Abstract
Pure cobalt aluminate (CoAl2O4) nanoparticles were successfully synthesized by novel sol–gel method with the aid of Co(NO3)2·3H2O, Al(NO3)3·9H2O, and gelatin without adding external surfactant or template. Besides, the effect of the dosage of gelatin on the particle size of final product was investigated. Moreover, gelatin plays role as capping agent, reducing agent, and chelate agent in the synthesis CoAl2O4 nanoparticles. The cobalt aluminate nanoparticles were characterized by using X-ray diffraction, scanning electron microscopy, ultraviolet–visible, Fourier transform infrared spectroscopy, and spectra energy dispersive analysis of X-ray techniques. To evaluate the catalytic properties of nanocrystalline cobalt aluminate, the photocatalytic degradation of methyl orange under visible light irradiation was carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the preparation of low-dimensional nanostructures has been intensively pursued because of their useful applications in various areas. The sol gel method of preparing oxide powders generally involves polymerization via hydrolysis and condensation of an alkoxide, gelation, and heat treatment under suitable conditions [1–3]. Aluminum-based spinels constitute an interesting class of oxide ceramics with important technological applications. The impressive optical (e.g., CoAl2O4 is well known as Thenard’s Blue) and chemical (catalytic applications) properties of transition-metal aluminates make them of significant interest as semiconductor and catalysts. For example spinel AMn2O4 (A=Cu, Zn) has been performed well for photocatalyzing water splitting into H2 and O2. Also, studies of aluminate system have focused on doping with the second activator, such as Nd, Eu and codoped with Er or Cr [4–6] that these studies are interesting. Transition metal aluminates are commonly prepared by a solid state reaction [7], coprecipitation method [8, 9], hydrothermal [10–12], combustion [13] and sol–gel [14–22]. The disadvantages of solid-state routes, such as inhomogeneity, lack of stoichiometry control, high temperature and low surface area, are improved when the material is synthesized using a solution-based method. Compared with other techniques, the sol–gel method is a useful and attractive technique for the preparation of aluminate spinels because of its advantage of producing pure and ultrafine powders at low temperatures. Transition metal-oxide spinels are important in many application fields because of their high thermal resistance and catalytic, electronic and optical properties. They are commonly used in semiconductor and sensor technology as well as in heterogeneous catalysis [23–27]. In this report, for the first time, we had presented the green approach for preparation of CoAl2O4 nanoparticles by novel sol–gel method in the presence of gelatin without adding external capping agent, capping agent or template. This approach is simple and friendly to the environment. The photocatalytic degradation was investigated using methyl orange (MO) under visible light irradiation (λ > 400 nm). The resulting degradation rates of the MO were measured to be as high as 90 % in 6 h.
2 Experimental
2.1 Characterization
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), aluminium nitrate nonahydrate (Al(NO3)3·9H2O), were purchased from Merck Company and used without further purification. X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10 < 2θ < 80. The electronic spectra of the cobalt aluminate were obtained on a Scinco UV–vis scanning spectrometer (Model S-10 4100). The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. Fourier transform infrared (FT-IR) spectrum was recorded on a magna Nicolet 550 spectrophotometer in KBr pellets.
2.2 Synthesis of CoAl2O4 nanoparticles
In a typical synthesis, in two beakers separately, 2 and 1 mmol of Co(NO3)2·6H2O, Al(NO3)3·9H2O were dissolved in 20 ml distilled water under stirring to form a homogeneous solution, respectively. Subsequently, different concentration of gelatin such as 0.5, 1, 2, and 4 g which are corresponding to the samples number 1–4, respectively were dissolved in 10 ml distilled water and was added to the Co(NO3)2·6H2O solution under stirring at room temperature. Then, above solution was added to the Al(NO3)3·9H2O solution. Afterwards, the final mixed solution was kept stirring to remove solvent and form a gel at 100 and 120 °C, respectively. Finally, the obtained product was calcinated at 800 °C for 135 min in a conventional furnace in air atmosphere. The synthesis conditions of CoAl2O4 nanoparticles are listed in Table 1.
2.3 Photocatalytic experimental
The MO photo degradation was examined as a model reaction to evaluate the photocatalytic activities of the CoAl2O4 nanoparticles. The photocatalytic experiments were performed under an irradiation wavelength of λ > 400 nm. The photo catalytic activity of nanocrystalline cobalt aluminate obtained from sample no. 4 was studied by the degradation of MO solution as a target pollutant. The photocatalytic degradation was performed with 0.001 g of MO solution containing 0.05 g of CoAl2O4. This mixture was aerated for 30 min to reach adsorption equilibrium. Later, the mixture was placed inside the photo reactor in which the vessel was 15 cm away from the visible source of 400 W Xeno lamp. The photo catalytic test was performed at room temperature. Aliquots of the mixture were taken at definite interval of times during the irradiation, and after centrifugation they were analyzed by a UV–vis spectrometer. The MO degradation percentage was calculated as:
where A0 and A are initial concentration and changed absorbencies of dye after visible light irradiation, respectively.
3 Results and discussion
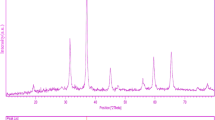
The XRD pattern of pure CoAl2O4 nanoparticles (sample 4) is shown in Fig. 1. Extremely broaden reflection peaks were observed in Fig. 1, which indicated fine particle nature of the obtained cubic phase of CoAl2O4 nanoparticles. No other crystalline phases were detected in the calcined product. All the reflection peaks in this pattern could be readily indexed to the pure crystallite phase CoAl2O4 (JCPDS No. 44-0160), with the calculated cell parameters of a = b = c = 8.1040 Å. According to XRD data, the crystallite diameter (Dc) of CoAl2O4 nanoparticles obtained from sample 4 is calculated to be 40 nm using the Scherer Eq. (1) [28]:
where β is the breadth of the observed diffraction line at its half intensity maximum (400), K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. To examine the effect of gelatin concentration on the morphology and particle size of final product four experiments were performed, as shown in Fig. 2a–d. According to the Fig. 2a, in the 0.5 gr concentration of gelatin, product mainly composed of inhomogeneous particles with average particle size 60–80 nm. Increase in the concentration of gelatin from 0.5 to 1 gr, the particle size of the CoAl2O4 nanoparticles decreases (Fig. 2b). If we continued to increase the concentration of gelatin from 1 to 2 and 4 gr, the size and homogeneity of CoAl2O4 nanoparticles decrease and increase, respectively (Fig. 2c, d); therefore, optimum concentration of gelatin is 4 g. In order to examine the quality and chemical composition of the as-synthesized CoAl2O4 nanoparticles (sample 4), FTIR analysis was performed at room temperature in the range of 400–4000 cm−1 (Fig. 3). According to the Fig. 3, bands at 3432 and 1628 cm−1 are attributable to the v(OH) stretching and bending vibrations, respectively [29]. Furthermore, the metal–oxygen stretching frequencies are reported in the range 500–900 cm−1, associated with the vibrations of M–O, Al–O, and M–O–Al bonds. Moreover, bands at 1119 and 1386 cm−1 are attributable to the C–O/C–C coupled stretching. EDS analysis was used to evaluate the chemical composition and purity of the as-synthesized CoAl2O4 nanoparticles (Fig. 3, sample 4). Based on the Fig. 4, Co, O, and Al elements are observed in the EDS spectrum. In addition, neither N nor C signals were detected in the EDS spectrum, which means the product is pure. The room temperature UV–vis absorption spectra of CoAl2O4 nanoparticles were also measured in the range of 200–600 nm. Figure 5a shows the diffuse reflection absorption spectra of the CoAl2O4 nanoparticles calcined at 800 °C. The figure indicates that the CoAl2O4 nanoparticles shows absorption maxima at 252 nm, the direct optical band gap estimated from the absorption spectra for the CoAl2O4 nanoparticles is shown in Fig. 5b. An optical band gap is obtained by plotting (αhυ)2 versus hυ where α is the absorption coefficient and hυ is photon energy. Extrapolation of the linear portion at (αhυ)2 = 0 gives the band gaps of 2.1 eV for CoAl2O4 nanoparticles. Photodegradation of MO under UV light irradiation (Fig. 6a–c) was employed to evaluate the photocatalytic activity of the as-synthesized CoAl2O4 (sample no. 4). No MO was practically broken down after 6 h without using Visible light irradiation or nanocrystalline CoAl2O4. This observation indicated that the contribution of self-degradation was insignificant. The probable mechanism of the photocatalytic degradation of MO can be summarized as follows:
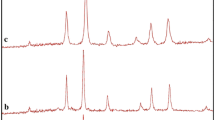
Using photo catalytic calculations by Eq. (1), the MO degradation was about 90 % after 6 h irradiation of visible light, and nanocrystalline CoAl2O4 presented high photo catalytic activity (Fig. 6a). The spectrofluorimetric time-scans of MO solution illuminated at 510 nm with nanocrystalline CoAl2O4 are depicted in Fig. 6b shows continuous removal of MO on the CoAl2O4 under visible light irradiation. It is generally accepted that the heterogeneous photocatalytic processes comprise various steps (diffusion, adsorption, reaction, and etc.), and suitable distribution of the pore in the catalyst surface is effective and useful to diffusion of reactants and products, which prefer the photo catalytic reaction. In this investigation, the enhanced photocatalytic activity can be related to appropriate distribution of the pore in the nanocrystalline CoAl2O4 surface, high hydroxyl amount and high separation rate of charge carriers 24 (Fig. 6c).
Photocatalytic methyl orange degradation of CoAl2O4 nanoparticles obtained from sample no. 4 under visible light (a), fluorescence spectral time scan of methyl orange illuminated at 510 nm with CoAl2O4 nanoparticles (b), and reaction mechanism of methyl orange photodegradation over CoAl2O4 under visible light irradiation
4 Conclusion
CoAl2O4 nanoparticles were synthesized successfully via a novel sol–gel method in the presence of gelatin as reducing agent and Chelate agent. To investigate effect of gelatin concentration on the particle size of final product several experiments were performed. The products were analyzed by SEM, and ultraviolet–visible (UV–Vis) spectroscopy to be round, about 45–55 nm in size and Eg = 2.1 eV. When as-prepared nanocrystalline CoAl2O4 was utilized as photocatalyst, the percentage of MO degradation was about 90 % 6 h irradiation of visible light.
References
F.M. Pontes, J.H.G. Rangel, E.R. Leite, E. Longo, J.A. Varela, E.B. Arau´ jo, J.A. Eiras, J. Mater. Sci. 36, 3565 (2001)
O. Yamamoto, Y. Takeda, R. Kanno, M. Noda, Solid State Ion. 22, 241 (1987)
H. Obayashi, Y. Sakurai, T. Gejo, J. Solid State Chem. 17, 299 (1976)
Y. Shimizu, K. Uemura, N. Miura, N. Yamzoe, Chem. Lett. 67, 1979 (1988)
T. Ivanova, A. Harizanova, M. Surtchev, Z. Nenova, Sol. Energy Mater. Sol. Cell. 76, 591 (2003)
R. Sirera, M.L. Calzada, Mater. Res. Bull. 30, 11 (1995)
D. Bersani, P.P. Lottici, A. Montenero, S. Pigoni, G. Gnappi, J. Non Cryst. Solids 490, 192 (1995)
P.M.T. Cavalcante, M. Dondi, G. Guarini, M. Raimondo, G. Baldi, Dyes Pigm. 80, 226 (2009)
L. Ji, S. Tang, H.C. Zeng, J. Lin, K.L. Tan, Appl. Catal. Gen. 207, 2473 (2001)
Y. Bessekhound, M. Trari, Int. J. Hydrogen Energy 27, 357 (2001)
X. Teng, W. Zhuang, Y. Hu, C. Zhao, H. He, X. Huang, J. Alloys Compd. 458, 446 (2008)
H. Ryu, K.S. Bartwal, J. Alloys Compd. 464, 317 (2008)
W.S. Hong, L.C. De Jonghe, X. Yang, M. Rahaman, J. Am. Ceram. Soc. 78, 3217 (1995)
T. Mimani, J. Alloys Compd. 315, 123 (2001)
M. Zawadzki, J. Wrzyszcz, Mater. Res. Bull. 35, 109 (2009)
W.M. Shaheen, Thermochim. Acta 385, 105 (2002)
Z. Chen, E. Shi, W. Li, Y. Zheng, N. Wu, W. Zhong, J. Am. Ceram. Soc. 85, 2949 (2002)
A.K. Adak, A. Pathak, P. Pramanik, J. Mater. Sci. Lett. 17, 559 (1998)
P. Thorma-hlen, E. Fridell, N. Cruise, M. Skoglundh, A. Palmqvist, Appl. Catal. B Environ. 31, 1 (2001)
N.J. Van der Laag, M.D. Snel, P.C.M.M. Magusin, G. With, J. Eur. Ceram. Soc. 24, 2417 (2004)
X.L. Duan, D.R. Yuan, X.Q. Wang, H.Y. Xu, J. Sol-Gel. Sci. Technol. 35, 221 (2005)
X.L. Duan, D.R. Yuan, Z.H. Sun, C.N. Luan, D.Y. Pan, D. Xu, M.K. Lv, J. Alloys Compd. 386, 311 (2005)
M. Salavati-Niasari, F. Davar, Z. Fereshteh, Chem. Eng. J. 146, 498 (2009)
C.O. Arean, M.P. Mentruit, E.E. Platero, F.X.L. Xamena, J.B. Parra, Mater. Lett. 39, 22 (1999)
W.S. Cho, M. Kahihana, J. Alloys Compd. 287, 87 (1999)
C. Wang, S. Liu, L. Liu, X. Bai, Mater. Chem. Phys. 96, 361 (2006)
A. Laobuthee, S. Wongkasemjit, E. Traversa, R.M. Laine, J. Eur. Ceram. Soc. 20, 91 (2000)
M.Y. Li, W.S. Dong, C.L. Liu, Z. Liu, F.Q. Lin, J. Cryst. Growth 310, 4628 (2008)
S. Ummartyotin, S. Sangngern, A. Kaewvilai, N. Koonsaeng, H. Manuspiya, A. Laobuthee, J. Sustain. Energy Environ. 1, 31 (2009)
Acknowledgments
Authors are grateful to council of University of Borujerd for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Rahnamaeiyan, S., Nasiri, M., Talebi, R. et al. Novel sol–gel method for synthesis of cobalt aluminate and its photocatalyst application. J Mater Sci: Mater Electron 26, 8720–8725 (2015). https://doi.org/10.1007/s10854-015-3548-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3548-4