Abstract
The photocatalytic degradation of methylene blue (MB) has been investigated under visible light irradiation with an incandescent light bulb using chromium doped TiO2 nanoparticles. Cr–TiO2 photocatalysts were successfully synthesized by sol–gel method at room temperature and characterized by X-ray diffraction (XRD), UV–Vis diffuse reflectance spectroscopy (UV–Vis DRS), Raman spectroscopy, Transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy. The band gap energy of the nanoparticles were estimated using UV–Vis DRS technique. With increasing Cr3+ cations content into TiO2 host lattice, the optical absorption band tuned in the visible region. XRD and TEM results reveal uniform and crystalline anatase TiO2 nanoparticles. The photodegradation of MB indicated that the photocatalytic activity of pure TiO2 nanoparticles increased with increasing Cr3+ cations concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor photocatalysis is a promising technology for solar water splitting and purification of air and water [1]. Among various candidates, TiO2 is a versatile material and has attracted great attention as an excellent photocatalyst for photocatalytic disinfection and degradation of various environmental contaminants. It is considered to be an ideal photocatalyst as it is chemically inert, photostable, highly reactive and inexpensive [2, 3]. However, TiO2 can only absorb ultraviolet (UV) light (λ < 390 nm) due to its wide band-gap (3.0–3.2 eV), leading to absorption in only a small region of the solar spectrum. The undesired recombination of photoexcited charge carriers limited the effective application of TiO2. To avoid recombination by increasing the lifetime of e/h+ pairs and to lower the band gap, doping with metals, non-metals or a combination of both is usually performed [4–6].
Metal doping in TiO2 for photocatalytic application has gained lot of attention since enhancing the visible light response by lowering the charge carrier recombination. It is well known that metal ions doping in TiO2 can influence the intrinsic properties of the semiconductor photocatalyst by extending its photoresponse into the visible. Importantly, metal-doped TiO2 facilitates electron-hole separation and promotes the interfacial electron transfer processes. The optical absorption edge of the different transition metal ion doped TiO2 is found to shift from UV to visible region of electromagnetic spectrum of light. This red shift in metal-doped TiO2 was attributed to the charge-transfer transition between the d electrons of the dopant and the conduction band (CB) of TiO2 [7].
The report came in 2010, Choi et al. [8] studied the effect of 13 different single metal ion dopants Ag+, Rb+, Ni2+, Co2+, Cu2+, V3+, Ru3+, Fe3+, Os3+, Y3+, La3+, Pt4+, Pt2+, Cr3+, and Cr6+ on visible light reactivity of TiO2. Among the various transition metal ions, Cr has been mostly used as a dopant since Cr doping extends the photoresponse to visible-light range by narrowing the band gap [9–13]. Doping TiO2 with Cr has found to promote photoelectrochemical water decomposition and photo-degradation of organic compounds using solar energy [11]. Zhang et al. [14] investigated the photocatalytic activities of Cr/TiO2 nanotubes prepared by the combination of sol–gel process with hydrothermal treatment. Buddee et al. [15] reported the improvement in the photocatalytic property of amorphous TiO2 by doping it with Cr(III) and Fe(III) ions for methylene blue degradation. Choudhury et al. [16] investigated the effect of Cr-doping on the structural, optical and magnetic properties of TiO2 nanoparticles. The photocatalytic behavior of chromium-doped titania and chromium supported on the surface of titania were studied by Ould-Chikh et al. [17].
However, despite the absorption in the visible-light region, chromium-doped TiO2 has been found to be photocatalytically inactive. This has been explained by the creation of additional oxygen vacancies upon chromium doping, as vacancies might constitute recombination sites for electron-hole pairs [18]. Though, it has been reported that the Cr-doped TiO2 show lower visible-light photocatalytic activity than other transition metal ions doped TiO2 but the Cr-doped TiO2 may even show lower UV-light photocatalytic activity than TiO2 [15]. Various factors, such as the physicochemical properties of TiO2 (including particle size and crystalline phase), preparation methods for doping and the organic probe for photocatalytic activity test, have been confirmed to influence the final results for photoactivity test [19].
To understand the aforementioned controversy, therefore, in this work Cr-doped TiO2 nanoparticles have been prepared with various doping concentration by modified sol–gel method at room temperature. The structural, optical and photocatalytic properties of the doped nanoparticles were also investigated.
2 Materials and methods
2.1 Materials
Titanium(IV) isopropoxide and sodium dodecyl sulfate were purchased from Sigma Aldrich. Chromium(III) nitrate nonahydrate were from SD Fine Chemicals (Mumbai, India). Ammonia, ethanol and methylene blue from Duksan Pure Chemicals Co. Ltd Korea. Solutions were prepared using water from a Millipore Water Milli Q purification system. All chemicals used as received.
2.2 Synthesis of photocatalyst
The Cr-doped TiO2 nanoparticles were synthesized with the slightly modified sol–gel method, which was reported previously [20]. In the preparation, titanium(IV) isopropoxide was mixed with glacial acetic acid and magnetically stirred for 5 min, and then aqueous solution of sodium dodecyl sulfate followed by 50 mL of deionized water was added and again stirred for 40 min. To get the desired concentration of Cr3+ ion as a dopant (1.0–3.0 mol%) in TiO2, required stoichiometric amount of aqueous solution of Cr(NO3)3·9H2O was then added and stirred for 60 min. Then 20 mL of ammonia solution was added and the mixture was further stirred at 60 °C for 2 h. The resulting product was filtered, washed with ethanol and dried at 110 °C in an oven. The catalyst was calcinated in air at 400 °C for 4 h. The different samples such as 1.0 mol% Cr-doped TiO2, 2.0 mol% Cr-doped TiO2, and 3.0 mol% Cr-doped TiO2 are denoted as Cr1–TiO2, Cr2–TiO2 and Cr3–TiO2, respectively.
2.3 Photocatalytic activity test
The photocatalytic activity of the prepared samples was evaluated with the degradation of MB under UV and visible light irradiations. In a glass beaker, 10 mg L−1 of photocatalyst and 100 mL of MB (1.6 × 10−5 mol L−1) were stirred magnetically. The distance between the applied lamps and the surface of MB solution was 15 cm. The photodegradation was carried out at 25 °C by using a 200 W incandescent lamp (NB 220-C 200 W, Lanxi China). A UV-cut-off filter was inserted just below the white light lamp to filter the UV radiations with wavelength λ < 410 nm. Prior to light irradiation the catalyst and MB solution were stirred magnetically in the dark for 45 min to reach adsorption equilibrium. At regular time intervals, 1.5 mL of the solution was aspirated and centrifuged. Concentration of residual solution was estimated using UV–Visible–NIR spectrophotometer (Shimadzu UV 3150). Since the degradation mechanism of MB is well known with high reliability, we have monitored photodegradation by using a UV–Vis spectrophotometer.
The photodegradation rate of MB was calculated by the following equation:
where C0 is the initial concentration of MB and Ct is the concentration of MB at the irradiation time t.
2.4 Characterizations
The prepared samples have been characterized by various techniques such as XRD, TEM, XPS, Energy Dispersive X-ray Spectroscopy (EDS), Raman spectroscopy and UV–Vis diffuse reflectance spectroscopy. The powder X–ray diffraction patterns of the nanoparticles were recorded at room temperature on a Bruker D8-advance diffractometer using Cu Kα (λ = 1.5418 Å) radiation to identify the crystal phase. The diffraction data were collected in the 2θ range of 10°–90° in step scan mode at a rate of 3°/min. TEM images of the samples were obtained with a Tecnai F30 field emission transmission electron microscope operating at 300 kV. EDS analysis was carried out to estimate the elemental composition of the sample. UV–Vis diffuse reflectance absorbance spectra of the samples were recorded with a UV–visible spectrophotometer (UV3600, Shimadzu, Japan) in the range of 200–800 nm. Raman spectra of the samples were obtained with a Raman spectrometer of Bruker make at a Laser power of 16 mV with 256 scans. X-ray photoelectron spectroscopy (XPS) measurements were carried out to examine the valence states of chromium element in Cr–TiO2 nanoparticles. The XPS spectra were recorded on a Physical Electronics 5600 Multi-technique System with monochromatic Al Kα radiation.
3 Results and discussion
3.1 X-ray diffraction analysis
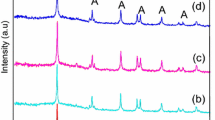
XRD patterns of TiO2 and Cr-doped TiO2 nanoparticles are shown in the Fig. 1. XRD was used to investigate phase structures and crystallite size of the prepared nanoparticles. The diffraction pattern of the all Cr-doped TiO2 nanoparticles matches with the standard anatase TiO2 (JCPDS 21-1272). All Cr-doped TiO2 nanoparticles consist of anatase as a unique phase. The crystallite size calculated by using Scherer’s equation range from 12 to 14 nm. The diffraction pattern does not show any peak of secondary phase or other impurities such as chromium oxides. The peak position and intensity were not significantly affected by doping of Cr into the TiO2 host lattice. The ionic radii of Cr3+ is 0.755 Å, which is very close to that of Ti4+ (0.745 Å), a TiO2 crystalline lattice [8]. This shows that substitutional doping of Cr3+ occurred rather than interstitial doping in the Ti4+ sites.
3.2 UV–Vis diffuse reflectance spectroscopy
UV–Vis diffuse reflectance spectra of TiO2 and Cr-doped TiO2 nanoparticles are presented in Fig. 2. The sol–gel synthesized Cr-doped TiO2 nanoparticles show absorption edge at 417, 436 and 477 nm for Cr1–TiO2, Cr2–TiO2 and Cr3–TiO2 samples, respectively. The band gap energy values were determined by linear fitting the absorption edge. The band gap energy values were calculated to be 3.21, 3.0, 2.86 and 2.60 eV for TiO2, Cr1–TiO2, Cr2–TiO2 and Cr3–TiO2 samples, respectively. The increase of Cr ions contents in TiO2 host lattice leads to linear increase in the optical absorption to the visible light region. The extended absorption in the visible region is due to the excitation of the electrons from the Cr to the conduction band of TiO2. [8] The observed red shift in Cr–TiO2 samples is consistent with the incorporation of Cr3+ into the titania matrix. The absorption spectra of the Cr–TiO2 samples show more absorption than TiO2 in the visible region, which indicates the existence of surface states. The oxygen vacancies should have been created to maintain charge neutrality due to metal doping, inducing bathochromic shift in the band gap transitions [12, 21]. Influence of doping on optical properties of TiO2 is clearly evident in UV–Vis spectra and it is manifested by change of the color of doped samples from light to dark brown. The extended absorbance of Cr–TiO2 nanoparticles into the visible region provides a possibility for enhancing the photocatalytic activity of TiO2 for visible or solar light induced applications.
3.3 Raman spectroscopy
We carried out Raman spectroscopy experiments for further investigation of structural phases of TiO2 and Cr-doped TiO2 nanoparticles. Raman spectra of the samples are consistent with XRD data and suggests a pure phase as seen in Fig. 3. All samples exhibited a Raman peak pattern similar to the characteristic feature of anatase structured TiO2.
The tetragonal anatase structured titania belongs to D 194h (I41/amd) space group and may have following normal lattice vibration modes.
The strongest Eg mode at 145 cm−1, arises due to the external vibration of the anatase structure is well resolved, which demonstrates that an anatase phase for all samples. A very small peak at 197 cm−1 is attributed to intrinsically weak vibrations in anatase TiO2. For all samples, the peaks at 147, 445 and 639 cm−1 belong to B1g, Eg and A1g modes, respectively. The peaks at 516 and 396 cm−1 can be attributed to B1g and the peaks at 196, 144 cm−1 correspond to Eg vibration modes [22]. Interestingly, any peaks related to other oxides are not detected for Cr–TiO2 nanoparticles. This reveals that the dopant is substitutionally interposed in the TiO2 frame-work replacing Ti4+ cations. The absence of Raman bands (235, 447, and 612 cm−1) corresponding to the rutile phase of TiO2 again confirms the phase purity of the samples [23].
3.4 Transmission electron microscopy
A typical TEM image of TiO2 and Cr3–TiO2 nanoparticles can be seen in the Fig. 4. The diameters obtained from TEM analysis are in good agreement with those obtained from XRD analysis, which distribution from 7 to 14 nm. This demonstrates that the all Cr-doped TiO2 nanoparticles were anatase crystalline, well dispersed and non-spherical shape. The selected area electron diffraction patterns (SAED) of TiO2 and Cr3–TiO2 are shown in inset of Fig. 4a, b, respectively. The bright spot in the center indicates that the electrons are diffracted randomly by the material and confirms crystalline nature of the TiO2 and Cr3–TiO2 nanoparticles [24].
The content of doped ions in the TiO2 matrices was evaluated from EDS analysis. Figure 4c shows the elemental compositions of the representative Cr3–TiO2 nanoparticles. From this figure, the peaks of corresponding dopant, Ti and O can be clearly seen, indicating that the nanoparticles contain dopants. Ti and O elements are found to be essentially pure and with the absence of any other impurities. All dopants of Cr were successfully identified, indicating that the doped metal ions have been integrated into TiO2 host lattice. The obtained amount in EDS analysis was in good agreement with the doping contents.
3.5 X-ray photoelectron spectroscopy
XPS technique was used to study the surface chemistry of the Cr3–TiO2 sample (Fig. 5a–d). In survey spectrum, peak observed at 284.6 eV corresponds to carbon impurities, arising due to the background of XPS test or the residual precursors [25].
The survey spectra for Cr3–TiO2 sample exhibit presence of Ti, O, and Cr elements. The core level binding energies of Ti 2p 3/2 and Ti 2p 1/2 are observed at 463.56 eV and 457.87 eV, respectively which are in good agreement with standard binding energies of anatase TiO2 [26]. The separation between the Ti 2p 3/2 and Ti 2p 1/2 peaks is 5.69 eV. These values are consistent with the values of Ti4+ cations of anatase TiO2 [26]. The Cr 2p XPS spectrum showed a major peak at 576.93 eV and a minor peak at 585.71 eV. The peak can be assigned to Cr3+ [25]. Figure 5c show the XPS spectrum of O 1s configuration. The binding energies at 531.73 and 526.46 eV reveal the existence of surface hydroxyl groups of TiO2 as well as O 1s of titanium lattice, respectively [27].
3.6 Photodegradation of MB under visible light
MB is a distinguished histological dye that has extensive applications in dying cottons, wools, temporary hair colorant, coloring papers and coating for paper stock [28, 29]. Although MB is not strongly dangerous, it can cause some harmful effects such as heart rate, shock, Heinz body formation, cyanosis, jaundice, vomiting, quadriplegia, and tissue necrosis in humans [30].
In this study, MB used as a model dye to demonstrate and compare the photocatalytic activity of metal modified TiO2 nanoparticles. The photocatalytic degradation activity was tested with nanoparticles and a definite dye concentration (1.6 × 10−5 mol L−1) under dark conditions and with visible light irradiations.
Figure 6 indicates the absorption spectra of Cr3–TiO2 nanoparticles which demonstrates the photodegradation of about 100 % of the MB solution after 10 h of the irradiation under visible light. It can be seen that about 20 % of the MB solution is mineralized under dark condition before irradiation of visible light.
The absorption maximum wavelength shift from 664 to 630 nm during the irradiation was observed. Such blue-shifted absorption is characteristic of N-demethylated derivatives of MB; the hypsochromic shifts occurred slowly. A mixture of N-demethylated analogs of MB broadens the absorption spectra in the visible region [31]. Moreover, a parallel decrease in intensities and slight blue shift of the bands located at 292 nm was observed; these are caused by the N-demethylated degradation concomitantly with the degradation of the phenothiazine [32, 33]. The absorption of MB decreased with irradiation time due to photocatalytic degradation process under visible light. The plausible reaction mechanism for the photocatalytic degradation is given below.
The absorption of a photon with energy greater than the band gap of the TiO2 excites a valence band electron to the conduction band, generating a positive hole in the valence band. The generated holes (h+) are trapped by H2O and OH− groups adsorbed on the surface of TiO2 to generate hydroxyl radicals, and the electrons (e−) are attracted to the metal particle due to the difference in the work functions between TiO2 and metal nanoparticles, thus preventing the recombination of photogenerated charge carriers [34, 35]. Hydroxyl radicals and superoxide anions produced by the photocatalytic process oxidize most the organic compounds until complete decomposition is achieved [36, 37]. Houas et al. [38] proposed that the decomposition of MB leads to the transformation of organic carbon into CO2 while that of nitrogen and sulfur into inorganic ions. The resulting degradation process is as shown the equation below.
The photocatalytic activities of different samples are evaluated with the degradation rate of MB. Figure 7 shows the variation of the photocatalytic degradation using various samples estimated at the maximum absorption wavelength of MB (664 nm) with the time of irradiation. After 15 h exposer, blue color of MB solution completely vanished in the presence of Cr-doped TiO2 nanoparticles under visible light irradiations. From figure it is observed that the Cr3–TiO2 exhibit much higher photocatalytic degradation of MB than other samples. This reveals that the photocatalytic activity of TiO2 can be enhanced by the doping with chromium metal ions in the host lattice of TiO2.
As shown in Fig. 8, the degradation efficiency was impressed with increasing Cr doping. For Cr–TiO2, MB degradation rate depends on the Cr loading. Cr3–TiO2 nanoparticles presented the highest activity with 100 % MB degradation at 10 h. In an extended time region, Cr1–TiO2 and Cr2–TiO2 were able to achieve complete MB degradation in about 13 and 15 h, respectively. Here, Cr3–TiO2 nanoparticles had higher visible light photocatalytic activity and this can be correlated with the enhanced visible light absorption of TiO2 by doping with 3.0 mol% chromium ions. The efficient absorption of visible light does not seem to be a key factor which controls the visible-light photocatalytic activity of metal ion doped TiO2. Although to initiate photoreactions a visible-light absorption is obviously essential [8]. The variable activity of Cr-doped TiO2 nanoparticles may be due to the dopant concentration. The concentration of dopant should be considered here, since a high concentration of Cr3+ in TiO2 may induce over-trapping of holes with Cr3+, which retards the recombination of photogenerated charge carriers [9]. The photocatalytic activity of metal ion doped TiO2 strongly depends on the crystalline nature, phase composition, morphology, chemical composition, band gap, energy, surface area, particle size, electron–hole recombination rate, the existence of several dopants ions and synthesis methodology [39–42].
The photoactivity can be enhanced by doping TiO2 with metal ions for improving the trapping-to-recombination rate ratio. However, when metal ions are incorporated into TiO2 host lattice, the impurity energy levels formed in the band gap of TiO2 can also lead to decrease the rate of recombination between photogenerated charge carriers. Not all metal doped TiO2 photocatalyst will exhibit positive effects. The dopant content directly influences the rate of electron and hole recombination [43]. There is an optimum concentration of dopant ions where the thickness of the space-charge layer is similar to the depth of light penetration.
The dopants like Cr3+ and Fe3+ ions at a low concentration promote the separation of photogenerated charge carriers, which benefit the photocatalytic reaction. Conversely, the reaction between metal ions and electrons are rapidly enhanced at higher concentration of dopants, which is disadvantageous to photocatalytic reaction [15]. The present investigation reveals that the addition of optimum concentration of chromium ion into TiO2 host lattice can tune the photocatalytic activity in the visible region of electromagnetic spectrum.
4 Conclusions
Cr doped anatase TiO2 nanoparticles with visible light activity were successfully synthesized by modified sol–gel method at room temperature. XRD and Raman analysis of Cr–TiO2 nanoparticles show characteristic features of nanocrystalline TiO2 in tetragonal anatase phase. The effect of low concentration chromium doping on the photocatalytic activity of TiO2 was investigated by performing the photodegradation of MB. The presence of chromium dopant plays a vital role in altering physiochemical and photocatalytic properties of TiO2. The optical band gap energy of Cr-doped TiO2 nanoparticles shifted in the visible light region. XPS analysis confirmed the proper substitution of Ti4+ cations by Cr3+ cations in the TiO2 matrix. Cr–TiO2 nanoparticles showed higher photoactivity than undoped samples. The photocatalytic degradation of MB is enhanced with increasing chromium concentration in TiO2 host lattice. The doping of Cr3+ cations into TiO2 facilitated its photocatalytic activity under visible light irradiation. Considering facile preparation process and visible light activity at low dopant concentration, Cr-doped TiO2 photocatalyst are found to be plausibly applicable for environmental remediation applications.
References
D.P. Macwan, P.N. Dave, S. Chaturvedi, A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 46, 3669–3686 (2011)
D.F. Ollis, E. Pelizzetti, N. Serpone, Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 25, 1522–1529 (1991)
Y. Yalçın, M. Kılıç, Z. Çınar, Fe+3-doped TiO2: a combined experimental and computational approach to the evaluation of visible light activity. Appl. Catal. B Environ. 99, 469–477 (2010)
J. Virkutyte, R.S. Varma, Visible light activity of Ag-loaded and guanidine nitrate-doped nano-TiO2: degradation of dichlorophenol and antibacterial properties. RSC Adv. 2, 1533–1539 (2012)
S.S. Soni, G.S. Dave, M.J. Henderson, A. Gibaud, Visible light induced cell damage of Gram positive bacteria by N-doped TiO2 mesoporous thin films. Thin Solid Films 531, 559–565 (2013)
S.S. Soni, M.J. Henderson, J.F. Bardeau, A. Gibaud, Visible-light photocatalysis in titania-based mesoporous thin films. Adv. Mater. 20, 1493–1498 (2008)
J.C.S. Wu, C.H. Chen, A visible-light response vanadium-doped titania nanocatalyst by sol–gel method. J. Photochem. Photobiol. A Chem. 163, 509–515 (2004)
J. Choi, H. Park, M.R. Hoffmann, Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 114, 783–792 (2010)
J. Zhu, Z. Deng, F. Chen, J. Zhang, H. Chen, M. Anpo et al., Hydrothermal doping method for preparation of Cr3+–TiO2 photocatalysts with concentration gradient distribution of Cr3+. Appl. Catal. B Environ. 62, 329–335 (2006)
P. Bouras, E. Stathatos, P. Lianos, Pure versus metal-ion-doped nanocrystalline titania for photocatalysis. Appl. Catal. B Environ. 73, 51–59 (2007)
C.C. Tsai, H. Teng, Chromium-doped titanium dioxide thin-film photoanodes in visible-light-induced water cleavage. Appl. Surf. Sci. 254, 4912–4918 (2008)
A.T. Vu, Q.T. Nguyen, T.H.L. Bui, M.C. Tran, T.P. Dang, T.K.H. Tran, Synthesis and characterization of TiO2 photocatalyst doped by transition metal ions (Fe3+, Cr3+ and V5+). Adv. Nat. Sci. Nanosci. Nanotechnol. 1, 015009 (2010)
X. Li, Z. Guo, T. He, The doping mechanism of Cr into TiO2 and its influence on the photocatalytic performance. Phys. Chem. Chem. Phys. 15, 20037–20045 (2013)
S. Zhang, Y. Chen, Y. Yu, H. Wu, S. Wang, B. Zhu et al., Synthesis, characterization of Cr-doped TiO2 nanotubes with high photocatalytic activity. J. Nanoparticle Res. 10, 871–875 (2007)
S. Buddee, S. Wongnawa, U. Sirimahachai, W. Puetpaibool, Recyclable UV and visible light photocatalytically active amorphous TiO2 doped with M(III) ions (M = Cr and Fe). Mater. Chem. Phys. 126, 167–177 (2011)
B. Choudhury, A. Choudhury, Structural, optical and ferromagnetic properties of Cr doped TiO2 nanoparticles. Mater. Sci. Eng., B 178, 794–800 (2013)
S. Ould-Chikh, O. Proux, P. Afanasiev, L. Khrouz, M.N. Hedhili, D.H. Anjum et al., Photocatalysis with chromium-doped TiO2: bulk and surface doping. ChemSusChem 7, 1361–1371 (2014)
R. Bechstein, M. Kitta, J. Schütte, H. Onishi, A. Kühnle, The effects of antimony doping on the surface structure of rutile TiO2(110). Nanotechnology 20, 264003 (2009)
Z. Zhang, C. Wang, R. Zakaria, J.Y. Ying, Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. 102, 10871–10878 (1998)
S.D. Delekar, H.M. Yadav, S.N. Achary, S.S. Meena, S.H. Pawar, Structural refinement and photocatalytic activity of Fe-doped anatase TiO2 nanoparticles. Appl. Surf. Sci. 263, 536–545 (2012)
H.M. Yadav, S.V. Otari, R.A. Bohara, S.S. Mali, S.H. Pawar, S.D. Delekar, Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 294, 130–136 (2014)
J.S. Chen, Y.L. Tan, C.M. Li, Y.L. Cheah, D. Luan, S. Madhavi et al., Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100 % exposed (001) facets for fast reversible lithium storage. J. Am. Chem. Soc. 132, 6124–6130 (2010)
K. Bhattacharyya, S. Varma, A.K. Tripathi, S.R. Bharadwaj, A.K. Tyagi, Effect of vanadia doping and its oxidation state on the photocatalytic activity of TiO2 for gas-phase oxidation of ethene. J. Phys. Chem. C 112, 19102–19112 (2008)
Y. Yu, X. Yin, A. Kvit, X. Wang, Evolution of hollow TiO2 nanostructures via the Kirkendall effect driven by cation exchange with enhanced photoelectrochemical performance. Nano Lett. 14, 2528–2535 (2014)
C.D. Wagner, W.M. Riggs, L.E. Davis, J.F. Moulder, G.E. Muilenberg, Handbook of X-Ray Photoelectron Spectroscopy (Perkin-Elmer Corp., Physical Electronics Division, USA, 1979)
J. Zhu, F. Chen, J. Zhang, H. Chen, M. Anpo, Fe3+-TiO2 photocatalysts prepared by combining sol–gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A Chem. 180, 196–204 (2006)
L. Li, C. Liu, Y. Liu, Study on activities of vanadium (IV/V) doped TiO2(R) nanorods induced by UV and visible light. Mater. Chem. Phys. 113, 551–557 (2009)
J.P. Tardivo, A. Del Giglio, C.S. de Oliveira, D.S. Gabrielli, H.C. Junqueira, D.B. Tada et al., Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2, 175–191 (2005)
B.H. Hameed, A.A. Ahmad, Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 164, 870–875 (2009)
Y. Bulut, H. Aydın, A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194, 259–267 (2006)
T. Zhang, T. Oyama, A. Aoshima, H. Hidaka, J. Zhao, N. Serpone, Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. A Chem. 140, 163–172 (2001)
F. Wang, S. Min, Y. Han, L. Feng, Visible-light-induced photocatalytic degradation of methylene blue with polyaniline-sensitized composite photocatalysts. Superlattices Microstruct. 48, 170–180 (2010)
T. Mohammad, H. Morrison, Simultaneous photoconjugation of methylene blue and cis-Rh(phen)2Cl2 + to DNA via a synergistic effect†. Photochem. Photobiol. 71, 369–381 (2007)
P. Sangpour, F. Hashemi, A.Z. Moshfegh, Photoenhanced degradation of methylene blue on cosputtered M:TiO2 (M = Au, Ag, Cu) nanocomposite systems: a comparative study. J. Phys. Chem. C 114, 13955–13961 (2010)
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemannt, Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995)
M.B. Fisher, D.A. Keane, P. Fernández-Ibáñez, J. Colreavy, S.J. Hinder, K.G. McGuigan et al., Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B Environ. 130–131, 8–13 (2013)
M. Pelaez, N.T. Nolan, S.C. Pillai, M.K. Seery, P. Falaras, A.G. Kontos et al., A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 125, 331–349 (2012)
A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard, J.M. Herrmann, Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 31, 145–157 (2001)
B. Ohtani, Y. Ogawa, S. Nishimoto, Photocatalytic activity of amorphous-anatase mixture of titanium(IV) oxide particles suspended in aqueous solutions. J. Phys. Chem. B. 5647, 3746–3752 (1997)
U.G. Akpan, B.H. Hameed, Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J. Hazard. Mater. 170, 520–529 (2009)
S. Liu, J.-H. Yang, J.-H. Choy, Microporous SiO2–TiO2 nanosols pillared montmorillonite for photocatalytic decomposition of methyl orange. J. Photochem. Photobiol. A Chem. 179, 75–80 (2006)
M.A. Rauf, S.B. Bukallah, A. Hamadi, A. Sulaiman, F. Hammadi, The effect of operational parameters on the photoinduced decoloration of dyes using a hybrid catalyst V2O5/TiO2. Chem. Eng. J. 129, 167–172 (2007)
J.M. Herrmann, Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 53, 115–129 (1999)
Acknowledgments
This research work was supported by a grant (14CTAP-C077607-01) from Infrastructure and transportation technology promotion research program funded by Ministry of Land, Infrastructure and Transport of Korean government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, H.M., Kolekar, T.V., Barge, A.S. et al. Enhanced visible light photocatalytic activity of Cr3+-doped anatase TiO2 nanoparticles synthesized by sol–gel method. J Mater Sci: Mater Electron 27, 526–534 (2016). https://doi.org/10.1007/s10854-015-3785-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3785-6