Abstract
The effects of trace mount of rare earth elements Pr and Nd addition on Sn–0.3Ag–0.7Cu–0.5Ga lead free solder were investigated by observation of microstructure and the morphology of interface layer, as well as the test of shearing strength of the solder joints. The results show that the microstructure of the solder matrix can be optimized by appropriate addition of Pr and Nd, but the scalloped and “region-like” compounds (IMCs) appear in the solder matrix with excessive Pr and Nd addition respectively which may result in the stress concentration and become the birthplace of the cracks. The behavior of absorption of rare earth elements can retard the interface reaction between solder and Cu substrate, refine the morphology of the interface layer. The shearing strength of the solder joints can be enhanced better with appropriate addition of Pr than Nd by improvement of nucleation rate and control of growth rate of the grains near the interface layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The concept of “lead-free” has been advocated for many years in consideration of human health and sustainable development in the field of electronics industry. For this “lead-free” purpose, Sn–Ag–Cu lead free solder (SAC) was chosen as one of the most promising substitutes of traditional Sn–Pb solders on the industrialized application area due to superior performances [1, 2]. But there are some disturbing issues about the new generation of solder. Firstly, the content of Ag is a key factor which restricts the application of SAC solders [3]. The solder joints fatigue strength can be improved with the addition of Ag, meanwhile the resistivity and melting point will reduce. But during the solidification of the solder with high Ag content, the excessive Ag atoms will be rejected by the grains formed earlier, then proceed long-range diffusion along the grain boundaries, finally remain there when the temperature drops which results in the generation and reproduction of the cracks. The properties of solid solution and precipitated phase will decrease due to the formation of Ag3Sn phase [4]. In addition, high Ag content makes the cost raising [5], in contrast, low Ag content will increase the liquidus and broaden the range of the melting temperature of the solder which may has a seriously impact on the wettability of the solder, reduce the mechanical properties of the solder joints [6]. Moreover, generally the SAC solders have relatively high melting temperature owing to more than 90 wt% Sn which gives a rise to the cost, increases the chance of being oxidized during soldering.
Gallium is a low-melting-point (29.78 °C) metal, it has significant effect on decreasing the melting point of the solder as an alloy component, besides, gallium has a prominent property of wetting most metals and their oxides [7]. The previous researches [8, 9] showed that the melting point of SAC solders declined, meanwhile, the wettability of them enhanced with the addition of Ga. But taking account of the increasing requirement of reducing Ag content, a small amount addition of Ga cannot compensate for the negative effects of the reduction of Ag. Instead, the amount of Ga should not be too much, studies [10] indicated that the microstructure of the solder distributed heterogeneously, as a consequence, the strength and ductility descended with more than 0.5 wt% addition of Ga.
In regard of previous investigation [11–14] that the oxidation resistance and microstructure as well as the mechanical properties of the lead-free solder could be improved by addition of trace mount of rare earth element Pr and Nd, the objectives of this work were to research the influence of Pr and Nd based on Ga addition on the microstructure of matrix and interface of SAC lead-free solder as well as the shearing strength of the solder joints, accompanied with the difference between Pr and Nd addition.
2 Experimental
2.1 Solders design and preparation
The compositions of the solder were designed according to 0.02, 0.05, 0.1, 0.2 (wt%) RE addition into Sn–0.3Ag–0.7Cu–0.5Ga (SACG) solder matrix respectively, the content of Ag, Cu, Ga were 0.3, 0.7, 0.5 (wt%) respectively, and the content of Sn was the rest. The crude materials for the designed solders were prepared from 99.9 % pure Sn, Ag, Cu, Ga and rare earth elements Pr and Nd with purity of 99.99 %. To prevent the RE from oxidation, the Sn-10RE alloys were fabricated in a vacuum furnace firstly then added into the SACG solders fabricated by the conventional method to make the designed solders.
2.2 Microstructure observation
The fabricated solders were cut into small pieces before soldering which was performed at 260 °C on the Cu substrate to get the interface layers. The specimens of the solder matrix and the cross-sectioned interface layer were prepared by polishing with 0.3 μm Al2O3 powder and etched with a solution of 4 % HNO3-alcohol. The microstructure of solder matrix observation was conducted with optical microscope and scanning electron microscope (SEM). The morphologies of the interface layers were examined by SEM and the chemical compositions were determined by energy dispersive X-ray (EDX).
2.3 Shearing strength test
For the sake of reflecting the effect of Pr and Nd addition on the shearing strength of SACG solders accurately and factually, the 0805 ceramic resistors were chosen to solder on Printed Circuit Board (PCB) with water-soluble flux according to Japan’s Industry Standard JIS Z 31986 to investigated.
3 Results and discussion
3.1 Microstructure observation
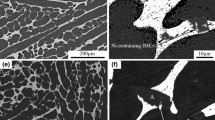
Figure 1 shows the microstructure morphologies of the solder matrix with different amount of Pr. In Fig. 1a, the addition amount is 0, the SACG solder is consist of β-Sn matrix phase and IMCs which distribute scarcely. With the increasing amount of Pr, the morphologies of solder matrix change relatively, the eutectic structure and IMCs become uniformly which present comparatively ideal microstructure as shown in Fig. 1b and c. In Fig. 1d, the IMCs mingle with the solder matrix along the grain boundaries due to the increasing number when the addition of Pr comes to 0.1 wt%. And in the case of 0.2 wt% addition (Fig. 1e), the matrix solder appears massive scalloped IMCs whose magnified picture is shown in Fig. 1f. This isolated IMCs with different properties compared with the solder matrix which appear locally are bound to deteriorate the shearing strength of the solder joints. The similar phenomenon can be found in the SACG solders with Nd addition shown in Fig. 2a–d. With the increasing Nd addition, the number of IMCs grows simultaneously, but the IMCs present regionally which is different from the circumstance with Pr addition. The magnified picture of the “region-like” IMCs is shown in Fig. 2e. And these IMCs whose volumes increase in company with content of Nd occupy the major part of the solder matrix. It can be estimated that this situation may bring serious influence on the comprehensive properties of the solder.
The major components of the IMCs spread along the grain boundaries are Sn, Ag, Cu, Ga and trace amount of RESn3 (RE is Pr and Nd respectively), they can reinforce the solder matrix since their inherent feature of hardness with no more than 0.05 wt% addition of RE. The critical nucleation energy of the nucleus expressed as followed:
In this function, ξ is the form factor of the nucleus, γ is the interface free energy per unit area, μα and μβ are the chemical potentials of liquid and solid phase respectively. Function 1 shows that ∆Gc is in direct proportion to γ3, the critical nucleation energy will be descended by the reduction of γ, and the nucleus superficial area of the rare earth elements is small as their relatively larger atomic radius which reduces the nucleation energy of nucleus/liquid interface, so the nucleation rate increase along with it. In addition, rare earth elements will absorb to the surface with high surface free energy of the IMCs along the boundaries due to their surfactivity to restrain the growth of the IMCs which results in the control of the rate of grains growth. As a consequence, the refinement will be achieved by restriction of both nucleation rate and growth rate. According to the binary phase diagrams of Nd–Sn and Pr–Sn, the melting point of NdSn3 is higher than that of PrSn3, furthermore, the radius of Nd atom is smaller than that of Pr atom. Consequently, the Nd atoms can assemble then grow up to critical nucleus more easily by reason of higher temperature and smaller atom radius during nucleation. These easy-to-form nucleuses become obstacles of other atoms when diffusion which lead to the stacking, so the phenomenon of “region-like” IMCs mentioned above emerges.
3.2 Interface layer
Figures 3 and 4 shows the morphology of the SACG/Cu interface with Pr and Nd addition. Some IMCs appear near the interface whose major components were determined as Sn, Ag, Cu, Ga, by EDX (Fig. 5). As we can see from Fig. 3a, the IMCs distribute widely around the interface, and has the trend of spreading to the solder matrix without Pr addition, and the interface layer is thick. Then, the IMCs diffuse around the solder matrix decreased slightly with a trace amount of 0.02 wt% Pr addition showed in Fig. 3b, but little effect on the reduction of the thickness of the interface layer was achieved. In the case of Fig. 3c, the formation of the IMCs and the growth of interface layer are both restricted well when the addition reach to 0.05 wt%. And in Fig. 3d, the IMCs re-emerges near the interface with 0.1 wt% Pr addition, but the layer thickness changes little. With the addition of Pr further increasing to 0.2 wt%, its inhibition effect on the formation of the IMCs and the reduction of the layer thickness almost disappears. The circumstance with Nd addition shown in Fig. 4 is slightly different from that of Pr. With 0.02 wt% addition of Nd, the IMCs trend to distribute around the interface in Fig. 4a. In Fig. 4b, when the content of Nd rises to 0.05 wt%, the reduction of the IMCs is achieved but not the ideal morphology of the interface. The numbers of the IMCs increase and the morphology of the interface still cannot be refined when the addition comes to 0.1 wt% as shown in Fig. 4c. After this figure rising to 0.2 wt%, the effect of Nd addition almost disappears which leads to the worst interface morphology shown in Fig. 4d.
The variation of the free energy of Cu6Sn5 which is one of the IMCs near the interface layer during formation is given by Function 2 as an example, in this function, ΔG is Gibbs formation energy, ΔG0 is the Gibbs free energy under the standard temperature and pressure, a is the activity of the reactant. Due to this function, if the activity of any reactants decreases, the value of the formula in the bracket will increase as well as that of ΔG which may hinder the chemical reaction. The IMCs usually have different physical and chemical properties from the matrix solder and Cu substrate whose appearance around the interface may affect the wettability of the solder seriously and also lead to the stress concentration problem. In this study, RE will be absorbed then gather around the grain boundaries and the interface on account of surfactivity. The absorption will decrease the concentration of Sn atoms reacted with Ag, Cu and Ga atoms. As a result, the addition of RE enhances the comprehensive properties through the restriction of the formation of excessive IMCs.
3.3 Mechanical properties
Figure 6 shows the shearing strength of the solder joints by application of different solders with addition of Pr and Nd. It can be seen that the shearing strength of the solder joints can be improved in different degree with RE addition. With the increasing of the amount of RE, the shearing strength of the joints increases firstly then decreases. The enhancement effect of the RE elements on the shearing strength increases gradually when the addition amounts of them are between 0 and 0.05 wt%, and the shearing strength reaches the maximum when the number is 0.05 wt% which are 20.4 and 17.7 % higher than the matrix solders for the addition of Pr and Nd respectively. Since then, the effects drop followed by the increasing RE content, but the degree of Nd addition is more obvious compared with the situation of Pr addition. Finally, the effects keep decreasing and the distinction between further Pr and Nd addition trend to minish on the improvement of shearing strength of the solder joints.
According to the binary phase diagrams of Nd–Sn and Pr–Sn, Pr can form a high melting point IMC Pr3Sn with Sn, but there exists no Nd3Sn. So the SACG solder with addition of Pr may refine the grains near the interface to enhance the shearing strength of the solder joints better by getting more nucleation centers when solidification due to the formation of Pr3Sn, along with the reduction of the growth rate of the grains resulting from the behavior of absorption of RE. Besides, the addition of RE has an optimum amount. It is hard to form the solid solution that the differential of the radius of two kinds of metal atoms is beyond 15 % due to relatively high lattice distortion energy according to Hume-Rothery law [15]. This makes the RE atoms distribute rarely in the grains, but mainly around the boundaries and the interface. The volume ratio of grain boundaries/grains is originally small, so the optimizing can be realized with trace amount of RE addition but not with plenty of it in the contrast. What’s more, the polarization of the RE atoms may occur in terms of the relatively lower first ionization energy which makes the RE atoms turn into ions with reduction of radius. Then, followed by the increasing amounts of RE addition, the activity of RE elements decrease resulting from the stress field formed through the lattice distortion caused by diffusion of the RE ions with smaller radius [16]. This is another factor for the limitation of RE addition.
4 Conclusion
This study focuses the comparative analysis on the microstructure observation and mechanical properties of Sn–0.3Ag–0.7Cu–0.5Ga with the addition of rare earth element Pr and Nd respectively. The outcomes of the present study are given as follows:
-
1.
Sound microstructure and mechanical properties of Sn–0.3Ag–0.7Cu–0.5Ga can be obtained after addition of rare earth elements Pr and Nd. The optimum addition of Pr and Nd are both 0.05 wt%. And the effect of addition of Pr is better than that of Nd.
-
2.
The IMCs of the solder matrix with the appropriate addition of rare earth elements Pr and Nd distribute homogeneously. However, the “region-like” IMCs with excessive Nd may result in the stress concentration and become the birthplace of the fracture when the solder joints are under external force which makes the effect of Nd addition is worse than that of Pr.
-
3.
The addition of RE can improve the wettability of Sn–0.3Ag–0.7Cu–0.5Ga solder and refine the morphology of interface layer by the behavior of absorption.
-
4.
The shearing strength of the solder joints can be enhanced with appropriate addition of rare earth elements by advance of nucleation rate as well as the reduction of growth rate of the grains. And the effect is more obvious with addition of Pr due to additional IMC formation.
References
N. Mookam, K. Kanlayasiri, Effect of soldering condition on formation of intermetallic phases developed between Sn–0.3Ag–0.7Cu low-silver lead-free solder and Cu substrate. J. Alloys Compd. 509(21), 6276–6279 (2011)
C.M.L. Wu, D.Q. Yu, C.M.T. Law et al., Properties of lead-free solder alloys with rare earth element additions. Mater. Sci. Eng. R: Rep. 44(1), 1–44 (2004)
D.X. Luo, S.B. Xue, S. Liu, Investigation on the intermetallic compound layer growth of Sn–0.5Ag–0.7Cu-xGa/Cu solder joints during isothermal aging. J. Mater. Sci.: Mater. Electron. 25(12), 5195–5200 (2014)
G. Zeng, S.B. Xue, L. Zhang et al., A review on the interfacial intermetallic compounds between Sn–Ag–Cu based solders and substrates. J. Mater. Sci.: Mater. Electron. 21(5), 421–440 (2010)
M. Reid, J. Punch, M. Collins et al., Effect of Ag content on the microstructure of Sn–Ag–Cu based solder alloys. Solder. Surf. Mt. Technol. 20(4), 3–8 (1989)
A.A. El-Daly, A.E. Hammad, G.S. Al-Ganainy et al., Properties enhancement of low Ag-content Sn–Ag–Cu lead-free solders containing small amount of Zn. J. Alloys Compd. 614(10), 20–28 (2014)
W.X. Chen, S.B. Xue, H. Wang et al., Investigation on properties of Ga to Sn–9Zn lead-free solder. J. Mater. Sci.: Mater. Electron. 21(5), 496–502 (2010)
D.X. Luo, S.B. Xue, Z.Q. Li, Effects of Ga addition on microstructure and properties of Sn–0.5Ag–0.7Cu solder. J. Mater. Sci.: Mater. Electron. 25(8), 3566–3571 (2014)
Q. He, R.G. Ren, X.P. Zhao, Modification of Sn Ag Cu Ga filler metals for electronic packaging. Electron. Packag. 7, 14–15 (2014)
Kang I. Chen, Shou C. Cheng, Chin H. Cheng et al., The effects of gallium additions on microstructures and thermal and mechanical properties of Sn–9Zn solder alloys. Adv. Mater. Sci. Eng. 26(4), 653–659 (2014)
L.L. Gao, S.B. Xue, H. Xu, Microstructure and properties of SnAgCu-xPr solder. Trans. China Weld. Inst. 33(1), 69–72 (2012)
L.L. Gao, S.B. Xue, L. Zhang et al., Effects of trace rare earth Nd addition on microstructure and properties of SnAgCu solder. J. Mater. Sci.: Mater. Electron. 21(7), 643–648 (2010)
P. Xue, S.B. Xue, Y.F. Shen et al., Study on properties of Sn–9Zn–Ga solder bearing Nd. J. Mater. Sci.: Mater. Electron. 21(7), 643–648 (2010)
P. Xue, S.B. Xue, Y.F. Shen et al., Effect of Pr on properties and Sn whisker growth of Sn–9Zn-xPr solder. Solder. Surf. Mt. Technol. 24(4), 280–286 (2012)
X. Chen, J. Zhou, F. Xue et al., Microstructures and mechanical properties of Sn–0.1Ag–0.7Cu-(Co, Ni, and Nd) lead-free solders. J. Mater. Sci.: Mater. Electron. 44(2), 725–732 (2015)
J.D. Luo, S.B. Xue, H. Ye et al., Interfacial microstructure and properties of SnCuNi-xPr/Cu solder joint. Trans. China Weld. Inst. 33(5), 49–54 (2012)
Acknowledgments
The Project was supported by the Key Laboratory of Advanced Welding Technology of Jiangsu Province, China (Foundation No. JSAWT-14-04). This work was also supported by the Fundamental Research Funds for the Central Universities and the Foundation of Graduate Innovation Center in NUAA (Foundation No. kfjj20150604) and this work was also supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Y., Xue, S., Yang, J. et al. Effects of trace amount praseodymium and neodymium on microstructure and mechanical properties of Sn–0.3Ag–0.7Cu–0.5Ga solder. J Mater Sci: Mater Electron 27, 351–358 (2016). https://doi.org/10.1007/s10854-015-3761-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3761-1