Abstract
Pure bismuth aluminate (Bi2Al4O9) nanoparticles were successfully synthesized by a facile and reliable method with the aid of Bi(NO3)3, Al(NO3)3·9H2O, and starch without adding external surfactant, capping agent or template. Moreover, starch plays role as capping agent, reducing agent, and natural template in the synthesis Bi2Al4O9 nanoparticles. The structural, morphological and optical properties of as obtained products were characterized by techniques such XRD, EDX, VSM, SEM, and UV–Vis. The as-synthesized Bi2Al4O9 nanoparticle was utilized as photo-anode material for the fabrication of FTO/TiO2/Bi2Al4O9/Pt-FTO and photocatalyst for degradation of methyl orange to investigation its light harvesting applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Materials at the nanometer scale have been studied for decades because of their unique properties arising from the large fraction of atoms residing on the surface, and also from the finite number of atoms in each crystalline core. Especially, because of the increasing need for high area density storage, the synthesis and characterization of semiconductor nanocrystals have been extensively investigated [1–11]. The sol gel method of preparing oxide powders generally involves polymerization via hydrolysis and condensation of an alkoxide, gelation, and heat treatment under suitable conditions. In recent years Bi2M4O9 (M = Al3+, Ga3+, Fe3+) compounds with mullite-type structures [12] have obtained considerable technical interest as potential candidates for applications as oxygen-ion conductors or mixed ionic–electronic conductors (MIEC). Common structural items are chains of edge-shared MO6 octahedra running parallel to the orthorhombic c-axis linked by (M2O7) dimers of MO4 tetrahedra Especially, Bi2Al4O9 does not show any phase transition until 1080 °C and has a high chemical stability in hydrogen atmospheres [13, 14], a necessary feature for the application in fuel cells. Tutov and Echerlin described the possibility of orthorhombic bismuth aluminate preparation in 1965 [15–21], as well as defined the synthesis conditions, temperature and melting character of the compound. Among the wet chemical routes, sol–gel technique has been used widely because it has the advantage of producing pure, ultrafine powders at low temperatures, High surface area and pore size distribution [22–28]. In this report, for the first time, we had presented the preparation of Bi2Al4O9 nanoparticles by novel sol–gel method at 800 °C in the presence of starch without adding external surfactant, capping agent or template. A green approach for Bi2Al4O9 nanoparticles synthesis by utilizing natural template permits the reaction to proceed usually in milder conditions. Although existing chemical approaches have effectively produced well defined Bi2Al4O9 nanoparticles, these processes are generally costly and include the employ of toxic chemicals. The synthesized Bi2Al4O9 nanoparticles were further used to investigate the photo-anode material for the fabrication of solar cell and photodegradation of methyl orange (MO).
2 Experimental
2.1 Characterization
The magnetic measurement of samples were carried out in a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) at room temperature in an applied magnetic field sweeping between ±10,000 Oe. X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10 < 2θ < 80. The electronic spectra of the bismuth aluminate were obtained on a Scinco UV–Vis scanning spectrometer (Model S-10 4100). The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy.
2.2 Synthesis of Bi2Al4O9 nanoparticles
At first, 1.71 g of Bi(NO3)3 was dissolved in 50 mL of distilled water. Then, 4.35 of starch was subsequently added to the above solution under magnetic stirring at 70 °C for 30 min. Afterwards, 2.65 g of Al(NO3) .3 9H2O was dissolved in 50 mL of distilled water and was added to the above solution under magnetic stirring. A solution was obtained and further heated at 90 °C for 1 h to remove excess water. During continued heating at 110 °C for 1 h, the solution became more and more viscous to become a gel. Finally, the obtained product was calcinated at 800 °C for 2 h in a conventional furnace in air atmosphere and then cooled it to room temperature.
2.3 Photocatalytic experimental
The methyl orange (MO) photodegradation was examined as a model reaction to evaluate the photocatalytic activities of the Bi2Al4O9 nanoparticles. The photocatalytic experiments were performed under an irradiation ultraviolet light. The photocatalytic activity of nanocrystalline Bi2Al4O9 obtained was studied by the degradation of methyl orange solution as a target pollutant. The photocatalytic degradation was performed with 50 mL solution of methyl orange (0.0005 g) containing 0.1 g of Bi2Al4O9. This mixture was aerated for 30 min to reach adsorption equilibrium. Later, the mixture was placed inside the photoreactor in which the vessel was 15 cm away from the ultraviolet source of 400 W mercury lamps. The photocatalytic test was performed at room temperature. Aliquots of the mixture were taken at definite interval of times during the irradiation, and after centrifugation they were analyzed by a UV–Vis spectrometer. The methyl orange (MO) degradation percentage was calculated as:
where At and A0 are the obtained absorbance value of the methyl orange solution at t and 0 min by a UV–Vis spectrometer, respectively.
2.4 Cell fabrication
The Bi2Al4O9 paste have been fabricated according to the procedure reported by Gratzel et al. The slurry of Bi2Al4O9 prepared via mixing and grinding 1.0 g of Bi2Al4O9 powder with ethanol and water in several steps. After that the slurry sonicated to prepare stable mixture of Bi2Al4O9 on ethanol and water. The sonicated slurry mixed with binders such as terpineol and ethyl cellulose and sonicated again. Then the ethanol and water removed from slurry with a rotary evaporator and the final paste was prepared [29]. The prepared uniform Bi2Al4O9 paste have been coated on fluorinated tin oxide glass (FTO) glass, Pilkington Glass, TEC7) by a doctor blade technique. The active area of electrodes was 0.4 cm2. After natural drying at room temperature, the electrodes coated with the pastes gradually heated under an air flow at 300 °C for 5 min. The Bi2Al4O9 electrodes immersed into ethanolic solution of natural sensitizers and kept at room temperature for 24 h to complete the sensitizer uptake. Electrodes rinsed with ethanol and dried under a nitrogen stream. A Pt coated FTO glass electrode prepared as a counter electrode. The Pt electrode placed over the dye-adsorbed Ag- Bi2Al4O9 electrode and the edges of the cell were sealed with 50 mm thick sealing sheet (Surlyn150, Dyesol). Sealing accomplished by pressing the two electrodes together on a double hot-plate at a temperature of about 80 °C. The redox electrolyte consisting of 0.05 M of LiI, 0.05 M of I2, and 0.5 M of 4-tert-butylpyridine in acetonitrile as a solvent was introduced into the cell through one of the two small holes drilled in the counter electrode. The electrolyte introduced into the cell through one of two small holes drilled in the counter electrode. Finally, these two holes sealed by a small square of sealing sheet.
3 Results and discussion
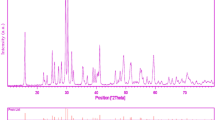
Crystalline structure and phase purity of as-prepared product has been determined using XRD. The XRD pattern of as-prepared Bi2Al4O9 is shown in Fig. 1. Based on the Fig. 1, the diffraction peaks observed can be indexed to pure monoclinic phase of Bi2Al4O9 (a = 7.7190 A°, b = 8.1090 A°, c = 5.6919 A°) with space group of Pbam and JCPDS No. 25-1048. No diffraction peaks from other species could be detected, which indicates the obtained sample is pure. From XRD data, the crystallite diameter (Dc) of Bi2Al4O9 nanoparticles was calculated to be 37 nm using the Scherer equation:
where β is the breadth of the observed diffraction line at its half intensity maximum (400), K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. The morphology of the nanoparticles was investigated using SEM which demonstrates uniform nanoparticles with spherical shape homogenously distributed all over the sample, as it could be clearly observed in Fig. 2. The Bi2Al4O9 nanoparticles with particle size of about 44–50 nm were observed. EDX analysis measurement was employed to investigate the chemical composition and purity of as-synthesized Bi2Al4O9 nanoparticles. The EDX pattern of Bi2Al4O9 in Fig. 3 exhibits that the only elements which existed were Bi, Al, and O. Furthermore, neither N nor C signals were detected in the EDS spectrum, which means the product is pure and free of any surfactant or impurity. The hysteresis loop of Bi2Al4O9 nanoparticles was studied to examine their magnetic properties (Fig. 4). At 300 K the remanent magnetization (Mr) is 0.001 emu/g, the coercive field (Hc) is 85 Oe and the magnetization at saturation (Ms) is estimated to be only 0.007 emu/g (the saturation magnetization Ms was determined from the extrapolation of curve of H/M vs. H). Using Tauc’s formula, the band gap can be obtained from the absorption data. The energy gap (Eg) of the Bi2Al4O9 nanoparticles has been estimated by extrapolating the linear portion of the plot of (αhν)2 against hν to the energy axis. The diffused reflectance spectrum of the as-prepared Bi2Al4O9 nanoparticles is shown in Fig. 5. The Eg value is calculated as 3.30 eV for the Bi2Al4O9 nanoparticles. Photodegradation of methyl orange as water contaminant under UV light illumination was employed to evaluate the properties of the as-synthesized Bi2Al4O9 nanoparticles. Figure 6 exhibits the obtained result. No methyl orange was practically broken down after 60 min without employing UV light illumination or as-prepared Bi2Al4O9 nanoparticles. This observation illustrated that the contribution of self-degradation was insignificant. The proposed mechanism of the photocatalytic degradation of the methyl orange can be assumed as:
Utilizing photocatalytic calculations by Eq. (1), the methyl orange degradation was about 65 % after 60 min illumination of UV light. This obtained result demonstrates that as-prepared Bi2Al4O9 nanoparticles have high potential to be applied as favorable and appropriate material for photocatalytic applications under illumination of UV light. The heterogeneous photocatalytic processes have diffusion, adsorption and reaction steps. It has been shown that the desirable distribution of the pore has effective and important impact on the diffusion of the reactants and products, and therefore effects on the photocatalytic activity. It seems that the enhanced photocatalytic activity of the as-obtained nanoparticles Bi2Al4O9 can be owing to desirable and appropriate distribution of the pore, high hydroxyl amount and high separation rate of charge carriers (Scheme 1). I–V characterization of a typical solar cell fabricated using in situ approach is shown in Fig. 7 The measurement of the current density voltage (I–V) curve for Bi2Al4O9 was carried out under the illumination of AM1.5 (100 mW/cm2). Device characteristics are as follows: Voc = 0.4 V, Jsc = 0.20 mA/cm2, FF = 34 %. The Voc and Jsc of this device are in order of those obtained using non-vacuum-based techniques. Furthermore, this route is facile to operate and very suitable for industrial production of Bi2Al4O9 nanoparticles. In addition, this process can be versatile to easily synthesize other aluminate based perovskite oxides.
4 Conclusions
In this work, bismuth aluminate nanoparticles were successfully synthesized by a novel sol–gel method at 800 °C for 120 min. High purity of the as-prepared nanocrystalline sample was proved by XRD, and EDS analyses. When as-prepared nanocrystalline bismuth aluminate was utilized as photocatalyst, the percentage of methyl orange degradation was about 65 % after 60 min irradiation of UV light. This result suggests that as-obtained nanocrystalline bismuth aluminate as favorable material has high potential to be used for photocatalytic applications under UV light. The nature of M–H curve represents ferromagnetic behaviour in Bi2Al4O9 nanoparticles. A preliminary study on the possibility of developing a solar cell having FTO/TiO2/Bi2Al4O9/Pt-FTO structure was also performed.
References
P. Bakhshaei, A. Ataie, H. Abdizadeh, J. NanosStruct. 3, 403 (2013)
R.R. Shahraki, M. Ebrahimi, J. NanosStruct. 2, 413 (2013)
F.S. Ghoreishi, V. Ahmadi, M. Samadpourc, J. NanosStruct. 3, 453 (2013)
S. Moshtaghia, D. Ghanbarib, M. Salavati-Niasari, J. NanosStruct. 5, 169 (2015)
A. Rahdar, M. Aliahmad, Y. Azizi, J. NanosStruct. 5, 145 (2015)
J. Safaei-Ghomi, S. Zahedi, M. Javid, M.A. Ghasemzadeh, J. NanosStruct. 5, 153 (2015)
M. Shakib Nahad, G. Mohammadi Ziarani, A. Badiei, A. Bananc, J. NanosStruct. 3, 395 (2013)
M. Rahimi-Nasarabadi, J. NanosStruct. 4, 211 (2014)
M. Najafi, H. Haratizadeh, M. Ghezellou, J. NanosStruct. 5, 129 (2015)
M. Ahmadzadeh, M. Almasi-Kashia, A. Ramazani, J. NanosStruct. 5, 97 (2015)
V.B.R. Boppana, D.J. Doren, R.F. Lobo, Chemsuschem 3, 814 (2010)
K. Gurunathan, J.O. Baeg, S.M. Lee, E. Subramanian, S.J. Moon, K.J. Kong, Int. J. Hydrogen Energy 33, 2646 (2008)
S.W. Cao, Y.J. Zhu, G.F. Cheng, Y.H. Huang, J. Hazard. Mater. 171, 431 (2009)
T. Debnath, C.H. Rüscher, P. Fielitz, S. Ohmann, G. Borchardt, Ceram. Trans. 217, 71 (2010)
T. Debnath, C.H. Rüscher, ThM Gesing, P. Fielitz, S. Ohmann, G. Borchardt, Ceram. Eng. Sci. Proc. 31, 81 (2010)
J. Maier, B. Bunsenges, Phys. Chem. 90, 26 (1986)
J.S. Piccin, C.S. Gomes, L.A. Feris, M. Gutterres, Chem. Eng. J. 183, 30 (2012)
H. Schneider, in Mullite, ed. by H. Schneider, S. Komarneni (Wiley-VCH, Weinheim, 2005), pp. 141–164
T.M. Gesing, C.H. Rüscher, J.C. Buhl, Z. Kristallogr. Suppl. 29, 93 (2009)
E.I. Speranskaya, V.M. Skorokov, G.M. Safronov, E.N. Gaidukov, Inorg. Mater. 6, 1201 (1970)
A.G. Tutov, I.E. Myl’nikova, I.N. Parfenov, V.A. Bokov, Phisika Tverdogo Tela. 6, 1240 (1964)
P. Eckerlin, J. Liebertz, Naturwissenschaften 52, 450 (1965)
N. Niizeki, M. Wachi, Z. Krist. 127, 173 (1968)
I. Bloom, M.C. Hash, J.P. Zebrowski, K.M. Myles, M. Krumpelt, Solid State Ion. 739, 53 (1992)
S. Zha, J. Cheng, Y. Liu, X. Liu, G. Meng, Solid State Ion. 156, 197 (2003)
S. Larose, S.A. Akbar, J. Solid State Electrochem. 10, 488 (2006)
ThM Gesing, R.X. Fischer, M. Burianek, M. Mühlberg, T. Debnath, C.H. Rüscher, J. Ottinger, J.C. Buhl, H. Schneider, J. Eur. Ceram. Soc. 31, 3055 (2011)
H.A. Harwig, A.G. Gerards, J. Solid State Chem. 26, 265 (1978)
K. Laurent, G.Y. Wang, S. Tusseau-Nenez, Y. Leprince-Wang, Solid State Ion. 178, 1735 (2008)
Acknowledgments
Authors are grateful to council of University of Science and Research for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, S.A. Facile and reliable synthesis and characterization of bismuth aluminate nanoparticles and its light harvesting applications. J Mater Sci: Mater Electron 27, 6517–6521 (2016). https://doi.org/10.1007/s10854-016-4594-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4594-2