Abstract
Transparent antireflective SiO2/TiO2 double layer thin films were prepared using a sol–gel method and deposited on glass substrate by spin coating technique. Thin films were characterized using XRD, FE-SEM, AFM, UV–Vis spectroscopy and water contact angle measurements. XRD analysis reveals that the existence of pure anatase phase TiO2 crystallites in the thin films. FE-SEM analysis confirms the homogeneous dispersion of TiO2 on SiO2 layer. Water contact angle on the thin films was measured by a contact angle analyzer under UV light irradiation. The photocatalytic performance of the TiO2 and SiO2/TiO2 thin films was studied by the degradation of methylene blue under UV irradiation. The effect of an intermediate SiO2 layer on the photocatalytic performance of TiO2 thin films was examined. SiO2/TiO2 double layer thin films showed enhanced photocatalytic activity towards methylene blue dye.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The photocatalysis technology is a promising alternative for environmental remediation because a global pollution has recognized to be a serious problem that needs to be addressed immediately [1, 2]. Titanium dioxide (TiO2) is an important semiconducting material with extraordinary physical and chemical properties. TiO2 is a versatile material and its photocatalytic properties have been utilized in various applications to remove contaminants from air and water and killing of harmful bacteria and cancer cells [3–6]. TiO2 is widely used as pigments, sunscreen, paints, toothpaste, ointments, electrochemical electrodes, capacitors, and solar cells [7, 8]. To extend its applicability in self-cleaning surfaces, many researchers have been developing various ways to apply TiO2 coating on different substrates [9].
Silica-titania (SiO2–TiO2) based coatings have gained extensive interest for optics application, including antireflective coatings and optical planar waveguides, due to their high, photoactivity, strong redox property, high thermal stability, high chemical durability, low thermal expansion coefficient, versatile refractive index and hydrophilicity [10, 11].
TiO2 coatings have a wide range of applications in catalysis, self-cleaning, antifogging, optics, flexible displays, biomedical, energy generation, decorative and barrier coatings for pharmaceutical and food products [12, 13]. Various metals, insulators, and semiconductors are mostly used to prepare thin film coatings. Thin films prepared from semiconducting metal oxides are well transparent in a broad wavelength range and have low (MgF2, SiO2), moderate (Al2O3, Ta2O5) or high (TiO2, Nb2O5) refraction index [12].
Anatase TiO2 is more appropriate for photochemical transformation application because of its high efficiency, non-toxicity, strong oxidizing power, high photochemical stability and exhibit a photo-induced super hydrophilicity under UV light irradiation [11, 14]. This photo-induced super hydrophilicity allows removing surface adsorbed organic pollutants without any detergent [11]. The main advantage of TiO2 is that the photocatalysis and hydrophilicity can occur simultaneously on the same surface even though the mechanisms are completely different [15].
Metal oxide thin films have been extensively used as anti-reflection, protective and self-cleaning coatings depending on their particular chemical composition and micromorphology [13]. Various methods have been developed to prepare thin films such as sol–gel [16], hydrothermal [17], chemical vapor deposition [18], sputtering [19], electrodeposition [20], chemical bath deposition [21], etc. Among these techniques, the sol–gel method has many advantages of relatively simple, inexpensive, low temperature, easy to control process parameters, homogeneity and well dispersion of TiO2 [22, 23].
Preparing a homogenous SiO2/TiO2 double layer is considered as a real challenge due to the cracks or delamination’s on the surface or interface of the films and more hydrophilic nature of SiO2 layer affects the quality of the produced films. Thus, in order to prepare homogeneous double layer thin films, removal of residual alkoxy groups is required [23]. Several methods have been used to eliminate residual alkoxy groups including, UV light illumination, plasma cleaning and thermal treatment for fabricating multilayer films [23–25]. Here an attempt was made to develop a good self-cleaning coating which also exhibits efficient photocatalytic activity.
In the present work, uniform TiO2, SiO2, and SiO2/TiO2 thin films were prepared by sol–gel spin coating on a glass substrate. The films were analyzed by X-ray diffraction, Field emission scanning electron microscopy (FE-SEM), atomic force microscopy (AFM) and UV–Vis spectroscopy. Photo-induced hydrophilicity of the thin films was studied by measuring the water contact angle before and after UV irradiation. The unique physiochemical properties of TiO2 were used to evaluate the photocatalytic activity and super hydrophilicity of the self-cleaning thin films. The photocatalytic degradation of methylene blue dye over thin films was estimated using a UV–Vis spectrophotometer.
2 Experimental
2.1 Preparation of thin films
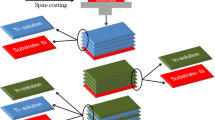
The SiO2, TiO2, SiO2/TiO2 double layer films were prepared by the sol–gel method at room temperature. Figure 1 shows the flow chart of the preparation of thin films. Prior to coating, the glass substrates were ultrasonically cleaned in acetone and ethanol, respectively. Initially, SiO2 sol and TiO2 sol were prepared separately. A SiO2 sol was prepared by hydrolyzing tetraethyl orthosilicate (TEOS) with ethanol and deionized water in the presence of HNO3 catalyst under magnetic stirring for 1 h. The molar ratio of TEOS:Ethanol:H2O:HNO3 was 1:13.5:11.5:0.3. A small quantity of surfactant Triton X-100 (2 wt%) was added to this mixture. Then, cleaned glass substrates were spin-coated by the SiO2 precursor sol (100 µL) with the rotation speed of 2000 rpm for 20 s. Then, the coatings were dried at room temperature and then heat-treated at 400°C for 1 h, at a heating rate of 10°C/min. Then, a TiO2 sol was prepared by mixing titanium tetra-isopropoxide (TTIP) with ethanol in the presence of HNO3 and deionized water and the relative molar ratio of each chemical in the precursor sol was TTIP:Ethanol:HNO3:H2O = 1:18.5:16:2.2. After magnetically stirring for 1 h in a glass beaker, TiO2 sol (100 µL) was deposited on the glass substrates with the rotation speed of 2000 rpm for 20 s. Then, the glass substrates were heat treated at 400°C for 1 h, at a heating rate of 10°C/min.
The double layer film was performed on previously fabricated SiO2 thin film by spin coating technique. The TiO2 sol (100 µL) was deposited on the SiO2 thin film with the rotation speed of 2000 rpm for 20 s. Finally, the double layer thin films were heat treated at 400°C for 1 h, at a heating rate of 10°C/min.
2.2 Characterization
X-ray diffractometer (Bruker D8 advance, Germany, CuKα (λ = 1.54056 Å) was used to investigate crystalline phase of thin films. Surface hydrophilicity of the films was performed at room temperature using a goniometer (Surface tech, GSTD, Korea) attached to a camera. Water contact angles were measured on the thin film surface by dropping water droplets (2 μL) at pH 7.0. The photo-induced hydrophilicity of the films was measured using a UV lamp (Daytime CFL 20W Blacklight, Korea). A field-emission scanning electron microscopy (FE-SEM, Hitachi Field Emission S-4300) was used to observe surface morphologies and the cross section of the thin films. The thickness of the thin films was evaluated by an FE-SEM cross section method. Surface observation of thin films was carried out by atomic force microscopy (AFM, XE-100 Park system, Korea). AFM images were analyzed using XEI program software. UV–Vis transmission spectra of the thin films were recorded on a UV–Vis spectrophotometer (S-4100 PDA Scinco, Korea).
2.3 Photocatalytic activity
The photocatalytic activity of the thin films was studied using degradation of MB solution under UV light irradiation. 10 mL of MB aqueous solution with the concentration of 5.34 × 10−6 M was taken in a small beaker. The TiO2 coated glass substrates (2.5 × 2.5 cm) were dipped into the MB solution horizontally. Before irradiation, the beaker was kept in the dark under magnetic stirring for 1 h to ensure adsorption–desorption equilibrium. Then, this beaker was irradiated by a 20 W UV lamp (Daytime CFL 20W Blacklight, Korea.). The distance from the lamp to the surface of the solution was 7 cm. The concentration of MB aqueous solution was determined every 60 min with a UV–Vis spectrophotometer.
3 Results and discussion
3.1 Structural analysis
The XRD patterns of SiO2, TiO2 and SiO2/TiO2 thin films deposited on a glass substrate are shown in Fig. 2. The XRD pattern of SiO2 thin films (Fig. 2a) reveals that there are no any characteristic peaks of SiO2. The diffraction pattern of SiO2 exhibits a broad peak in the range 2θ of 10°–40° indicates the amorphous nature of SiO2 thin films. The well-defined reflection of both thin films (TiO2 and SiO2/TiO2) could be indexed to anatase phase. The diffraction peaks of both thin films located at 2θ = 25.27°, 37.91°, 48.17°, 53.89°, 54.99° and 62.48° can be indexed to the planes 101, 004, 200, 105, 211 and 204 of anatase TiO2 (JCPDS 21-1272). This indicates that TiO2 is well dispersed on SiO2 layer and crystallite size is appropriate for the detection by XRD. No other phases such as rutile or brookite were observed. The average crystallite size was calculated from the Scherrer’s equation using the broadening of the (1 0 1) anatase reflection. The average crystallite sizes of nanoparticles for TiO2 and SiO2/TiO2 thin films were about 13 and 16 nm, respectively. Thus, XRD analysis confirms the existence of anatase phase, which is considered as most suitable for the photocatalytically active phase of TiO2 [14, 22, 26].
3.2 Morphological analysis
Figure 3a–c shows the FESEM micrographs of the SiO2, TiO2, and SiO2/TiO2 thin films. All films are homogeneous and without cracks over a wide area. The SiO2 thin film has flat texture and consists of very fine particles. For TiO2 thin film, the dispersion of TiO2 grains was less tightly packed and a significant grain growth was noticed. In contrast, the SiO2/TiO2 double layer is composed of densely packed and uniform nanoscale grains (10–15 nm) of TiO2 layer. From FESEM micrographs shown in Fig. 3, it can be observed that the TiO2 grains were embedded on SiO2 layer which forms homogenous SiO2/TiO2 double layer thin films. No any separate SiO2 and TiO2 clusters were observed in double thin films.
Figure 3d–f shows cross-sectional SEM micrographs for SiO2, TiO2 and SiO2/TiO2 thin films. The average thickness of the thin films was estimated according to an FE-SEM cross section. The thickness for the SiO2, TiO2, and SiO2/TiO2 thin films was about 260, 175 and 420 nm, respectively. The cross-sectional analysis confirms the formation of uniform structures.
Figure 4 shows the three-dimensional AFM images of SiO2, TiO2 and SiO2/TiO2 thin films deposited on the glass substrate. The arithmetic roughness (Ra) and the root-mean-square roughness (Rrms), were calculated using the topographical data and listed in Table 1. According to the AFM images, the root mean square roughness values (Rrms) of the SiO2, TiO2, and SiO2/TiO2 thin films are 0.1378, 0.998 and 1.219 nm, respectively. The surface skewness (Rsk) of the TiO2 thin film is positive which confirms the presence of few bumps, whereas a negative value of Rsk indicates that the surface is made up of valleys [27, 28]. The distribution of the sample height can be obtained from Kurtosis (Rku) value [28]. The AFM analysis indicates that the SiO2/TiO2 double layer thin film has the slightly higher roughness with a uniform coating layer.
3.3 UV–visible transmittance spectra
Figure 5 shows the UV–visible transmittance spectra for three different thin films. The SiO2 thin films does not exhibit a sharp absorption edge while TiO2 and SiO2/TiO2 films show a sharp absorption edge in the visible region. The average transmittance of SiO2, TiO2, and SiO2/TiO2 thin films were 95, 75, and 85 %, respectively. Transmittance is mainly dependent on thickness and surface structure of the thin films. For double layer coating, the transmittance (%) was not significantly different from the SiO2 coated glass. The transmission of TiO2 decreased by about 10 % comparing to the other glasses. Since anatase TiO2 has a high refractive index (n = 2.52), it reflected a large portion of incident light, resulting in low transmittance [29]. The transmission of the SiO2/TiO2 double layer thin films was found to be higher than that of a pure TiO2 thin film, due to the decrease of light scattering by the addition of small SiO2 layer [30].
3.4 Hydrophilic and self-cleaning properties
Photo-induced hydrophilic property of the thin films was evaluated by measuring the water contact angle before and after UV irradiation. The water contact angle of the as-prepared SiO2 thin film was 30°. After heat treatment at 400°C, the water contact angle of the thin film was decreased to below 5°. The thermal treatment can eliminate the oxygen defects in the thin film and also enhance roughness [23, 31]. The SiO2/TiO2 double layer thin films were effectively fabricated by forming the hydrophilic surface of the SiO2 (bottom) layer with thermal treatment. Initially, the water contact angle of TiO2 thin films was 14° and after UV irradiation it decreased to below 5°. In the case of SiO2/TiO2 double layer thin film, the water contact angle was 24°. Upon UV irradiation, the water contact angle was decreased to 7° and further reached to almost 0° within 10 min of irradiation. This indicates that SiO2/TiO2 double layer thin films show photo-induced super hydrophilicity (Fig. 6).
Additionally, the photocatalytic performance of the thin films was studied by degradation of an aqueous solution of methylene blue under UV irradiation. Figure 7 shows the photocatalytic performance of the TiO2 and SiO2/TiO2 thin films as a function of irradiation time. In the absence of thin films, MB solution was stable when irradiated with UV light. Both TiO2 containing thin films were found to be photocatalytically active for the photocatalytic degradation of MB solution under UV light irradiation. The MB degradation reaction followed pseudo-first order kinetics. The apparent rate constant of the photocatalytic reaction was determined by plotting ln(C0/C) against time, where C0 is the initial concentration of MB solution and C is the concentration of MB after irradiation of the thin films in the corresponding time interval (Fig. 8). The reaction rate constants of TiO2 and SiO2/TiO2 thin films under UV light irradiations were 0.0088 and 0.1395 min−1, respectively. The obtained results showed that the photocatalytic performance of SiO2/TiO2 double layer thin films was superior to single layer TiO2 thin films. The superior photocatalytic activity of SiO2/TiO2 double layer thin films can be attributed to its higher optical transmission than that of a pure TiO2 thin film. The higher optical transmission of double layer thin films was due to the decrease in light scattering by the addition of thin SiO2 layer. The combination of low and high refractive materials SiO2 and TiO2 are suitable for a broad range wavelength which increases the spectral bandwidth and has more lifetimes [32]. In addition to these, the changes in microstructure and surface roughness enhance the photocatalytic performance of the TiO2 thin films. As shown in Fig. 4, surface roughness and specific surface area of SiO2/TiO2 thin films is higher than that of TiO2 only.
4 Conclusions
A facile sol–gel coating method has been used for coating TiO2, SiO2, and SiO2/TiO2 on the glass substrate. The prepared TiO2 and SiO2/TiO2 thin films are highly transparent and antireflective. The existence of an intermediate SiO2 layer plays a vital role in the photocatalytic performance of the TiO2 thin films coated on glass substrates. The photocatalytic degradation of methylene blue is enhanced because of intermediate SiO2 layer. FE-SEM and optical analysis confirmed the changes in microstructure, surface roughness and optical properties attributed to the superior photocatalytic performance of the SiO2/TiO2 double layer thin films. This double layered thin films with enhanced self-cleaning and antireflective properties have potential value in the field of energy harvesting as well water and air pollution applications.
References
G. Palmisano, V. Augugliaro, M. Pagliaro, L. Palmisano, Chem. Commun. 33, 3425–3437 (2007)
S.-H. Nam, S.-J. Cho, C.-K. Jung, J.-H. Boo, J. Šícha, D. Heřman, J. Musil, J. Vlček, Thin Solid Films 519, 6944–6950 (2011)
D.P. Macwan, P.N. Dave, S. Chaturvedi, J. Mater. Sci. 46, 3669–3686 (2011)
R. Daghrir, P. Drogui, D. Robert, Ind. Eng. Chem. Res. 52, 3581–3599 (2013)
H.M. Yadav, T.V. Kolekar, S.H. Pawar, J.-S. Kim, J. Mater. Sci. Mater. Med. 27, 57 (2016)
D.M. Blake, P.-C. Maness, Z. Huang, E.J. Wolfrum, J. Huang, W.A. Jacoby, Sep. Purif. Rev. 28, 1–50 (1999)
X. Chen, S.S. Mao, Chem. Rev. 107, 2891–2959 (2007)
U.G. Akpan, B.H. Hameed, Appl. Catal. A Gen. 375, 1–11 (2010)
L. Zhou, S. Yan, B. Tian, J. Zhang, M. Anpo, Mater. Lett. 60, 396–399 (2006)
C.F. Song, M.K. Lü, P. Yang, D. Xu, D.R. Yuan, Thin Solid Films 413, 155–159 (2002)
M. Houmard, G. Berthomé, J.C. Joud, M. Langlet, Surf. Sci. 605, 456–462 (2011)
M. Mazur, D. Wojcieszak, J. Domaradzki, D. Kaczmarek, S. Song, F. Placido, Opto Electron. Rev. 21, 233–238 (2013)
Y. Situ, T. Huang, Y. Chen, W. Huang, H. Huang, Ceram. Int. 40, 919–927 (2014)
H.M. Yadav, T.V. Kolekar, A.S. Barge, N.D. Thorat, S.D. Delekar, B.M. Kim, B.J. Kim, J.S. Kim, J. Mater. Sci. Mater. Electron. 27, 526–534 (2015)
A. Soklič, M. Tasbihi, M. Kete, U.L. Štangar, Catal. Today 252, 54–60 (2015)
H. Dislich, P. Hinz, J. Non Cryst. Solids 48, 11–16 (1982)
Q. Chen, Y. Qian, Z. Chen, W. Wu, Z. Chen, G. Zhou, Y. Zhang, Appl. Phys. Lett. 66, 1608 (1995)
D. Li, M. Carette, A. Granier, J.P. Landesman, A. Goullet, Thin Solid Films 522, 366–371 (2012)
M. Mazur, D. Wojcieszak, D. Kaczmarek, J. Domaradzki, S. Song, D. Gibson, F. Placido, P. Mazur, M. Kalisz, A. Poniedzialek, Appl. Surf. Sci. 380, 165–171 (2016)
D. Eisenberg, H.S. Ahn, A.J. Bard, J. Am. Chem. Soc. 136, 14011–14014 (2014)
A.T. Rajamanickam, P. Thirunavukkarasu, K. Dhanakodi, J. Mater. Sci. Mater. Electron. 26, 8933–8938 (2015)
S.D. Delekar, H.M. Yadav, S.N. Achary, S.S. Meena, S.H. Pawar, Appl. Surf. Sci. 263, 536–545 (2012)
K. Han, J.H. Kim, Appl. Surf. Sci. 263, 69–72 (2012)
M. Mennig, P. Oliveira, H. Schmidt, Thin Solid Films 351, 99–102 (1999)
N.R. Armstrong, C. Carter, C. Donley, A. Simmonds, P. Lee, M. Brumbach, B. Kippelen, B. Domercq, S. Yoo, Thin Solid Films 445, 342–352 (2003)
H.M. Yadav, S.V. Otari, V.B. Koli, S.S. Mali, C.K. Hong, S.H. Pawar, S.D. Delekar, J. Photochem. Photobiol. A Chem. 280, 32–38 (2014)
F. Bensouici, T. Souier, A. Iratni, A.A. Dakhel, R. Tala-Ighil, M. Bououdina, Surf. Coat. Technol. 251, 170–176 (2014)
A.I. Gómez-Varela, Y. Castro, A. Durán, P.A.A. De Beule, M.T. Flores-Arias, C. Bao-Varela, Thin Solid Films 583, 115–121 (2015)
B. Moongraksathum, Y.-W. Chen, J. Sol–Gel Sci. Technol. 77, 288–297 (2015)
R. Fateh, R. Dillert, D. Bahnemann, Langmuir 29, 3730–3739 (2013)
Y.J. Xu, J.X. Liao, Q.W. Cai, X.X. Yang, Sol. Energy Mater. Sol. Cells 113, 7–12 (2013)
N. Lari, S. Ahangarani, A. Shanaghi, J. Mater. Eng. Perform. 24, 2645–2652 (2015)
Acknowledgments
This research work was supported by a Grant (14CTAP-C077607-01) from Infrastructure and transportation technology promotion research program funded by Ministry of Land, Infrastructure, and Transport of the Korean government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, H.M., Kim, JS. Fabrication of SiO2/TiO2 double layer thin films with self-cleaning and photocatalytic properties. J Mater Sci: Mater Electron 27, 10082–10088 (2016). https://doi.org/10.1007/s10854-016-5082-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5082-4