Abstract
Nanostructured manganese doped magnesium aluminate (Mg1−xMnxAl2O4, where x = 0.0, 0.25, 0.50, 0.75, 1.0) were prepared by a sol–gel method. All the samples were characterised by TGA, XRD, SEM, EDAX, TEM and IR spectroscopy techniques. The semiconducting nature of materials were investigated by DC resistivity measurement. The TGA curves show that spinel the oxides are formed at 600 °C. The XRD studies reveal formation of cubic spinel phase with average crystallite size of 28 nm. The composition of Mg1−xMnxAl2O4, (x = 0.0, 0.50, 1.0) shows spherical interlinked fibrous morphology. The elemental compositions determined by energy dispersive X-ray analysis (EDAX) indicate desired composition. Particle size obtained from TEM analysis was found to be ~23 nm. The IR spectra show two strong characteristic absorption bands at tetrahedral and octahedral sites. The temperature dependent variation of dc resistivity of material reflects semiconducting nature of materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnesium aluminate oxide spinel has been widely studied as it has a specific combination of desirable properties like, excellent optical properties [1, 2], good mechanical strength at room temperature as well as high temperatures. Magnesium aluminate has a low density (3.58 g/cm3), high melting point (2135 °C), high chemical inertness against both acidic and basic slag’s, and excellent strength at extremely high temperature [3]. Therefore, it has been extensively used for various purposes such as refractory material [4, 5], humidity sensors [6], structural material in fusion reactors [7, 8] catalyst or catalyst support [9, 10] and optical devices [1, 2].
Due to their excellent corrosion and thermal shock resistance, corundum-MgAl2O4 spinel-based castables are widely used as ladle linings, purging plugs and well blocks in steel-making processes [11–14]. The characteristics of manganese aluminates such as high purity, low particle size, chemical homogeneity and uniform size distribution depend substantially on preparation methods. Manganese aluminate has been synthesized by various methods such as solid state [15], co-precipitation [16, 17], spray drying [18], freeze drying [19], micro-pulling-down (μ-PD) method [20], Czochralski [21] and sol–gel [22, 23].
However, most of these methods are either complex or expensive which diminishes preparation of the nano-sized materials in a large scale as compared to the sol–gel method. Moreover, other disadvantages include the necessity of high temperature, non-homogeneity, and low surface area of the nano-sized products. Generally, smaller particle size results in higher surface area which is required for different catalytic applications [24]. Hence, using a sol–gel auto combustion method at relatively low processing temperature is a new and good approach to prepare nanosized magnesium aluminate particles suitable for application in the above-mentioned different fields especially the electrical properties. From the literature survey, it is found that very limited research were done on MnAl2O4 and related material. Seok et al. [25] prepared Mn-promoted Ni/Al2O3 catalysts for stable carbon dioxide reforming of methane. Shaheen et al. [26] studied thermal characterization and physicochemical properties of Fe2O4-Mn2O3/Al2O3 system. Gritsyna et al. [21] investigated effect of doping with manganese on the optical properties of magnesium aluminate spinel crystals. They doped 0.02, 0.04 and 0.1 % of manganese in magnesium aluminate. Also no research work has been done on Mn substituted magnesium aluminate. Therefore our attention was made to prepare novel spinel material of manganese substituted magnesium aluminate by sol–gel method. The spinel material prepared by this method showed a high degree of chemical homogeneity, better control of stoichiometry and improved sinterability, when compared with those synthesized by the other conventional methods. The aim of the present research work is to study the structural, electrical and morphological properties of manganese substituted magnesium aluminates.
2 Experimental
Nanocrystaline Mg1−xMnxAl2O4 (x = 0.0, 0.25, 0.50, 0.75, 1.0) were prepared by simple sol–gel method. The A. R. grade citric acid [C6H8O7·2H2O], alluminium nitrate [Al(NO3)3·6H2O], magnesium nitrate [Mg(No3)2·6H2O], manganese nitrate [Mn(No3)2·4H2O] and ammonia solution [NH4OH] were used as precursor materials.
The metal nitrate solutions were prepared in double distilled water. Then citric acid as a chelating agent was added to metal nitrate solutions. The molar ratio of citrate to metallic ions in the solution was maintained at 1:1. Citric acid has two different roles, (1)Citric acid as a fuel helps to progress the synthesis at relatively lower temperature, (2) as a chelating agent binds metallic ions (Mg2+, Mn2+ and Al3+) and restricts precipitation of solution during variation in pH. The solutions of metal nitrates were mixed in their stoichiometric ratio followed by agitation using a magnetic stirrer at room temperature for 1 h. To increase the efficiency of chelating agent, pH of the solution was adjusted to 9.5 by adding ammonia solution drop by drop. This solution was gelled by heating at 120 °C. By further heating the citric acid melts at around 173 °C and converts into aconitic acid (Reaction 1) and then aconitic acid converts into itaconic acid (Reaction 2). The itaconic acid swells with the decarboxylation. Thus the gel was heated at 180 °C in an electric oven to get a precursor. The obtained precursor was ground into powder by a pestle and mortar and then calcined in furnace at 600 °C for 6 h with a heating rate of 10 °C min−1 to obtained desired nanomaterial.
The thermal stability of samples were checked by taking thermogravimetric (TGA) curves by heating the sample of Mg1−xMnxAl2O4 (x = 0.0, 0.50, 1.0) at a rate of 10 °C min−1 from room temperature to 1000 °C in nitrogen atmosphere. The crystal size, phase identification and lattice constants were determined by using X-ray diffraction (XRD) studies (Philips PW-1710 X-ray diffractometer with CuKα radiation). The surface morphology of the sample was observed by the use of scanning electron microscopy (SEM Model JEOL-JSM 6360). The elemental analysis was determined using an energy dispersive X-ray spectroscope (EDS). The fourier trnsform infra-red spectra (FTIR) spectra were recorded in the range of 350–700 cm−1 using KBr pellets (Perkin Elmer FTIR). The dc resistivity of all the samples was measured from room temperature to 350 °C.
3 Results and discussion
3.1 Thermogravimetric analysis
The TGA analysis of dried Mg1−xMnxAl2O4 (x = 0.0, 0.50, 1.0) powders were presented in Fig. 1. All TG curves show a thermal decomposition process involving several steps that from room temperature to 1000 °C. The weight loss from room temperature to about 1000 °C was 46.36, 34.06 and 31.09 % for x = 0.0, x = 0.50 and x = 1.0 respectively. The weight loss below 200 °C is due to loss of water vapors of adsorbed water. The weight loss from 200 to 400 °C is due to loss of organic matter including citric acid. The weight loss above 400 °C is due to loss of unreacted nitrates. The TGA curve shows that formation of spinel oxides is at about 600 °C.
3.2 X-ray diffraction analysis
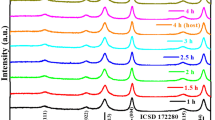
The XRD patterns of Mg1−xMnxAl2O4 (x = 0.0, 0.25, 0.50, 0.75, 1.0) sintered at 6000 C using Ni-filter CuKα radiation are shown in Fig. 2. The diffraction patterns and data proved that the samples are formed in a cubic spinel structure because of their small size giving rise to high specific surface area. The crystallite size of the prepared powders is calculated from XRD line broadening of (311) peak using Scherrer equation.
Where β is the FWHM of the most intense peak (311), θ is the Bragg angle for the (311) peak and λ is the wave length of Cukα = 1.54 Å. From above equation the average crystal size of the Mg1−xMnxAl2O4 was estimated to be 28 nm. The lattice constant of this composition lie in the range 8.090–8.210 Å (Table 1). Ali Saberi et al. [27] reported lattice constants of MgAl2O4 were 8.08 Å. It is observed that the lattice constant increases linearly with increase in Mn content obeying the Vegard’s law [28]. The increase in lattice parameters with Mn can be explained on the basis of ionic size difference of Mn2+ (0.80 Å) and Mg2+ (0.65 Å). The lattice constant, crystallite size and X-ray density increase linearly with increase in manganese content.
3.3 Scanning electron microscopy analysis
In order to investigate the morphology and grain size of material, SEM analysis were performed. The SEM micrograph of Mg1−xMnxAl2O4 (x = 0.0, 0.5 and 1.0) are shown in Fig. 3a–c. The average grain size was calculated by Cottrell’s method [29]. It is clearly seen that grain size increases with increase in manganese content. It is observed that with increasing Mn contents the particles get aggregated and appears to have soften grain boundaries. The morphology can be correlated with our thermogravimetric analysis which shows that the single phase formation occurs at lower temperatures with addition of Mn in MgAl2O4.
3.4 Energy dispersive X-ray analysis
The elemental analysis of the material Mg1−xMnxAl2O4 (x = 0.0, 0.5 and 1.0) has been checked using energy dispersive X-ray spectroscopy (EDS) is shown in Fig. 4a–c. From figure, it is seen that Mg, Mn, Al and O were presented in their stoichiometric ratio without any impurity. The theoretical and observed data (Table 2) shows that the unreacted precursors like nitrates and citrates have been completely removed from the material.
3.5 Transmission electron microscopy analysis
TEM images of the nanoparticle of Mg0.50Mn0.50Al2O4 composition is shown in Fig. 5. The image shows that spinel grains are equiaxed in nature. The particle size estimated using TEM is ~23 nm which is in good agreement with those obtained from line broadening analysis of X-ray diffraction.
3.6 FTIR analysis
The FTIR spectra of the Mg1−xMnxAl2O4 were recorded in the range of 350–700 cm−1 are shown in Fig. 6, which indicates presence of two fundamental absorption band ν1 (625–550 cm−1) and ν2 (425–400 cm−1). The band ν1 corresponds to intrinsic stretching vibrations of the metal at the tetrahedral site, Mtetra ↔ O, where as ν2 lowest band is assigned to octahedral-metal stretching, Mocta ↔ O are shown in Table 3. It is known that the frequency is inversely proportional to the reduced mass. From Table 3, it is clear that the band ν1 and ν2 systematically decreases with increasing Mn2+ concentrations. The slight decrease of ν1 and ν2 with increase in Mn2+ may not only due to the difference in the ionic radius between Mn2+ and Mg2+ but also due to the difference of their masses in the A-site. The frequency ν1 decreases with increases in manganese content indicating that Hook’s law is obeyed.
3.7 Electrical properties
The temperature dependent variation of dc resistivity for Mg1−xMnxAl2O4 is shown in Fig. 7. Linear decrease in resistivity with increasing temperature reflects semiconducting nature of aluminates. The decrease in resistivity with increase in temperature can be attributed to increase in drift mobility of the charge carriers.
DC resistivity of the compounds at room temperature for the system Mg1−xMnxAl2O4 when measured as a function of temperature varied between 106 and 103 Ω cm. A plot of log σ versus 103/T shows linear nature for all the compositions indicating that Wilson’s law is obeyed. The activation energy is calculated using exponential form of Arrhenius equation.
The activation energy values decreases with increasing magnesium content. It has been observed that electrical resistivity in transition metal oxides is low if the material contains cation of the same element with valences differing by unity are situated at similar sites in the lattice.
In the spinel structure distance between tetrahedral–octahedral cations is so large that the overlap between the electronic wave functions of such adjacent pairs is negligible and probability of electron exchange between cations of these sites is very small. Thus electrical conductivity can take place by the electron exchange between tetrahedral ions. Thus resistivity and activation energy decreases with increase in manganese contents.
4 Conclusion
Spinel aluminate of Mg1−xMnxAl2O4 systems were synthesized by simple sol–gel method. XRD analysis shows that, as the composition of Mn+2 increases with increase in lattice constants, crystal size and X-ray density. The average crystallite size of the system was 28 nm. SEM analysis shows fine grained microstructure. EDAX analysis of the nanomaterial confirms that the material is composed of Mg, Mn, Al and O without any impurity. Particle size obtained from TEM analysis (23 nm) was found to be in good agreement with the value obtained from XRD analysis. Unit cell parameter increases with increases in Mn concentration. The linear decrease in resistivity with increasing temperature reflects semiconducting nature of aluminates.
References
A. Goldstein, J. Eur. Ceram. Soc. 32, 2869 (2012)
F.S. Al-Hazmi, W.E. Mahmoud, J. Eur. Ceram. Soc. 34(12), 3047 (2014)
B. Ismail, S.T. Hussain, S. Akram, Chem. Eng. J. 219, 395 (2013)
A.E. Lavat, M.C. Grasselli, E.G. Lovecchio, Ceram. Int. 36, 15 (2010)
N.M. Khalil, M.B. Hassan, E.M.M. Ewais, F.A. Saleh, J. Alloys Compd. 496, 600 (2010)
A. Laobuthee, S. Wongkasemjit, E. Traversa, J. Eur. Ceram. Soc. 20, 91 (2000)
M.J. Iqbal, B. Ismail, C. Rentenberger, H. Ipser, Mater. Res. Bull. 46, 2271 (2011)
T. Shiono, K. Shiono, K. Miyamoto, G. Pezzotti, J. Am. Ceram. Soc. 83, 235 (2000)
J. Guo, H. Lou, H. Zhao, X. Wang, X. Zheng, Mater. Lett. 58, 1920 (2004)
R. Jiang, Z. Xie, C. Zhang, Q. Chen, Catal. Today 93–95, 359 (2004)
M.A.L. Braulio, M. Rigaud, A. Buhr, C. Parr, V.C. Pandolfelli, Ceram. Int. 37(6), 1705 (2011)
F.R. Perez, C.A. Barrero, A.R.H. Walker, K.E. García, K. Nomura, Mater. Chem. Phys. 117(1), 214 (2009)
B.Q. Zhu, B.X. Fang, X.C. Li, Ceram. Int. 36, 2493 (2010)
B.Q. Zhu, B.X. Fang, X.C. Li, X. Jiang, J. Chin. Ceram. Soc. 38, 730 (2010)
A. Rahman, R. Jayaganthan, J. Nanostruct. Chem. 3, 147 (2015)
M.F. Zawrah, H. Hamaad, S. Meky, Ceram. Int. 33, 969 (2007)
R.B. Jotania, P.A. Patel, Int. J. Eng. Res. Appl. 2, 494 (2012)
Y. Suyama, A. Kato, Ceram. Int. 8, 17 (1982)
C.T. Wang, L.S. Lin, S.J. Yang, J. Am. Ceram. Soc. 75, 2240 (1992)
A. Jouini, A. Yoshikawaa, T. Fukudaa, G. Boulonb, J. Cryst. Growth 293, 517 (2006)
V.T. Gritsyna, YuG Kazarinov, V.B. Kol’ner, L.A. Lytvynov, K.E. Sickafus, Funct. Mater. 12, 719 (2005)
P.P. Hankare, V.T. Vader, U.B. Sankpal, R.P. Patil, A.V. Jadhav, I.S. Mulla, J. Mater. Sci. Mater. Electron. 22, 1109 (2011)
A.S. Tapase, R.P. Patil, S.D. Delekar, I.S. Mulla, P.P. Hankare, J. Mater. Sci. Mater. Electron. 25, 369 (2014)
P.V.M. Kutty, S. Dasgupta, Ceram. Int. 39, 7891 (2013)
S.H. Seok, S.H. Choi, E.D. Park, S.H. Han, J.S. Lee, J. Catal. 209, 6 (2002)
W.M. Shaheen, K.S. Hong, Thermochim. Acta 381, 153 (2002)
A. Saberi, F. Golestani-Fard, H. Sarpoolaky, M. Willert-Porada, T. Gerdes, R. Simon, J. Alloys Compd. 462, 142 (2008)
K.J. Standley, Oxide Magnetic Materials (Clarendon press, Oxford, 1962)
A. Cottrell, An Introduction to Metallurgy (Edward Arnold, London, 1967)
Acknowledgments
Author (PPH) is very thankful to UGC, New Delhi for financial assistance through UGC-BSR fuculty fellowship F. No. 18-1(46)/2013 (BSR). ISM is grateful to CSIR India for granting him Emeritus Scientist scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mali, A.V., Wandre, T.M., Sanadi, K.R. et al. Synthesis, characterization and electrical properties of novel Mn substituted MgAl2O4 synthesized by sol–gel method. J Mater Sci: Mater Electron 27, 613–619 (2016). https://doi.org/10.1007/s10854-015-3796-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3796-3