (ionic radius is 0.74 Å) by the Fe3+ (ionic radius is 0.63 Å) in their ZnO lattice site. Similar kind of result were found in Fe doped ZnO NPs [15].
Furthermore, Cu doped ZnO NPs (1.13 Å for Cu2+) have one additional peak emerged at 38.90° corresponding to CuO phase along (111) plane (matched with JCPDS data card no: 05-0661) which may be due to the formation of CuO from remaining un-reacted Cu2+ ions present in the solution [16].
The micro-strain analysis is performed for the samples from the X-ray diffraction pattern. The breadth of the Bragg peak is a combination of both instrument and sample-dependent effects. To decouple these contributions, it is necessary to collect a diffraction pattern from the line broadening of a standard material such as silicon to determine the instrumental broadening. The instrument-corrected broadening βhkl [22] can be represented by
The crystallite size of ZnO NPs was determined by the X-ray line broadening method using the Scherrer’s equation,
where D is the size in nanometers, λ is the wavelength of the radiation (1.5406 Å for CuKα), k is a constant (0.94), βD is the peak width at half-maximum intensity and θ is the peak position.
The average crystallite size (Table 1) decreases in the Cu2+ and Fe3+ doped ZnO NPs as compared to pure ZnO NPs [21]. The reduction in the particle size are mainly due to the distortion in the host ZnO lattice by the foreign impurities i.e., Cu2+ and Fe3+, which decrease the nucleation and subsequent growth rate of the ZnO NPs.The dislocation density of ZnO NPs was calculated from the equation [23]
where D is the crystallite size of the particles.
The substitution of Cu2+ and Fe3+ ions in the interstitial position of host ZnO lattice sites would affect the concentration of the interstitial Zn, oxygen and Zn vacancies. The observation of small changes in 2θ values of diffraction peaks and the peak broadening are due to the increase in micro-strain and the line broadening. This effect is mainly caused by the size and micro-strain [24] of the nanoparticles.
The strain-induced broadening βε is given by the Williamson formula βε = 4εtan θ, where ε is the root mean square value of the micro-strain. Assuming that the particles size and strain contributing to the line broadening, independent of each other and both have a Cauchy like profile. The observed line width is simply the sum of these two, i.e., βhkl = (kλ/Dcos θ) + 4εtan θ, which becomes as
When plotting the Williamson–Hall equation between β cos θ versus 4sin θ as shown in Fig. 2, the micro strain ε values are given in Table 1. As compared to the pure ZnO NPs, the strain increases in the Cu2+ and Fe3+ doped ZnO NPs, these effects due to change in size and shape of the particles.
3.2 Field emission scanning electron microscopic analyses
The FESEM images of the Cu and Fe-doped ZnO NPs are shown in Fig. 3a–d. The pure ZnO nanoparticles are found to be spherical and rod like mixed phase [21]. The Cu and Fe doped ZnO NPs are formed spherical structure. The particles sizes are found to be 74, 37 and 60 nm for pure ZnO [21], Cu and Fe doped ZnO NPs, respectively. The particles sizes of the Cu and Fe doped ZnO NPs are found to be reduced as compared to that of pure ZnO NPs. This reduction in particles size are due to the distortion in the host material incorporated with Cu2+ and Fe3+ metal ions in ZnO NPs surface area. Hence the sizes of ZnO NPs are reduced by Cu and Fe doping.
3.3 Energy dispersive analysis X-ray (EDAX) studies
The typical EDAX spectra of the Cu and Fe doped ZnO samples are shown in Fig. 4a, b. From the EDAX analysis, the amounts of transition metal ions present in the doped ZnO NPs as shown in Fig. 4a, b. In the doped samples, the concentrations of Cu and Fe were found to be 3.25 and 2.20 % respectively. In the pure ZnO samples, the chemical compositions of Zn and O were found to be 56.42 and 43.58 % respectively [21]. From the EDAX result, the zinc percentage decreased whereas the oxygen percentage increases and the values are given in Fig. 4a, b. The Cu and Fe doped ZnO NPs as compared to the undoped ZnO NPs, reverse treat in the chemical composition.
3.4 UV–Vis spectroscopy studies
UV–visible absorption Spectroscopy is the potential method to discover the optical properties of semiconducting nanoparticles [25]. The UV–Vis absorption spectra of Cu and Fe doped ZnO NPs have been calcination at 600 ◦C. In our early report, the pure ZnO NPs excitonic peak was observed around 376 nm [21]. But in the case of Cu and Fe doped ZnO NPs cutoff wavelength at 373 and 361 nm as shown in Fig. 5. The position of the absorption spectra were observed to blue shift towards the lower wavelength side in Cu and Fe doped ZnO NPs as compared to the pure ZnO NPs respectively. This indicates the band gap of ZnO NPs increases due to incorporation of Cu and Fe in ZnO lattice site. The optical energy band gap of Cu and Fe doped ZnO NPs are calculated by classical Tauc relation as given below [26].
where Eg is the optical band gap. A is a constant and the exponent n depends on the transition [27]. The value of n = 1/2, 3/2, 2, or 3 depends on the nature of the electronic transition (1/2 for allowed direct transition, 2 for allowed in-direct transition, 3/2 and 3 for forbidden direct and forbidden indirect transition, respectively). Considering direct band transition in Cu and Fe doped ZnO NPs, a plot between (αhυ)2 versus photon energy (hυ) drawn calcination at 600 °C is shown in Fig. 5a, b. Extrapolation of linear region of these plots to (αhυ)2 = 0 gives corresponding direct energy band gap. The optical band gap of pure ZnO NPs 3.30 eV [21], and transition metal like Cu and Fe doped ZnO NPs 3.32 and 3.43 eV respectively. As comparing it with that of the pure ZnO NPs, the increase in the band gap or blue shift can be explained on the basis of the Burstein–Moss effect [28], while the Fermi level shifts close to the conduction band due to increase in the carrier concentration. The low energy transition are blocked and the value of band gap increases due to the incorporation of Cu2+ and Fe3+ ion in ZnO lattice. The important parameter noticed in Cu and Fe doped ZnO NPs is the large difference of energy gap which assists the optoelectronic applications.
3.5 FT-IR spectroscopic studies
The incorporation of Cu and Fe ions in the ZnO lattice and the vibration states were characterized by FTIR spectra. Table 2 represents the assignment values of ZnO, Cu and Fe doped ZnO NPs. FTIR spectra of Cu and Fe doped ZnO NPs is given in the Fig. 6. The O–H stretching of H2O in the Cu–Zn–O and Fe–Zn–O lattice around 3434 and 3439 cm−1 [29]. The asymmetric C–H stretching occurs at 2923 cm−1 for Cu doped ZnO and 2951 cm−1 for Fe doped ZnO NPs [24]. The symmetric and asymmetric stretching vibration of C=O are observed at 1434, 1441, 1543 and 1557 cm−1 [30] for Cu and Fe doped ZnO NPs respectively. Another sharp peak around 1643 and 1651 cm−1 is attributed to H–O–H bending vibration of Cu and Fe doped ZnO NPs, which is assigned to a small amount of H2O in the ZnO NPs [31]. The weak ZnO vibration frequency shift from 881 [21], 871 and 863 cm−1 by the substitution of Cu and Fe ions into the ZnO lattice site [32]. The absorption bands of Zn–O stretching are exhibited at 416 and 431 cm−1 for Cu and Fe doped ZnO NPs [33].
3.6 Photoluminescence (PL) spectroscopic studies
In common, ZnO NPs were observed in ultraviolet (UV) and visible emission region. The UV emission is due to band-edge emission and visible emission, because of different intrinsic defects such as oxygen Vacancies, zinc vacancies, oxygen interstitial, zinc interstitials and oxygen antisites [34].
The photoluminescence spectra of the Cu and Fe doped ZnO NPs are shown in Fig. 7a, b. The PL spectra of the Cu and Fe doped ZnO NPs samples recorded with the excited wavelength of 325 nm. The PL emission are observed for doped ZnO samples covering from the very short wavelength of 340 nm to long wavelength 575 nm. A good fit of seven peaks Gaussian function is obtained for all the PL spectra of the samples at the bottom labeled as G1, G2, G3, G4, G5, G6 and G7.
Pure, Cu and Fe doped ZnO NPs values are given in the Table 3. From the PL spectra three near band edge emission (G1, G2, and G3) wavelength at (349, 364, and 397 nm), (349, 364, and 396 nm) and (349, 364, and 392 nm) for pure, Cu and Fe doped ZnO respectively. This UV peak corresponds to the radiative recombination of the free exciton–exciton collision process in the ZnO NPs. The G4 peak is the origin of the violet emission centered at 423 nm for ZnO and doped ZnO NPs. This peak attributed to an electron transition from a shallow donor level of the natural zinc interstitials to the top level of the valence band [35]. The Peak G5, two blue emission wavelength at (443 and 458 nm) (443 and 460 nm) and (444 and 458 nm) for ZnO, Cu and Fe doped ZnO nanoparticles respectively; it’s attributed to singly ionized Zn vacancies [36]. Finally, blue–green emission bands (G7) at 484, 491 and 484 nm due to a surface defects in the ZnO NPs corresponding to the transition between oxygen vacancy and oxygen interstitial defect.
The Cu and Fe doped ZnO NPs as compared to pure ZnO NPs; the small shift may be occur from different origins, such as electron phonon coupling, lattice distortion, localization of charge carriers due to interface effects and point defects. Due to the substitution of impurities impacts, near band edge UV emission and blue–green emission shift from 397 to 392 nm and 491 to 484 nm for pure and doped ZnO NPs, which confirmed the substitution of Cu2+ and Fe3+ into the ZnO lattice sites.
4 Conclusions
In conclusion, the Cu and Fe-doped ZnO NPs were prepared by co-precipitation method. The X-ray diffraction study confirmed that the prepared nanoparticles were hexagonal wurtzite structure. The average crystallite size of pure ZnO NPs, Cu and Fe doped ZnO NPs were found to be 35, 28 and 30 nm respectively. This size reductions mainly due to the distortion in the host ZnO lattice by the foreign impurities. From the FESEM images, the Cu and Fe doped ZnO NPs were formed spherical structure. From the EDAX analysis, the chemical compositions of Cu and Fe concentration doped with ZnO NPs were found to be 3.25 % (Cu) and 2.20 % (Fe). From the recorded FT-IR spectra, absorption peak of Zn–O stretching were exhibited at 438, 416 and 431 cm−1 for pure ZnO, Cu, and Fe doped ZnO NPs respectively. The PL spectra showed that, near band edge UV emission and blue–green emission shift from 397 to 392 nm and 491 to 484 nm for pure and doped ZnO NPs, which confirmed the substitution of Cu and Fe into the ZnO lattice. In PL spectra the defect level emission was reduced only for Fe doped ZnO NPs but not in Cu doped ZnO NPs. Because of Fe doped ZnO NPs showed no additional peaks, which is mainly due to the replacement of Zn2+ ions by the Fe3+ ions in their ZnO lattice site. But in the case of Cu doped ZnO NPs (1.13 Å for Cu2+) have one additional peak appeared due to the impact of higher ionic radius of Cu ion. The optoelectronic properties mainly depends on reduction of defect level in material, which influenced by electron phonon coupling interaction. In present investigation, Fe doped ZnO NPs defect level was decreased as compared to Cu doped ZnO NPs. In PL results provides strong support for the further development of extensive optical device application.
References
S.D. Kshirsager, D. Inamdar, I.K. Gopalakrishnan, S.K. Kulshreshtha, S. Mahamuni, Solid State Commun. 143, 457–460 (2007)
D.J. Norris, A.L. Efros, S.C. Erwin, Science 319, 1776–1779 (2008)
H.P. He, H.P. Tang, Z.Z. Ye, L.P. Zhu, B.H. Zhao, L. Wang, X.H. Li, Appl. Phys. Lett. 90, 023104-1–023104-3 (2007)
P. Kadam, C. Agashe, S. Mahamuni, J. Appl. Phys. 104, 103501-1–103501-4 (2008)
H. Wang, S. Baek, J. Song, J. Lee, S. Lim, Nanotechnology 19, 075607-1–075607-6 (2008)
S.Y. Li, P. Lin, C.Y. Lee, T.Y. Tseng, C.J. Huang, J. Phys. D Appl. Phys. 37, 2274 (2004)
J.J. Liu, M.H. Yu, W.L. Zhou, Appl. Phys. Lett. 87, 173119–173119-3 (2005)
L.P. Zhu, M.J. Zhi, Z.Z. Ye, B.H. Zhao, Appl. Phys. Lett. 88, 1–3 (2005)
B. Chavillon, L. Cario, A. Renaud, F. Tessier, F. Chevire, M. Boujtita, Y. Pellegrin, E. Blart, J. Am. Chem. Soc. 134, 464 (2012)
S.B. Rana, A. Singh, N. Kaur, J. Mater. Sci. Mater. Electron. 24, 44–52 (2012)
D.C. Look, Recent advances in ZnO materials and devices. Mater. Sci. Eng. B 80, 383–387 (2001)
J.G. Lu, S. Fujita, T. Kawaharamura, H. Nishinaka, Y. Kamada, T. Ohshima, Z.Z. Ye, Y.J. Zeng, Y.Z. Zhang, L.P. Zhy, H.P. He, B.H. Zhai, J. Appl. Phys. 101, 083705–083707 (2007)
A.B. Djurisic, Y.H. Leung, Small 2, 944–961 (2006)
D.L. Leslie-Pelecky, R.D. Rieke, Chem. Mater. 8, 1770–1783 (1996)
T. Pandiyarajan, R. Udaya Bhaskar, B. Karthikeyan, Spectrochim. Acta. A 103, 173–178 (2013)
S. Muthukumaran, R. Gopalakrishnan, Opt. Mater. 34, 1946–1953 (2012)
Z. Liu, Q. Zhang, G. Shi, Y. Li, H. Wang, J. Magn. Magn. Mater. 323, 1022–1026 (2011)
S. Gao, D. Li, X. Lv, J. Wang, H. Li, Q. Yu, F. Guo, L. Zhao, J. Alloys Compd. 539, 200–204 (2012)
G. Vijayaprasath, G. Ravi, A.S. HajaHameed, T. Mahalingam, J. Phys. Chem. C 118, 9715–9725 (2014)
M. Arshad, A. Azama, A.S. Ahmed, S. Mollah, A.H. Naqvi, J. Alloys Compd. 509, 8378–8381 (2011)
P. Geetha Devi, A. Sakthi Velu, J. Adv. Appl. Sci. Res. 1, 130–137 (2015)
S. Singhal, J. Kaur, T. Namgyal, R. Sharma, Phys. B 407, 1223–1226 (2012)
G. Vijayaprasath, R. Murugan, T. Mahalingam, G. Ravi, J. Mater. Sci. Mater. Electron. 26, 7205–7213 (2015)
A.S. Haja Hameed, C. Karthikeyan, S. Sasikumar, V. Senthil Kumar, S. Kumaresan, G. Ravi, J. Mater. Chem. B 1, 5950–5962 (2013)
S.S. Lin, J.L. Huang, Surf. Coat. Technol. 185, 222–227 (2004)
J. Tauc, Amorphous and Liquid Semiconductor (Plenum Press, London, 1974), p. 159
L. Xiao-Bo, S. Hong-Lie, Z. Hui, L. Bin-bin, Trans. Nonferr. Met. Soc. China 17, 3814–3817 (2007)
S. Suwanboon, T. Ratana, W.T. Ratana, J. Sci. Technol. 4, 111–121 (2007)
K. Nakamoto, IR Spectra of Inorganic and Coordination Compounds, Part A and B (Wiley, New York, 1997)
S.S. Alias, A.B. Ismail, A.A. Mohamad, J. Alloys Compd. 499, 231–237 (2010)
A. JagannathReaddy, M.K. Kokila, H. Nagabhushan, R.P.S. Chakradhar, C. Shivakumar, J.L. Rao, B.M. Nagabhushan, J. Alloys Compd. 509, 5349–5355 (2011)
S. Muthu Kumaran, R. Gopalakrishan, J. Sol-Gel Sci. Technol. 62, 193–200 (2012)
N.R. Yogamalar, S. Anitha, R. Srinivasan, A. Vinu, K. Ariga, A. Chanda Bose, J. Nanosci. Nanotechnol. 9, 5966–5972 (2009)
P. Mishra, R.S. Yadav, A.C. Pandy, Struct. Chem. 22, 1281–1286 (2011)
X.M. Fan, J.S. Lian, L. Zhao, Y.H. Liu, Appl. Surf. Sci. 252, 420–424 (2005)
J. Zhang, L.D. Sun, J.L. Su, C.S. Liao, C.H. Yan, Chem. Mater. 14, 4172–4177 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geetha Devi, P., Sakthi Velu, A. Structural, optical and photoluminescence properties of copper and iron doped nanoparticles prepared by co-precipitation method. J Mater Sci: Mater Electron 27, 10833–10840 (2016). https://doi.org/10.1007/s10854-016-5190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5190-1