Abstract
Bismuth layer-structured ferroelectric ceramics of SrCaBi4−x Er x Ti5O18 (SCBT-xEr, x = 0.00, 0.02, 0.04 and 0.06) were prepared by a conventional solid-state reaction method, and their ferroelectric, dielectric, piezoelectric and photoluminescence properties were investigated. The results indicated that all ceramics have a bismuth oxide layered with a dense structure. After Er3+ doping, samples show a bright up-conversion photoluminescence while simultaneously obtaining an enhanced ferroelectric and piezoelectric properties. A bright green (548 nm) and a weak red (681 nm) emission bands were obtained under excitation (980 nm) at room temperature, which correspond to the transitions from 4S3/2 to 4I15/2 and 4F9/2 to 4I15/2, respectively. Ferroelectric and piezoelectric measurements show that the Er3+-doped ceramics showed an increase in remnant polarization and piezoelectric constant. At x = 0.04, the piezoelectric constant d 33 reaches up to 13pC/N, together with a large remnant polarization (2P r = 16 μC/cm2) and high Curie temperature (T c = 573 °C). As a multifunctional material, Er3+-doped SCBT ferroelectric oxide showed great potential in optical electro integration and coupling device applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, Aurivillius bismuth-layer structure ferroelectrics (BLSFs) are widely used for high-temperature sensors, thin-film memory devices, and lead-free piezoelectric resonators because of their high Curie temperature and fatigue-free and lead-free nature [1–3]. The structure of BLSFs can be described as (Bi2O2)2+ (A m−1B m O3m+1)2− which is built by regular intergrowth of (Bi2O2)2+ layers and perovskite slabs, where A is a mono-, di- or tri-valent ion (or their combination) such as B3+, Pb2+, Ba2+, Sr2+, Ca2+, Na+, K+, La3+, Y3+, U3+, Th4+, B is a transition element suited to octahedral coordination such as Co3+, Cr3+, Zr4+, Ti4+, Nb5+, Ta5+, W6+, Mo6+, and m usually varies from 1 to 5 [4, 5]. It has been shown that the ferroelectric properties of the Aurivillius bismuth-layer compounds are strongly dependent on the number of layers and the chemical elements in the A and B sites [6, 7].
The M 2Bi4Ti5O18 (M = Ca, Ba, Sr) compounds with the layer number m = 5 are a subgroup of this BLSFs family. Sr2Bi4Ti5O18 (SBT) ceramics has good piezoelectric coefficient (d 33 = 26 pC/N), but the Curie temperature is only 267 °C [8]. Comparing to SBT, Ca2Bi4Ti5O18 (CBT) ceramics has higher Curie temperature (T c = 775 °C) and lower piezoelectric coefficient (d 33 = 8.83 pC/N) [9]. Nevertheless, SrCaBi4Ti5O18 (SCBT) was found with relatively higher Curie temperature, relatively larger piezoelectric constant d 33 and relatively better temperature stability [10], indicating that this compound is very promising piezoelectric materials for high-temperature applications. Therefore, the SCBT ceramic has been given more attentions in recent years.
Recently, it was considered that the introduction of rare earth ions (RE3+) in BLSFs is an efficient approach to improve its physical properties. To date, much research works related with the RE3+ doped in BLSFs have been reported in the literature [11–13]. However, these works mainly focus on the structural, dielectric, and ferroelectric properties of BLSFs system, although the RE ions have a well potential luminescent properties such as high brightness, high efficiency and long working time [14–17]. Therefore, it is of interest and significance to study the PL properties of RE3+ doped BLSFs from the aspect of development of multi-functional materials.
Up-conversion (UC) is an anti-stokes emission that emission energy is higher than excitation photon energy. Recently, there has been an intense interest in the investigation of the UC of rare earth doped phosphors due to its large number of device applications, which broadly involved in the physics, chemistry and biomedicine areas. Recently, the development of highly efficient and stable materials that can up-convert at room temperature with low excitation density thresholds is urgently required. In this sense, oxide materials such as BLSFs are usually chemically, mechanically, and thermally stable, and therefore can be promising hosts for light UC applications [18].
In the present study, we selected SrCaBi4Ti5O18 (SCBT) ceramic, which is a well-known typical material of BLSFs with m = 5 as a base material with small amounts of Er3+ being introduced as doping species. We found that the Er-modified SCBT exhibited bright UC at room temperature. Studies of dielectric and piezoelectric properties have also been carried out. Introduction of Er3+ increased the remnant polarization P r and piezoelectric constant, while maintaining high Curie temperature, thus making this ceramic suitable for piezoelectric sensor applications at higher temperatures.
2 Experimental procedure
SrCaBi4−x Er x Ti5O18 (SCBT-xEr, x = 0.00–0.06) ceramics were prepared using a traditional solid-state reaction. The high purity oxides and carbonates powders, TiO2 (98 %, Sinopharm Chemical Reagent), Bi2O3 (98 %, Sinopharm Chemical Reagent), Er2O3 (98 %, Sinopharm Chemical Reagent), SrCO3 (99 %, Sinopharm Chemical Reagent), CaCO3 (99.8 %, Sinopharm Chemical Reagent) were used as the starting raw materials, which had been treated carefully by a drying process. Then, we need weighing the materials according to the composition, and wet milled in polyethylene bottles with ZrO2 balls for 15 h with 180 round per minute in alcohol. Following, the milled powders were dried and calcined at 850 °C for 2 h. The calcined powders were milled again for 15 h with 180 round per minute. Next, the obtained dry powders were mixed with an appropriate amount of PVA (8 wt% of SCBT) binder and then were pressed into 12 and 15 mm in diameter, 1 mm in thickness disks under a uniaxial pressure of 200 Mpa. The discs with 12 mm in diameter and 0.3 mm in thickness was prepared for the measurement of ferroelectric, piezoelectric and photoluminescence properties, and the discs with 15 mm in diameter and 1 mm in thickness was used for the dielectric properties. The green pellets were fired at 800 °C for 2 h to burn out PVA. Following, the samples were sintered at 1180 °C for 3 h. Finally, the sintered pellets were polished and covered with silver paste on both sides, then fired at 850 °C for 20 min.
The crystalline structure of the crushed samples was analyzed by X-ray diffraction (XRD) methods (D8 Advanced, Bruker. Inc., Germany). The microstructure evolution was observed using a scanning electron microscope (SEM, JSM-6380LV, Tokyo, Japan). The temperature dependences of the dielectric properties were measured using an Agilent 4294A precision impedance analyzer (Agilent Inc., USA). The ferroelectric properties such as the coercive field and the remnant polarization have been evaluated with the TF Analyzer 2000 FE-Module (aixACCT, Aachen, Germany). The piezoelectric coefficient d 33 was measured with a Piezo-d 33 meter (Sznocera Piezotronics INC, China). For upconversion luminescence measurement, a 980 nm laser diode (MDL-III-980- 100 mW) was used to excite the ceramic samples.
3 Results and discussion
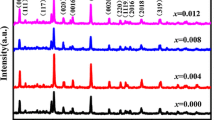
The XRD patterns of Er3+-modified SCBT piezoelectric ceramic samples at room temperature were shown in Fig. 1. According to the XRD analysis, we can see that all the ceramics have a bismuth oxide layer-type structure, and no secondary phases are detected in the range detected, suggesting that Er3+ have diffused into crystal lattice of the SCBT ceramics and formed a solid solution. The highest diffraction peak (1011) is indicated that it have a pure five-layer structure, compared with the pure SCBT ceramics, the peak position of the Er3+-modified SCBT ceramics in XRD patterns shifts to lower angle side as shown in Fig. 1b. It is believed that Er3+ ions will preferentially substitute for the Bi3+ ions in the pseudo-perovskite blocks (SCBT) when Er3+ ions concentration is relatively low. If referring to the ion radii of Er3+ (0.89 Å, CN12) and Bi3+ (1.30 Å, CN12) [19], the lower angle shift of diffraction peak with x increase is unreasonable. Maybe the Er3+ ions will inevitably enter the (Bi2O2)2+ layers. Owing to different chemical character between Er3+ and Bi3+, the substitution of Er3+ at Bi3+ sites in (Bi2O2)2+ layers may break the crystalline structure of (Bi2O2)2+ layers which should be attributed to the low angle shift of diffraction peak.
The SEM images of surface for Er3+-modified SCBT ceramics sintered at 1180 °C for 3 h were shown in Fig. 2. It can be seen from the image that the ceramic samples are dense and have plate-like grain morphology without forming aggregates or a precipitate, which is the typical morphology of BLSFs [20], further proved that the Er ions in the sample do not form minority phases or segregate from the interior grain but dissolve into the SCBT host lattice [18].
The dielectric constant ε r versus the temperature of the Er3+-modified SCBT piezoelectric ceramics at 1 kHz were shown in Fig. 3. Two dielectric anomalies are observed in the samples. The similar phenomenon was also found in mixed-layered Bi7Ti4NbO21 ferroelectric ceramics [21]. It was disclosed that two dielectric anomalies of Bi7Ti4NbO21 ferroelectric ceramics was induced by the intergrowth structure of perovskite-like (Bi2Ti3O10)2−, (BiTiNbO7)2− and (Bi2O2)2+ layer along c direction, which caused Bi7Ti4NbO21 ferroelectric ceramics to undergo a ferroelectric–ferroelectric and a ferroelectric–paraelectric phase transition corresponding to the two dielectric anomalies [21]. In the present work, we assumed that two dielectric anomalies in Er3+-modified SCBT ferroelectric ceramics were also led by intergrowth structure of perovskite-like (Sr2Bi2Ti5O16)2−, (Ca2Bi2Ti5O16)2− and (Bi2O2)2+ layer along c direction and the materials underwent a ferroelectric–ferroelectric and a ferroelectric– paraelectric phase transition at two dielectric anomalies [10]. In addition, it clearly show that the dielectric constant maximums of all the specimens are increase at first then decrease, and the dielectric maximal peak becomes broad with the increasing of Er3+-modified content. The broadened peak can be attributed to the compositional fluctuation or substitution disordering in the arrangement of cations at one or more crystallographic sites in the lattice structure [22]. The Curie temperatures (T c) of the ceramics in this work changes slightly with the increasing of Er3+ content, which is in the range of 570–573 °C.
The ferroelectric hysteresis loop of the Er3+-modified SCBT piezoelectric ceramic samples recorded at 10 Hz were shown in Fig. 4. Each sample exhibits a well saturated hysteresis loop with a relatively large remanent polarization (P r). The optimum Er3+ content for maximum 2P r (~14 μC/cm2) is x = 0.02. It is resulted in the Er3+-modified lead to the lattice distortions, the turning of the electric domain is restricted, it is necessary to add a larger voltage to make its full polarization, so the residual polarization of the ceramic 2P r gradually rise. The piezoelectric constant d 33 of the Er3+-modified SCBT piezoelectric ceramic samples were shown in Fig. 5. With the Er3+-modified content increasing, the piezoelectric constant d 33 increased at first and then decreased, giving a maximum d 33 value of ~13pC/N when the Er3+ content is 0.04 mol.
Figure 6 shows the room temperature up-conversion emission of the Er3+-modified SCBT piezoelectric obtained under 980 nm NIR excitation. It shows two emission bands: the strong green emission peaks near 523 and 548 nm are attributed to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions respectively, and the weak red emission located at 674 nm is caused by the 4F9/2 → 4I15/2 transition. The room-temperature up-conversion emission obtained under 980 nm excitation was so strong that it was easily observed by the naked eye, as shown in the inset of Fig. 6.
The energy level diagram of the green up-conversion emissions for the Er3+-modified SCBT ferroelectric oxide is shown in Fig. 6. Er3+ ions can be excited to 4I11/2 through ground state absorption (GSA) from pumping source or energy transfer (ET) from neighboring Er3+ ions under a 980 nm laser excitation. The Er3+ ions at 4I11/2 may further rise up to the higher 4F7/2 level through ET, i.e., excited state absorption (ESA), followed by nonradioactive decaying to 4S3/2 and 4F9/2 levels through multi-phonon assisted relaxation. Some Er3+ ions at 4S3/2 levels produced green up-conversion emissions and some at 4F9/2 levels produced red ones.
4 Conclusions
The Er3+-modified SCBT piezoelectric ceramics were prepared by the traditional solid-state method. The ferroelectric, dielectric, piezoelectric and photoluminescence properties were investigated. After Er3+ doping, samples show a bright up-conversion photoluminescence while simultaneously obtaining an enhanced ferroelectric and piezoelectric properties. A bright green (548 nm) and a weak red (681 nm) emission bands were obtained under excitation (980 nm) at room temperature, which correspond to the transitions from 4S3/2 to 4I15/2 and 4F9/2 to 4I15/2, respectively. Moreover, an increase in remnant polarization and piezoelectric constant was observed in Er3+-modified SCBT. At x = 0.04, the piezoelectric constant d 33 reaches up to 13pC/N, together with a large remnant polarization (2P r = 16 μC/cm2) and high Curie temperature (T c = 573 °C). These results suggest that the Er3+-modified SCBT system presents multifunctional properties and significant technological potential in novel multifunctional devices.
References
A. Megriche, L. Lebrum, M. Troccaz, Sens. Actuators A 78, 88 (1999)
B.H. Park, B.S. Kang, S.D. Bu, T.W. Noh, J. Lee, W. Jo, Nature (London) 401, 682 (1999)
A. Ando, M. Kimura, Y. Sakabe, Jpn. J. Appl. Phys. Part 1 42, 150 (2003)
L. Ma, K. Zhao, J. Li, Q. Wu, M. Zhao, C. Wang, J. Rare Earths 27, 496–500 (2009)
Z. Yao, R. Chu, Z. Xu, J. Hao, D. Wei, G.J. Li, Mater. Sci. Mater. Electron. 26(11), 8740–8746 (2015)
V.A. Isupov, Ferroelectrics 189, 211 (1996)
D.Y. Suarez, I.M. Reaney, W.E. Lee, J. Mater. Res. 16, 3139 (2001)
P. Ferrer, M. Algueró, J.E. Iglesias, A. Castro, J. Eur. Ceram. Soc. 27, 3641–3645 (2007)
C. Moure, V. Gil, J. Tartaj, P. Duran, J. Eur. Ceram. Soc. 25, 2447–2451 (2005)
Z. Xu, R. Chu, J. Hao, Y. Zhang, Q. Chen, L. Zhao, G. Li, Q. Yin, J. Alloys Compd. 487, 585–590 (2009)
Z.G. Yi, Y.X. Li, Y. Wang, Q.R. Yin, Appl. Phys. Lett. 88, 152909 (2006)
J. Zhu, X.B. Chen, W.P. Lu, X.Y. Mao, R. Hui, Appl. Phys. Lett. 83, 1818 (2003)
J.S. Zhu, D. Su, X.M. Lu, H.X. Qin, Y.N. Wang, D.Y. Wang, H.L.W. Chan, K.H. Wong, C.L. Choy, J. Appl. Phys. 92, 5420 (2002)
V. Lupei, A. Lupei, A. Ikesue, J. Appl. Phys. Lett. 86, 111–118 (2005)
D. Mohr, A.S.S. de Camargo, J.F. Schneider, T.B. Queiroz, H. Eckert, E.R. Botero, D. Garcia, J.A. Eiras, Solid State Sci. 10, 1401–1407 (2008)
B. Yan, X.Q. Su, Opt. Mater. 29, 547–551 (2007)
V.S. Sastri, J.C. Biinzli, V.R. Rao, G.V.S. Rayudu, J.R. Perumareddi. Modern Aspects of Rare Earths and their Complexes. (Elsevier, Amsterdam, 2003)
D. Peng, H. Zou, C. Xu, X. Wang, X. Yao, J. Lin, T. Sun, AIP Adv. 2, 042187 (2012)
R.D. Shannon, Acta Crystallogr. Sect. A Found. Crystallogr. 32(5), 751–767 (1976)
G.R. Li, L.Y. Zheng, Q.R. Yin, B. Jiang, W.W. Cao, J. Appl. Phys. 98, 064108 (2005)
L. Zhang, S. Zhao, L. Zheng, G. Li, Q. Yin, Acta Phys. Sin. 54, 2346–2351 (2005)
X. Jiang, X. Fu, C. Chen et al., J Adv. Ceram. 4, 54–60 (2015)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51402144, 51372110, 51502127 and 51302124), the Project of Shandong Province Higher Educational Science and Technology Program (Grant Nos. J14LA11 and J14LA10), the National High Technology Research and Development Program of China (No. 2013AA030801), Science and Technology Planning Project of Guangdong Province, China (No. 2013B091000001), Independent innovation and achievement transformation in Shandong Province special, China (No. 2014CGZH0904), the Natural Science Foundation of Shandong Province of China (Grant No. ZR2014JL030), and the Research Foundation of Liaocheng University (No. 318011306).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, L., Hao, J., Chu, R. et al. Bright upconversion emission and enhanced piezoelectric properties in Er-modified bismuth layer-structured SrCaBi4Ti5O18 ceramics. J Mater Sci: Mater Electron 27, 5259–5263 (2016). https://doi.org/10.1007/s10854-016-4422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4422-8