Abstract
The Mg4Nb2O9 co-doped with 20 wt% TiO2 (MNT) and x wt% LiF (1 ≤ x ≤ 8) composite ceramics were prepared by a solid-state reaction method for developing ultralow-fired (≤800 °C) microwave dielectric ceramics. The sintering temperatures of MNT ceramics were successfully lowered to 750 °C due to the formation of liquid phase (LiF). A secondary phase of MgTiO3 was observed at above 700 °C for MNT-8 wt% LiF ceramics. All the composite ceramics have the optimal bulk densities at 800 °C, which corresponds to the maximum Q × f value of 32,000 GHz and ɛ r value of 15.7. The well microwave dielectric properties of ɛ r = 15.6, Q × f = 25,000 GHz, and τ f = −56 ppm/°C were obtained at 750 °C for 5 h for MNT-8 wt% LiF ceramics. This material is compatible with Ag electrodes, suitable for low-temperature co-fired ceramics applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Low-temperature co-fired ceramics (LTCC) have recently been widely investigated because of the necessity for miniaturization of electronic components and reduction the size of wireless system to achieved integration [1, 2]. A high relative permittivity (≥20) is needed for miniaturization [3], whereas a low relative permittivity (ε r ≤ 20) material is preferred for substrate applications to avoid signal delay [4]. In the past, several microwave dielectric systems, such as Al2O3, MgTiO3, Y2BaCuO5, Mg2SiO4, Mg2TiO4, Zn2SiO4, MgAl2O4, have been studied for developing the low dielectric materials. Al2O3 filled with La2O3–B2O3 glass and sintered at 950 °C were reported by Seo et al. (ε r = 8.4, Q × f = 12,400 GHz) [5]. Dai et al. [6] developed a low loss and near zero τ f LTCC (T2000) based on Al2O3 by adding glass and TiO2 (ε r = 9.1, Q × f = 2500 GHz). Naoya et al. [7] found that MgAl2O4 doped with Li–Mg–Zn–B–Si–O glass exhibited low sintering temperatures of 1000 °C and a good microwave dielectric properties (ε r = 7.4, Q × f = 48,000 GHz). Gu [8] and Chen et al. [9] reported that the ultra-low firing BiVO4/Li0.5Re0.5WO4 (Re = Sm, La, Nd) ceramics with dielectric properties well were obtained.
Mg4Nb2O9 (MN) ceramics with a corundum-type structure possesses good microwave dielectric properties (ε r = 12.6, Q × f = 197,000 GHz, τ f = −77 ppm/°C) at relatively high temperatures (1350–1400 °C) [10–13]. Recently, Lim et al. found that MN exhibited the microwave dielectric properties of ε r = 11.2, Q × f = 15950 GHz, τ f = 6.7 ppm/°C at 1300 °C by addition 20 wt% nanosize-TiO2 [14]. In addition, using the nano-size MN powders produced by high-energy ball milling or chemistry syntheses methods, the sintering temperatures of MN was also decreased lowered [15, 16]. MN nanoceramics were prepared at 1300 °C by high-energy ball milling method and subsequent microwave sintering [17]. Moreover, Zhu et al. [18] reported that the sintering temperatures of MN ceramics were lowered to 1125 °C by using 2 wt% CaO–B2O3–SiO2 glass addition. Additions of Li2CO3, V2O3, and LiF compounds with low melting temperatures are found to be effective in reducing the sintering temperatures of MN ceramics below 960 °C [19]. In the present work, MN doped with nano-size TiO2 (MNT) and x wt% LiF (1 ≤ x ≤ 8) composite ceramics were prepared by a solid-state reaction method for the development of ultra-low-temperature sintering(≤800 °C) ceramics. The sintering behavior, phase constitutes, microstructures, microwave dielectric properties of MNT ceramics and relationships among them were systematically investigated.
2 Experimental procedure
The starting materials to prepare MN ceramics were MgO (99.99 %, Guo-Yao Co. Ltd., Shanghai, China), Nb2O5 (99.99 %, Guo-Yao Co. Ltd., Shanghai, China). They were weighed and milled in a nylon jar with zirconia balls for 7 h, and then dried and calcined at 1000 °C for 10 h. After re-milling with the LiF (≥99.96 %, Guo-Yao Co. Ltd., Shanghai, China) and TiO2 (rutile structure with a small amount of anatase, purity 99.99 %, and average particle size ca. 80 nm, Guo-Yao Co. Ltd., Shanghai, China) additives, the power was dried, added with of polyvinyl alcohol (PVA), pressed into discs with 11.5 mm in diameter and 5.7 mm in thickness under a pressure of 200 kg/cm2. Samples were sintered from 700 to 950 °C for 5 h.
The sintering behavior was determined by thermogravimetric analysis (TGA) and differential thermal analyzer (TG/DTA; SDTQ600, USA) with heating rate of 20 °C/min. The bulk densities of the sintered ceramics were measured by Archimedes method. The crystal structures of sintered samples were analyzed by X-ray diffraction (XRD; Rigaku D/MAX2550X, Japan) using CuKa radiation. The microstructures of samples were observed by scanning electron microscopy (SEM; QUANTA200, Holland). The microwave dielectric properties were measured by the Hakki-Coleman dielectric resonator method [20], using a network analyzer (Agilent Tech., HP8720ES). The temperature coefficient of the resonator frequency was also measured by the same method by changing temperatures from 20 to 80 °C and calculated with the following Eq. (1):
where f 80 and f 20 were the resonant frequency measured at 80 and 20 °C, respectively.
3 Results and discussion
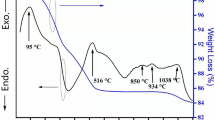
Figure 1 shows the TG and DTA curves of the MNT doped with 8 wt% LiF. The DTA curve exhibited the four significant peaks, noted as points A, B, C and D, respectively. At around 200 °C (point A), one endothermic peak was observed consistent with the loss of water in the first weight loss. The exothermic peak (point B) is due to decomposition of PVA accompany with the second weight loss at around 300 °C. The endothermic peaks appeared at 480 °C (point C) is corresponding to transformation of nano-sized TiO2 crystal structure from the polymorphic anatase to rutile [21], and the peak D at 750 °C is related to the melting of LiF compound. These results provide evidence of helping sintering MN ceramics as shown below.
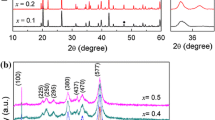
Powders XRD patterns of MNT specimens with 8 wt% LiF sintered at 500–950 °C for 5 h are shown in Fig. 2. The peaks were indexed to be MN (JCPDS #38-1459) and TiO2 phases with a minor of LiF phase at the temperatures ranging from 500 to 650 °C. For the specimens sintered at temperatures exceeding 700 °C, an impurity ilmenite phase MgTiO3 was detected (JCPDS #79-0831), accompanying with the disappearing of TiO2 and LiF phases. Thus, the chemical reaction (2) should occur at temperatures ≥600 °C:
In addition, as shown in Fig. 2, with an increase of sintering temperatures, LiF phase gradually was disappeared at temperatures higher than 700 °C due to the evaporation of LiF or existence in amorphousness.
The MNT specimens doped with various LiF and sintered at 750 °C for 5 h are shown in Fig. 3. MN, TiO2, MgNb2O6 phases are detected in the specimens with 1 wt% LiF. With increasing LiF content from 1 to 8 wt%, the peak intensity of MgTiO3 phase increase accompany with disappearing of MgNb2O6 and TiO2 phases. Thus, the chemical reaction (3) was supposed to occur:
As shown in the inset of Fig. 3, compared with pure MN phase, the 2θ values of the strongest diffraction peak (104) of MNT phase obviously shifted from 32.25° to 32.55°, due to some of Ti4+ ions (ionic radius = 0.605 Å, coordination number CN = 6) enter the Nb5+ (0.64 Å, CN = 6) site. Moreover, with LiF content increasing from 1 to 8, (104) peak turns toward the lower angles, which is related to a substitution of larger Li+ (0.76 Å, CN = 6) for Mg2+ (0.72 Å, CN = 6) site of MN, as we ever discussed in [15]. Finally, the (101) peak positions of MgTiO3 phase shift to lower angles with increasing LiF content, implying that Nb5+ ions originating from reactions (2) and (3) enter the Ti4+ site or Li+ ions substitute Mg2+ of MgTiO3 compound. The chemical reactions and ion diffusions process taking place among MN, TiO2, and LiF compounds during the sintering process strongly depend on the temperatures, powder size, and other processing factors. The chemical reactions provide the best wetting condition of MN powders, and MNT can be sintered by a liquid sintering process at low temperatures [22].
SEM pictures of fractured surface of the MNT ceramics with 8 wt% LiF additions sintered at 700–950 °C are shown in Fig. 4. It shows that the grains grew and congregated evidently as increasing sintering temperatures. All samples showed porous microstructures, which may be related to volatilization of LiF [23]. For the samples sintered at 800 °C, the average grain sizes reached the minimum values (~700 nm). The energy dispersive spectroscopy (EDS) analysis of the specimens sintered at 750 °C showed that the dark phase primarily contained Mg, Nb, O and small amounts of Ti elements, while the white phase contained more amounts of Ti element than the deep-color phase. Thus, the dark regions are mainly composed of MN phase and the MgTiO3 phase was mainly in the white regions. The inter-diffusion occurred between the two phases during firing process, resulting in a shift of XRD peak positions of both MN and MgTiO3 phases as shown in Fig. 3.
Figure 5 shows the bulk densities (ρ), dielectric constants (ε r ) and Q × f values of MNT ceramics doped with LiF as a function of sintering temperatures. As shown in Fig. 5a, all the sintered samples have their optimal bulk densities at about 800 °C except specimens with 5 wt% LiF, corresponding to their maximum Q × f values and the maximum dielectric constant in Fig. 5b, c. This is due to a high density and a low porosity which leads to a high dielectric constant and Q × f value [24]. The densification of ceramics at low temperatures originated from the formation of liquid phase LiF as sintering agent (Fig. 1). In Fig. 5b, the higher ε r values than that of pure MN (ε r = 12.6) are related to the existence of MgTiO3 phase (ε r = 17.17) [24]. The variation of Q × f value is affected by many factors such as lattice irrational modes, porous, grain boundaries, and secondary phases [25]. In Fig. 5c, Q × f values of all compositions have the maximum values at 800 °C, which is in agreement with their highest densities. With an increase of temperature from 800 to 950 °C (seen in Fig. 2), the decrease of Q × f values may be due to an increase of MgTiO3 phase (Q × f = 20,780 GHz) [24]. Since the smallest average grain size (~700 nm) is observed for the MNT ceramics sintered at 800 °C (Fig. 4c), the dielectric loss of MNT ceramics is supposed to be mainly caused by the porous and secondary phases rather than grain boundaries, similar to that ever found in Al2O3, MgO, and LaAIO3 dielectric materials [26, 27]. In addition, the oxygen vacancy and lattice defects, which are caused by the substitution of Li+ for Mg2+ and Ti4+ for Nb5+ in the solid solution of (Mg,Li)4(Nb,Ti)2O9-δ (confirmed by Fig. 3), should also contribute to the decrease of Q × f values.
The temperature coefficient resonant frequency of MNT with 8 wt% LiF content is not tuned appropriately (−56 ppm/°C) because of the small τ f value of secondary phase MgTiO3 (−50 ppm/°C) [24].
Figure 6 shows the XRD patterns of mixtures (MNT powders with 8 wt% LiF and Ag powders) and that after heat-treated at 800 °C. None impurity phases were observed in the XRD patterns, demonstrating a good chemical compatibility between the MNT-8 wt% LiF ceramics and Ag.
4 Conclusions
The Mg4Nb2O9 co-doped with 20 wt%TiO2 (MNT) and x wt% LiF (1 ≤ x ≤ 8) composite ceramics were primarily prepared by a solid-state reaction method. It was found that addition of 8 wt% LiF and 20 wt% TiO2 effectively reduced the sintering temperature of Mg4Nb2O9 ceramics from 1400 to 750 °C due to the formation of liquid phase (LiF). The grain size of MNT-8 wt% LiF ceramics grew as the increasing sintering temperatures and a secondary phase MgTiO3 was formed due to a chemical reaction between MN and TiO2 compounds. All the samples had optimal bulk densities at about 800 °C, which is corresponds to the maximum Q × f value of 32,000 GHz and the maximum dielectric constant of 15.7. The decrease of Q × f values MNT ceramics are related to the formation of lattice defects, porous, and secondary phases rather than grain boundaries. The MNT specimens with 8 wt% LiF sintered at 750 °C had the good microwave dielectric properties of ε r = 15.6, Q × f = 25,000 GHz, and τ f = −56 ppm/°C. The good compatibility of MNT ceramics with Ag makes it a suitable candidate for LTCC application.
References
N.X. Xu, J.H. Zhou, H. Yang, Ceram. Int. 40, 15191–15198 (2014)
G.G. Yao, P. Liu, H.W. Zhang, J. Am. Ceram. Soc. 96, 1691–1693 (2013)
K.W. Long, K.M. Luk, Int. J. Antennas. Propag. 43, 517–519 (1995)
R.R. Tummala, J. Am. Ceram. Soc. 74, 895–908 (1991)
Y.J. Seo, D.J. Shin, Y.S. Cho, J. Am. Ceram. Soc. 89, 2352–2355 (2006)
S.X. Dai, R.-F. Huang, D.L. Wilcox, J. Am. Ceram. Soc. 85, 828–832 (2002)
N. Mori, Y. Sugimoto, J. Harada, Y. Higuchi, J. Eur. Ceram. Soc. 26, 1925–1928 (2006)
F.F. Gu, G.H. Chen, X.L. Kang et al., J. Mater. Sci. 50, 1295–1299 (2015)
G.H. Chen, F.F. Gu, M. Pan et al., J. Mater. Sci. Mater. Electron. 26, 6511–6517 (2015)
N. Kumada, K. Taki, N. Kinomura, Mater. Res. Bull. 35, 1017–1021 (2000)
A. Kan, H. Ogawa, A. Yokoi, Y. Nakamura, J. Eur. Ceram. Soc. 27, 2977–2981 (2007)
A. Kan, H. Ogawa, A. Yokoi, Mater. Res. Bull. 41, 1178–1184 (2006)
H. Ogawa, A. Kan, S. Ishihara, Y. Higashida, J. Eur. Ceram. Soc. 23, 2485–2488 (2003)
C.L. Huang, J.Y. Chen, C.C. Liang, Mater. Res. Bull. 44, 1111–1115 (2009)
Z.F. Fu, P. Liu, X.M. Chen et al., J. Alloys Compd. 493, 441–444 (2010)
H.T. Wu, L.X. Li, Q. Zou et al., J. Alloys Compd. 509, 2232–2237 (2011)
M.H. Sarrafi, H.B. Bafrooei, M. Feizpour et al., J. Mater. Sci. Mater. Electron. 25, 946–951 (2014)
H. Zhu, W. Shen, Y. Jin et al., J. Mater. Sci. Mater. Electron. 24, 3546–3550 (2013)
A. Yokoi, H. Ogawa, A. Kan, H. Ohsato, Y. Higahsida, J. Eur. Ceram. Soc. 25, 2871–2875 (2005)
B.W. Hakki, P.D. Coleman, IRE Trans. Microwave Theory Tech. 8, 402–410 (1960)
A.A. Gribb, J.F. Banfield, Am. Mineral. 82, 717–728 (1997)
M.J. Wang, H. Yang, Q.L. Zhang et al., J. Mater. Sci. Mater. Electron 26, 162–167 (2015)
X.M. Wu, S. Chen, Z.Q. He et al., Thin Solid Films 589, 574–577 (2015)
L. Li, X.M. Chen, X.C. Fan, J. Eur. Ceram. Soc. 26, 3265–3271 (2006)
Q.L. Zhang, H. Yang, J.L. Zou, H.P. Wang, Mater. Lett. 59, 880–884 (2005)
S.J. Penn, N.M. Alford, A. Templeton et al., J. Am. Ceram. Soc. 80, 1885–1888 (1997)
J.D. Breeze, J.M. Perkins, D.W. McComb et al., J. Am. Ceram. Soc. 92, 671–674 (2009)
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant Nos. 51272150, 51572162 and 51132003), the Scientific Research Foundation of Shaanxi Normal University (Grant No. X2013YB01), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120202110004), and the Fundamental Research Funds for the Central Universities (GK201401003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fu, Z., Liu, P., Wu, Y. et al. Dielectric properties of ultralow-fired Mg4Nb2O9 ceramics co-doped with TiO2 and LiF. J Mater Sci: Mater Electron 27, 1553–1557 (2016). https://doi.org/10.1007/s10854-015-3923-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3923-1