Abstract
In this study, the hydrothermal synthesis of TiO2 nanoparticles from its coordinated metal precursor via a novel modified hydrothermal method inside stainless steel autoclave has been reported. The synthesized nanoparticles were characterized via different techniques such as: XRD, XPS, TEM and SEM. The mechanism of formation has been proposed and this method is advantageous by producing pure anatase crystalline structure at mild conditions with a relatively high surface area of 208.7 m2 g−1 and 19 nm in size. Finally we assessed its photocatalytic activity toward the photocatalytic degradation of methylene blue dye. The results revealed a photoefficiency of 96 % with a degradation rate of 0.013 min−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor photocatalysis with a primary focus on TiO2 is widely used for the treatment of air and water pollutants [1]. Photocatalysis aided by Titanium dioxide nanoparticles is used in removing the organic chemicals which occur as pollutants in wastewater effluents from industrial and domestic sources [2, 3]. There are various “sensitizers” that accelerate the process. This process is a combination of heterogeneous catalysis and solar technology [4–6]. The Photocatalytic process breaks down the compounds such as alcohols, carboxylic acids, amines, herbicides and aldehydes into carbon dioxide, water and simple mineral acids [7, 8]. The main advantage of photocatalysis is that there is no further requirement for secondary disposal methods. Other treatment methods such as adsorption by activated carbon and air stripping merely concentrate the chemicals present by transferring them to the adsorbent or air and they do not convert them to non toxic wastes [9, 10]. Also as compared to other oxidation technologies, expensive oxidation methods are not required as ambient oxygen is used [11]. TiO2 is the most promising semiconductor used in photolysis since it provides a good compromise between catalytic activity and stability in aqueous media [12–14].
The anatase form possesses a band gap of 3.2 eV with an absorption edge at 386 nm and the rutile form has a lower band gap of 3.02 eV with an absorption edge at 416 nm. Both the crystal forms exhibit significant photocatalytic activity, however, the anatase form has found to exhibit higher photoactivity compared to the rutile. This makes anatase have a slightly higher redox driving force than rutile, although the range of the light absorption by the former is slightly less than that by the latter [15, 16]. Anatase also has much higher surface area than rutile, leading to enhanced adsorption capability and even generation of much more active sites (such as oxygen vacancies) [17]. Furthermore, although rutile has better charge carrier mobility due to its higher crystallinity than anatase, the latter can generate more efficient charge separation due to the existence of more oxygen vacancies. Because of these advantages of anatase, it usually shows much higher photocatalytic activity than rutile [18, 19].
The key requirement of any novel study of metal oxide nanoparticles is the synthesis method. The development of systematic, facile and reproducible methods for the synthesis of metal oxide nanoparticles is a current challenge. There are several acceptable methods for the preparation of metal oxide nanoparticles such as sol–gel method [20, 21], microemulsion method [22, 23], precipitation method and thermal decomposition [24]. Thermal decomposition is a chemical decomposition transformation caused by heat. Thermal decomposition, as a technique broadly employed in nanotechnology, is an endothermic chemical disintegration caused by heat. Heat is required to break chemical bonds in the precursor undergoing decomposition. This is a promising and easy ‘‘top down’’ technique to prepare metal oxide nanoparticles and it is essentially based in the breakdown of the material into smaller units [25, 26].
In this work, the synthesis of nanosized anatase TiO2 has been reported via simple and novel hydrothermal method based on its analogue coordinated precursor. TFAA was assessed as both a scavenger and a morphological control agent for obtaining pure anatase nanoparticles. The synthesized nanoparticles with very high surface area revealed a relatively high photocatalytic activity toward the photodegradation of methylene blue dye.
2 Experimental
2.1 Synthesis of TiO2 NPs
In atypical procedure, 24 ml of Trifuloroacetic acid was added to a 30 ml of a mixture of ethanol: water with molar ratio 2:1 (v/v) while stirring at 80 °C. To this mixture, 6 ml of Titanium Tetraisopropoxide was added drop wise and the mixture was kept stirring then transferred to a Teflon-lined stainless steel autoclave for 2 h at 110 °C. The obtained gel precipitate was dried in open air oven at 50 °C.
2.2 Characterization of nanoparticles
The X-ray diffraction (XRD) measurements were conducted by using X’Pert PRO Panalyticaldiffractometer with copper target and nickel filter with CuKα radiation (λ = 0.154056 nm). Measurements were performed in the range 20°–80° (2θ).
The morphology of the particleswas obtained by Scanning electron microscopy (SEM) using JEOL JSM-7001F operating at 120 kV and by transmission electron microscopy (TEM) using a Jeol JEM 1230 operating at 120 kV. The average particle size was determined statistically by counting manually 90 particles.
X-ray photoelectron spectroscopy (XPS) surface elemental analysis was conducted using a model Thermo ESCA Lab 250xi equipped with MgKα radiation (1253 eV) and operated at 23 kV and 13 mA. The binding energy was referenced to C1 s line at 284.76 eV for calibration. N2 adsorption–desorption isotherms were measured on test samples at liquid nitrogen temperature (−195 °C) using a model ASAP 2010 automatic Micromeritics sorptiometer (USA), equipped with an out gassing platform.
2.3 Photocatalytic activity
Methylene blue was used as representative of organic pollutant to evaluate the photocatalysts. In a 250 ml beaker 0.01 g of the catalyst was dispersed in 100 ml of 0.01 g/l methylene blue aqueous solution. The powder was dispersed in the solution dye using ultrasonic bath for 10 min in dark afterward. After another15 min of stirring in dark, to achieve adsorption–desorption equilibrium with catalysts, the zero measurement was taken then the solution was irradiated with 254 nm UV lamb while stirring. 5 ml from the solution was taken on regular intervals to monitor absorbance change.
3 Results and discussions
Trifluoroacetic acid, TFAA, can act as a capping agent, and as an acid catalyst, it can also promote the hydrolysis and inhibit the condensation reactions [27]. Like Hydrofluroic acid, HF, TFAA has shown to be a good electron scavenger too, mainly due to the strong electron withdrawing role of the –CF3 group [28]. So it seems that TFAA is a promising candidate to substitute HF in the preparation of anatase nanoparticles with enhanced photocatalytic activity. TFAA species are mainly bonded to the Ti atoms in a bidentate mode (bridging or chelating) as shown in scheme 1 to yield pure anatase TiO2 nanoparticles.
3.1 XRD of TiO2 nanoparticles
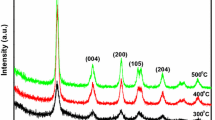
The XRD pattern of the as-synthesized sample was shown in Fig. 1 The peaks of the powder materials are identified to corresponding (101), (004), (200), (105), (211), (204), (116), (220), (215), and (303) crystal planes. All diffraction peaks are well defined and can be perfectly assigned to the anatase TiO2 (JCPDS-21-1272). The anatase TiO2 nanoparticles were known to be very photoactive and practical for water treatment and water purification [29]. No characteristic peaks associated with other crystalline forms were detected in the XRD pattern, indicating the anatase phase-pure nature of the product.
The crystallite size was calculated from the full width at half-maximum at the value of 2θ = 25.4° using the Scherrer equation:
where d is the average crystallite size and the number K = 0.9 is a coefficient, λ = 0.1541 nm is the X-Ray wavelength, β is the full width half maximum (FWHM) of the catalyst, θ is the diffracting angle. The average crystallite size of as-prepared sample was calculated to be around 17 nm from the peak broadening.
3.2 N2 adsorption–desorption isotherms of TiO2
It is well known that the surface area, the type of adsorption isotherms, and the pore sizes/shapes are key factors in catalysis. Hence, such properties were measured using N2sorptiometry technique. The results revealed that the Brunauer–Emmett–Teller (BET) surface area, SBET, for TiO2. The surface area, pore volumes and pore diameters of the samples are reported in Table 1. As shown in Fig. 2 the obtained isotherms are of type-IV (indicative of mesopores in the range of 4 nm ≤ Dp ≤ 100 nm). The samples also found to exhibit type-H2 hysteresis loops as a direct indication of cylindrical porosity of ink-bottle neck shape that is commonly found associated with compact non-uniform particles [30].
3.3 Electron microscopy of TiO2
TEM analysis of TiO2 in Fig. 3a shows that the synthesized NPs are spherical in shape. As can be seen from the TEM image that the average particle size is ~19 nm, which is in agreement with the crystallite, size obtained from XRD. Figure 3a reveals that the prepared titania nanoparticles are spherical and monodispersed. Also Fig. 3b presents the SEM image of TiO2 which confirm the homogeneity in shape and particle size.
3.4 XPS of TiO2 nanoparticles
To confirm the surface composition and oxidation state(s) of TiO2, X-ray photoelectron spectroscopic study, XPS, was performed and the results are shown in Fig. 4 the surface oxygen (O 1 s spectrum) is attributed to O2− species in TiO2 (B.E. = 529.8 eV), a small peak appears at 531.1 eV and is assigned to OH (surface hydroxyl) [31, 32]. The binding energies of Ti 2p3/2 and Ti 2p1/2 are observed approximately at 458.8 and 464.5 eV, respectively [33]. The ratio of the areas of the two peaks A(Ti 2p1/2 )/A(Ti 2p3/2 ) is equal to 0.5 and the binding energy difference due to the spin-orbital coupling, ΔE b = E b (Ti 2p1/2 ) − E b (Ti 2p3/2 ) is 5.7 eV in good agreement with the expected and reported value [34]. These two peaks are the attributes of Ti4+ (TiO2).
3.5 Photodegradation of methylene blue dye
Generally speaking, the high photocatalytic degradation rate corresponds to the high photocatalytic activity [35]. The photocatalytic ability of TiO2 nanoparticles was evaluated by UV-degradation MB dye. Figure 5 illustrates the degradation rate of MB dye in the presence of TiO2. The results exhibit that the obtained TiO2 reveals superior higher photocatalytic activities up to 96 %. The photocatalytic activity of semiconductor nanoparticles depends mainly on its surface area, particle size, and band gap energy. TiO2 has three well-known crystallographic phases in nature: anatase, rutile, and brookite [36]. It is well known that the anatase phase exhibits the maximum photoactivity. The pure anatase phase obtained in our as-prepared TiO2 and its relatively high surface area of 209 m2/g endow the TiO2 nanoparticles a relative higher photocatalytic activity [37].
The bleaching reaction follows a pseudo-first-order reaction kinetics for the photocatalytic process, the degradation rate constant was determined from (Eq. 3.2):
where C0 is the initial concentration and C is the concentration at time t. k is the apparent first-order rate constant. A plot of Ln Co/C versus time represents a straight line, the slope of which upon linear regression equals the apparent first-order rate constant k (Fig. 6). The correlation constant for the fitted line was calculated to be R = 0.994 for TiO2. The rate constants were calculated to be 0.013 min−1 for TiO2.
The degradation efficiency (E %) has been calculated using:
where C0 is the initial concentration of the dye and C is the concentration of the dye after irradiation in selected time interval. The observed maximum degradation efficiency of MB is about 95.8 %.
3.6 Effect of dye concentration and catalyst dose on the photocatalytic degradation
Figure 7a shows the degradation efficiency (% D) of dye decreases with an increase in the concentration of dye. The degradation rate is directly proportional to the probability of formation of hydroxyl radicals (OH·) on the catalyst surface and the probability of hydroxyl radicals reacting with the dye molecules [38]. As the initial concentration of the dye increases, the interaction of OH radical with dye decreases. Further, increase in concentration also reduces the light penetration and the relative formation of hydroxyl radicals and super oxide radical anions decreases leading to the decreased photo degradation efficiency [39].
It is also possible that with an increase in the concentration of the dye, the amount of light that is available to excite the catalyst may decrease due to an increase in the light absorption by the dye molecules; in addition to this, for a fixed catalyst amount, the saturation of the adsorption of the dye on the surface of the catalytic particles can also be expected [39].
Figure 7b shows the photodegradation efficiency of MB (% D) as a function of the amount of the catalyst varying from 3 to 15 mg l−1. Results show that % D value increases with an increase in the catalyst amount up to the optimum concentration (100 mg l−1) and a further increase in the amount of the catalyst then decrease % D value, Fig. 7b. The observed enhancement in this range is probably due to an increased number of available adsorption and catalytic sites on TiO2. When the dosage of TiO2 was increased above the limiting value, the decolourization rate decreased due to an increase in the turbidity of the suspension and a decrease in UV light penetration [40]. Therefore, the penetration depth of the photons is decreased and less catalysts nanoparticles could be activated.
4 Conclusion
Successful preparation of pure anatase TiO2 via hydrothermal method has been reported. The characterization of the synthesized nanoparticles revealed small particle size of 19 nm with a semispherical shape and high surface area. The hydrothermally modified TiO2 nanoparticles with its anatase from exhibited superior photocatalytic activity toward the photocatalytic degradation of MB dye. The superior photoactivity was referred to its better charge separation and high surface area.
References
D. Beydoun, R. Amal, G. Low, S. Mcevoy, J. Nanopart. Res. 1, 439 (1999)
N. Murakami, Y. Fujisawa, T. Tsubota, T. Ohno, Appl. Catal. B: Environ. 92, 56 (2009)
F. Meng, F. Lu, Z. Sun, J. Lü, Sci. China, Technol. Sci. 53, 3027 (2010)
M. Ni, M.K.H. Leung, D.Y.C. Leung, K. Sumathy, Renew. Sustain. Energy Rev. 11, 401 (2007)
M. Behpour, S.M. Ghoreishi, F.S. Razavi, Dig. J. Nanomater. Biostruct. 5, 467 (2010)
P.C. Lansåker, J. Backholm, G.A. Niklasson, C.G. Granqvist, Thin Solid Films 518, 1225 (2009)
A. Fujishima, T.N. Rao, D.A. Tryk, J. Photochem. Photobiol. C Photochem. Rev. 1, 1 (2000)
S. Ahmed, M.G. Rasul, R. Brown, M.A. Hashib, J. Environ. Manage. 92, 311 (2011)
V. Fierro, V.T. Torne Fernandez, D. Montane, A. Celzard, Micropor. Mesopor. Mater 111, 276 (2008)
F. Woodard, Industrial Waste Treatment Handbook (Butterworth-Heinemann, Boston, 2001)
N. Nakada, H. Shinohara, A. Murata, K. Kiri, S. Managaki, N. Sato, H. Takada, Water Res. 41, 4373 (2007)
G. Tian, H. Fu, L. Jing, C. Tian, J. Hazard. Mater. 161, 1122 (2009)
J. Yu, J. Zhang, Dalton Trans. 39, 5860 (2010)
M. Mahlambi, C. Ngila, B. Mamba, J. Nanomater. 2, 1 (2015)
H.C. Liang, X.Z. Li, J. Hazard. Mater. 162, 1415 (2009)
G. Li, L. Chen, M.E. Graham, K.A. Gray, J. Mol. Catal. A275, 30 (2007)
F. Li, Y. Jiang, M. Xia, M. Sun, B. Xue, D. Liu, X. Zhang, J. Phys. Chem. C 113, 18134 (2009)
D.O. Scanlon, C.W. Dunnill, J. Buckeridge, S.A. Shevlin, A.J. Logsdail, S.M. Woodley, C.R.A. Catlow, M.J. Powell, R.G. Palgrave, I.P. Parkin, G.W. Watson, T.W. Keal, P. Sherwood, A. Walsh, A.A. Sokol, Nat. Mater. 12, 798 (2013)
Z. Luo, A.S. Poyraz, C.-H. Kuo, R. Miao, Y. Meng, S.-Y. Chen, T. Jiang, C. Wenos, S.L. Suib, Chem. Mater. 27, 6 (2014)
R. Kaur, A. V. Singh, and R. M. Mehra, J. Non-Cryst. Solids 352(23–25), 2335 (2006)
J. Zhang, H. Feng, W. Hao, and T. Wang, J. Sol–Gel Sci. Technol. 39(1), 37 (2006)
W. Dong, C. Zhu, Opt. Mater. 22, 227 (2003)
D. Sarkar, S. Tikku, V. Thapar, R. S. Srinivasa, K. C. Khilar, Colloids Surf. A 381(1–3), 123 (2011)
M. Ristic, S. Popovic, S. Music, Mater. Lett. 58, 2494 (2004)
R. F. K. Gunnewiek, C. F. Mendes, R. H. G. A. Kiminami, Mater. Lett. 129, 54 (2014)
M. I. Khalil, M. M. Al-Qunaibit, A. M. Al-zahem, J. P. Labis, Arabian J. Chem. 7, 1178 (2014)
X. Meng, L. Qi, Z. Xiao, S. Gong, Q. Wei, Y. Liu, M. Yang, F. Wang, J. Nanopart. Res. 14, 1 (2012)
S.C. Padmanabhan, S.C. Pillai, J. Colreavy, S. Balakrishnan, D.E. McCormack, T.S. Perova, Y. Gunko, S.J. Hinder, J.M. Kelly, Chem. Mater. 19, 4474 (2007)
K. Lv, B. Cheng, J. Yu, G. Liu, P.C.C.P. Phys, Chem. Chem. Phys. 14, 5349 (2012)
X. Wei, G. Zhu, J. Fang, J. Chen, Int. J. Photoenergy 2013, 6 (2013)
S.J. Gregg, K.S.W. Sing, Adsorption, Surface Area, and Porosity (Academic Press, New York, 1982)
X. Deng, A. Verdaguer, T. Herranz, C. Weis, H. Bluhm, M. Salmeron, Langmuir 24, 9474 (2008)
S. Zhu, S. Liang, Q. Gu, L. Xie, J. Wang, Z. Ding, Appl Catal B 119–120, 146 (2012)
J.-G. Kang, Y. Sohn, J. Mater. Sci. 47, 824 (2012)
C.D. Wagner, W.M. Riggs, L.E. Davis, J.F. Moulder, G.E. Mullenberg, in Handbook of X-ray Photoelectron Spectroscopy, ed. by G.E. Muilenberg (Perkin-Elmer, Eden Prairie, MN, 1979)
B. Ohtani, O.O. Prieto-Mahaney, D. Li, R. Abe, J. Photochem. Photobiol. A 216, 179 (2010)
T.Y. Han, C.F. Wu, C.T. Hsieh, J. Vac. Sci. Technol. B 25, 430 (2007)
W.Z. Tang, H. An, Chemosphere 31, 4157 (1995)
P. Kuma, R. Sivakumar, S. Anandan, J. Madhavan, P. Maruthamuthu, M. Ashokkumar, Water Res. 42, 4878 (2008)
M.A. Behnajady, N. Modirsahla, M. Shokri, B. Rad, Global NEST J. 10, 1 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Monem, Y.K. Efficient nanophotocatalyt of hydrothermally synthesized Anatase TiO2 nanoparticles from its analogue metal coordinated precursor. J Mater Sci: Mater Electron 27, 5723–5728 (2016). https://doi.org/10.1007/s10854-016-4484-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4484-7