Abstract
Lutetium doped nickel ferrite nanoparticles were successfully synthesized by novel sol–gel method with the aid of nickel(II) nitrate, iron(III) nitrate, lutetium(III) nitrate and lactose without adding external surfactant. Moreover, lactose plays role as capping agent, reducing agent, and natural template in the synthesis NiFe2–xLuxO4 nanoparticles. The as-synthesized NiFe2–xLuxO4 nanoparticles were characterized by means of several techniques such as X-ray diffraction, scanning electron microscopy, energy dispersive X-ray microanalysis and UV–Vis diffuse reflectance spectroscopy. The magnetic properties of as-prepared NiFe2–xLuxO4 nanoparticles were also investigated with vibrating sample magnetometer. To evaluate the photocatalyst properties of nanocrystalline NiFe2–xLuxO4, the photocatalytic degradation of methyl orange under ultraviolet light irradiation was carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Physical properties and potential applications of nanostructures and nanomaterial have been studied intensively [1–3]. This interest results from the special properties of materials at the nanoscale, such as a large surface-to-volume ratio and increased surface activity, as compared with that of the bulk material. The properties of bulk materials usually depend on the size of the primary particles. Thus, the control of particle size and morphology plays a crucial role in the manufacturing process [4–8]. In ferrite MFe2O4, the choice of rare-earth ions allows a relative tenability of the magnetic properties such as magnetization or anisotropy. The presence of rare-earth ions influences mainly the magnetic anisotropy of the system. The octahedral and tetrahedral sublattice magnetizations are antiparallel and therefore a noncompensated magnetic moment occurs. This structure is called ferrimagnetic. Various properties such as superparamagnetism [9, 10] and spin canting [11, 12] are observed when the particle size is much reduced compared to the bulk materials. Spinel-type ferrites powders can be prepared by ceramic technique [13], evaporative decomposition of metal organic solution [14], co-precipitation [15], sol–gel [16–18], microemution [19], micelle and hydrothermal methods [20, 21]. However, some methods encounter problems such as the formation of the undesirable phase, the requirement of complicated equipment or time-consuming caused by multiple steps, etc. Photocatalysis is a subject of current interest related to its application in effluent decontamination. Photocatalytic degradation of organic pollutants is becoming one of the most promising green chemistry technologies. In most of the industries, phenolic compounds are widely used and have become common pollutants in waste water bodies. The phenolic compounds are quite stable and remain in the environment for a long period of time. Due to their toxicity and carcinogenic character, they are dangerous to the ecosystem in water bodies and human health. A representative of this class of compounds is phenol. Sources of phenol include the process or waste solutions in chemical process industries, agriculture production, etc. [4, 6, 7]. In the present study, an attempt has been made to synthesis Lu3+ doped NiFe2O4 nanoparticles by the novel sol gel method. The photocatalytic degradation was investigated using methyl orange (MO) under ultraviolet light irradiation.
2 Experimental
2.1 Characterization
X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu K α radiation at scan range of 10 < 2θ < 80. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope. The magnetic measurement of samples were carried out in a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) at room temperature in an applied magnetic field sweeping between ±10,000 Oe. Spectroscopy analysis (UV–Vis) was carried out using shimadzu UV–Vis scanning UV–Vis diffuse reflectance spectrometer.
2.2 Synthesis of NiFe2–xLuxO4 nanoparticles
Nickel(II) nitrate, iron(III) nitrate, and lutetium(III) nitrate with a stoichiometric ratio of 1:1.95:0.05, were dissolved separately in 30 ml of water under magnetic stirring to form a homogeneous solution. Then, a solution containing 1 mmol of lactose was added into a solution involving 1 mmol of Ni(NO3)2·6H2O. Subsequently, above mention solution was mixed with solution containing 1.95 mmol of Fe(NO3)3·9H2O, and 0.05 mmol Lu(NO3)3·6H2O. Afterwards, the final mixed solution was kept stirring to form a gel at 110 °C. Finally, the obtained product was placed in a conventional furnace in air atmosphere for 2 h and calcine at 800 °C. After thermal treatment, the system was allowed to cool to room temperature naturally, and the obtained precipitate was collected.
2.3 Photocatalysis experiments
The methyl orange (MO) photodegradation was examined as a model reaction to evaluate the photocatalytic activities of the NiFe2–xLuxO4 nanoparticles. The photocatalytic experiments were performed under an irradiation ultraviolet light. The photocatalytic activity of nanocrystalline NiFe2–xLuxO4 obtained was studied by the degradation of methyl orange solution as a target pollutant. The photocatalytic degradation was performed with 50 ml solution of methyl orange (0.0005 g) containing 0.1 g of NiFe2–xLuxO4. This mixture was aerated for 30 min to reach adsorption equilibrium. Later, the mixture was placed inside the photoreactor in which the vessel was 15 cm away from the ultraviolet source of 400 W mercury lamps. The photocatalytic test was performed at room temperature. Aliquots of the mixture were taken at definite interval of times during the irradiation, and after centrifugation they were analyzed by a UV–Vis spectrometer. The methyl orange (MO) degradation percentage was calculated as:
where At and A0 are the obtained absorbance value of the methyl orange solution at t and 0 min by a UV–Vis spectrometer, respectively.
3 Results and discussion
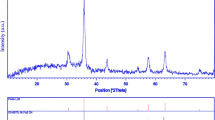
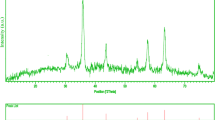
Crystalline structure and phase purity of as-prepared product has been determined using XRD. The XRD pattern of the NiFe2O4 in presence of La3+ doped is shown in Fig. 1. The XRD pattern of the as-synthesized NiFe2–xLuxO4 nanoparticles (Fig. 1) indicates the formation of cubic phase of NiFe2–xLuxO4 (JCPDS No. 03-0875) with the calculated cell parameter of a = b = c = 8.3400 Å. According to XRD data, the crystallite diameter (Dc) of NiFe2–xLuxO4 nanoparticles are calculated to be 48 nm using the Scherer Eq. (1):
where β is the breadth of the observed diffraction line at its half intensity maximum, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. The morphology of the nanoparticles was investigated using SEM which demonstrates uniform nanoparticles with spherical shape homogenously distributed all over the sample, as it could be clearly observed in Fig. 2. The NiFe2–xLuxO4 nanoparticles with particle size of about 55–60 nm were observed. The EDS analysis measurement was used to investigate the chemical composition and purity of NiFe2–xLuxO4 nanoparticles. According to the Fig. 3, the product consists of Ni, Fe, Lu, and O elements. Furthermore, neither N nor C signals were detected in the EDS spectrum, which means the product is pure and free of any surfactant or impurity. The hysteresis loop of NiFe2–xLuxO4 nanoparticles was studied to examine their magnetic properties (Fig. 4). At 300 K the remanent magnetization (Mr) is 4 emu/g, the coercive field (Hc) is 90 Oe and the magnetization at saturation (Ms) is estimated to be only 23 emu/g (the saturation magnetization Ms was determined from the extrapolation of curve of H/M vs. H). The diffused reflectance spectrum of the as-prepared NiFe2–xLuxO4 nanoparticles is shown in Fig. 5. The fundamental absorption edge in most semiconductors follows the exponential law. Using the absorption data the band gap was estimated by Tauc’s relationship:
where α is absorption coefficient, hν is the photon energy, α0 and h are the constants, Eg is the optical band gap of the material, and n depends on the type of electronic transition and can be any value between ½ and 3. The energy gap of the NiFe2–xLuxO4 nanoparticles is determined by extrapolating the linear portion of the plots of (αhν)2 against hν to the energy axis, as shown in Fig. 5. The Eg value is calculated as 2.8 eV for the NiFe2–xLuxO4 nanoparticles. Photodegradation of methyl orange under UV light irradiation (Fig. 6a–c) was employed to evaluate the photocatalytic activity of the as-synthesized NiFe2–xLuxO4. No methyl orange was practically broken down after 60 min without using UV light irradiation or nanocrystalline NiFe2–xLuxO4. This observation indicated that the contribution of self-degradation was insignificant. The mechanism for the enhanced photocatalysis of NiFe2–xLuxO4 could be proposed as follow. Under the irradiation, the electrons (e −cb ) are excited from the valence band to the conduction band of NiFe2–xLuxO4 leaving behind h +νb . Lu doping in NiFe2–xLuxO4 being lewis acid due to the presence of partially filled orbital can effectively trap the e −cb and inhibit the recombination with h +νb . This suggested that the Lu dopant can serve as an effective charge carrier trap and facilitated the excited e −cb transfer under visible light irradiation. The degradation mechanism for the NiFe2–xLuxO4 can be given as:
Using photocatalytic calculations by Eq. (1), the methyl orange degradation was about 63 % after 60 min irradiation of UV light, and nanocrystalline NiFe2–xLuxO4 presented high photocatalytic activity (Fig. 6a). The spectrofluorimetric time-scans of methyl orange solution illuminated at 510 nm with nanocrystalline NiFe2–xLuxO4 are depicted in Fig. 6b. Figure 6b shows continuous removal of methyl orange on the NiFe2–xLuxO4 under UV light irradiation. It is generally accepted that the heterogeneous photocatalytic processes comprise various steps (diffusion, adsorption, reaction, and etc.), and suitable distribution of the pore in the catalyst surface is effective and useful to diffusion of reactants and products, which prefer the photocatalytic reaction. In this investigation, the enhanced photocatalytic activity can be related to appropriate distribution of the pore in the nanocrystalline NiFe2–xLuxO4 surface, high hydroxyl amount and high separation rate of charge carriers (Fig. 6c). Furthermore, this route is facile to operate and very suitable for industrial production of NiFe2–xLuxO4 nanoparticles.
Photocatalytic methyl orange degradation of NiFe2–xLuxO4 nanoparticles under ultraviolet light (a), fluorescence spectral time scan of methyl orange illuminated at 510 nm with NiFe2–xLuxO4 nanoparticles (b), and reaction mechanism of methyl orange photodegradation over NiFe2–xLuxO4 under ultraviolet light irradiation (c)
4 Conclusions
In this work, NiFe2–xLuxO4 nanoparticles were successfully synthesized by a novel sol-gel method at 800 °C for 120 min. The stages of the formation of NiFe2–xLuxO, as well as the characterization of the resulting compounds were done using X-ray diffraction and energy dispersive X-ray spectroscopy. The products were analyzed by scanning electron microscopy (SEM), and ultraviolet–visible (UV–Vis) spectroscopy to be round, about 55–60 nm in size and Eg = 2.8 eV. The magnetic properties of the as-synthesized products were also studied. When as-prepared nanocrystalline NiFe2–xLuxO was utilized as photocatalyst, the percentage of methyl orange degradation was about 63 % after 60 min irradiation of UV light.
References
Y. Zhang, Y. Zhang, B. Fu, M. Hong, M. Xiang, Z. Liu, J. Leng, J. Mater. Sci. Mater. Electron. 25, 5475 (2014)
Z. Khayat Sarkar, F. Khayat Sarkar, Int. J. Nanosci. Nanotechnol. 7, 197 (2011)
A. Ghasemi, A.M. Davarpanah, M. Ghadiri, Int. J. Nanosci. Nanotechnol. 8, 207 (2012)
F.S. Ghoreishi, V. Ahmadi, M. Samadpourc, J. Nanostruct. 3, 453 (2013)
S. Moshtaghi, D. Ghanbari, M. Salavati-Niasari, J. Nanostruct. 5, 169 (2015)
A. Rahdar, M. Aliahmad, Y. Azizi, J. Nanostruct. 5, 145 (2015)
J. Safaei-Ghomi, S. Zahedi, M. Javid, M.A. Ghasemzadeh, J. Nanostruct. 5, 153 (2015)
D. Li, R. Shi, C. Pan, Y. Zhu, H. Zhao, Cryst. Eng. Comm. 13, 4695 (2011)
C.P. Bean, J.D. Livingston, J. Appl. Phys. 30, 120 (1959)
D.L. Leslie-Pelecky, R.D. Rieke, Chem. Mater. 8, 1770 (1996)
A.T. Ngo, P. Bonville, M.P. Pileni, J. Appl. Phys. 89, 3370 (2001)
S.A. Oliver, H.H. Hamdeh, J.C. Ho, Phys. Rev. B 99, 3400 (1829)
A. Lakshman, P.S.V. Subba Rao, K.H. Rao, J. Magn. Magn. Mater. 284, 352 (2004)
Q. Song, Z.J. Zhang, J. Am. Chem. Soc. 126, 6164 (2004)
S. Dey, A. Roy, J. Ghose, J. Appl. Phys. 90, 4138 (2001)
Z. Yue, J. Zhou, X. Wang, Z. Gui, L. Li, J. Eur. Ceram. Soc. 23, 189 (2003)
X.M. Liu, S.Y. Fu, C.J. huang. J. Magn. Magn. Mater. 281, 234 (2004)
K.P. Chae, J.G. Lee, H.S. Kweon, Y.B. Lee, J. Magn. Magn. Mater. 283, 103 (2004)
M. Bonini, A. Wiedenmann, P. Baglioni, Phys. A 339, 86 (2004)
X. Li, C. Kutal, J. Alloys Comput. 349, 264 (2003)
H.W. Wang, S.C. Kung, J. Magn. Magn. Mater. 270, 230 (2004)
Acknowledgments
Authors are grateful to council of University of Central Tehran for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Talebi, R., Alborzi, A. Synthesis, characterization and optical properties of lutetium doped nickel ferrite nanoparticles prepared by novel sol–gel method. J Mater Sci: Mater Electron 27, 4321–4325 (2016). https://doi.org/10.1007/s10854-016-4299-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4299-6