Abstract
SrAl2O4:Eu2+ films were fabricated on the Si substrates by metal–organic solution deposition method and subsequent treatment up to 1100 °C. The phase and microstructure of the obtained films were characterized by DTA-TG, XRD, SEM and AFM. The composition of the film was confirmed by XPS. The luminescence properties of the films were measured by spectrophotometer. The effect of annealing temperature on the structure and luminescence was studied. It showed that the films had the pure monoclinic phase and the depth about 2 µm. Under the excitation of 254 nm, SrAl2O4:Eu2+ films exhibited an intense green emission band peaking at 528 nm, which originates from the 4f–5d transition of the Eu2+ ions. The quenching temperature of the emission is about 400 K. The results indicated that the SrAl2O4:Eu2+ films have good potential for use as a green phosphor for displays and/or white light emitting diodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Strontium aluminates activated by Eu2+ with long afterglow, such as Sr4Al14O25 [1], SrAl2O4 [2], Sr3Al2O6 [3], and Sr4Al2O7 [4], have been studied extensively in recent years because of their excellent properties including high quantum efficiency [5], long persistence of phosphorescence and good stability [6]. In the strontium aluminates hosts, Eu2+ ions usually show broad band luminescence originating from the 4f 65d 1 → 4f 7 transitions. The interaction of the 4f orbitals with their surrounding ions is weak, while the 5d orbitals are exposed to the surroundings and therefore the 4f 7 → 4f 65d 1 transitions are strongly influenced by the chemical environment of Eu2+. It is known that the covalency increases with decreasing Al content in the series of Sr-aluminates [7]. As a result, the emission of Eu2+ in strontium aluminates hosts can vary from ultraviolet to red, depending on the Al/Sr ratio. And SrAl2O4:Eu2+ phosphors often show green emission when they are excited by the ultraviolet light [2, 8–10].
The films play an important role in some technological applications, such as microelectronic devices [11], magnetic storage media [12], and surface coating [13]. For example, a luminescence layer on the dye-sensitized solar cell (DSSC) composing of down-conversion phosphor materials has the ability to transform higher energy photons into visible photons, which extends light absorption and enhances photovoltaic performance of DSSC [14]. In addition, the phosphor films have advantages of higher lateral resolution from smaller grains, better thermal stability, reduced out gassing and better adhesion to solid substrates [15]. Therefore, for various industrial applications, it is important to study the properties of phosphors in the form of film.
Synthesis from a solution affords excellent chemical stoichiometry and compositional homogeneity, thus reduces the required diffusion path length [16]. Moreover, films deposited by metal–organic solution deposition method (MOSD) are of great and increasing interest for a wide number of materials ranging from metals to insulators because of advantages of large area deposition with good thickness uniformity, precise control of the stoichiometry, and simple equipment [17]. In the present work, we report on the fabrication and optical properties of SrAl2O4:Eu2+ films on Si substrates by MOSD. Film structure and morphological characterization have been carried out using X-ray diffraction (XRD), scanning electron microscopy (SEM), and atomic force microscopy (AFM).
2 Experiments
The SrAl2O4:Eu2+ metal–organic solution was prepared using strontium chloride (SrCl2·6H2O), aluminum isopropoxide (C9H21AlO3), and europium chloride (EuCl3·6H2O) as starting materials. The doping concentration of Eu2+ is 2 mol%. At first, stoichiometric amounts of strontium chloride and aluminum isopropoxide were dissolved in ethyl alcohol. The mixed solution was stirred and refluxed at room temperature, and then the desired amount of europium chloride was added for doping and hydrolysis. The resulting mixtures were further stirred for 30 min to yield clear and homogeneous solution, which served as the coating solution after aging at room temperature. The obtained sols were transparent and were dropped onto silicon wafer substrate and spun at 2000 rpm. After being deposited by spin coating, the films were dried at 300 °C in an oven. These coating and drying procedures were repeated 15 times to obtain a desirable film thickness, and the resulting films were annealed in the reducing atmosphere of active carbon at temperatures of 900, 1000 and 1100 °C for 2 h.
The different thermal analysis (DTA) and thermo gravimetric (TG) analysis were measured by the NETZSCH STA 449C thermal analyzer. The XRD patterns of thin films were examined using a Japan RigaKu D/max-g X-ray diffractometer system with graphite monochromatized Cu Kα irradiation. The surface morphology of thin films was observed by AFM (Innova, Veeco) and SEM (S-4800, Hitachi) attached with energy dispersive X-ray spectroscopy (XPS). The luminescence was performed on a HitachiF-7000 spectrophotometer equipped with a 150 W xenon lamp as the excitation source. The decay curves were obtained from a Lecroy Wave Runner 6100 digital oscilloscope (1 GHz) using a tunable laser (pulse width = 4 ns, gate = 50 ns) as excitation source (Continuum Suncite OPO). A FJ427-A1 thermally stimulated spectrometer was used to reveal the afterglow behavior.
3 Results and discussion
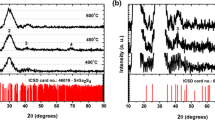
Figure 1 gives the DTA-TG curves of SrAl2O4:Eu2+ phosphor films. There are three regions of mass loss in the TG curve, corresponding to the three exothermic peaks in the DTA curve. The first region originates from the dehydration of the precursor. The second region is due to the pyrolysis of organics. The third region relates to the formation and crystallization of SrAl2O4:Eu2+. Figure 2 shows the XRD patterns of SrAl2O4:Eu2+ phosphor films annealed at 900, 1000 and 1100 °C for 2 h. All of reflection planes are well according with the JCPDS card No. 74-0794, which indicates the formation of monoclinic phase SrAl2O4. With the increases of annealing temperature, the diffraction peaks become stronger and sharper, reflecting greater crystallization. No other significant changes are observed. No impurity phase can be observed in the XRD patterns. This fact clearly implies that the little amount of doped Eu2+ ions have no effects on the SrAl2O4 phase composition. SrAl2O4 adopt a stuffed tridymite-type structure consisting of a corner sharing AlO4 tetrahedral which connect together to form six-member rings. Each oxygen ion is shared by two aluminum ions so that each tetrahedron has one net negative charge. The charge balance is achieved by the large divalent Sr2+, which occupies interstitial site within the tetrahedral framework [18].
Figure 3 presents the surface morphology of SrAl2O4:Eu2+ films annealed at 1100 °C for 2 h. As shown in Fig. 3a, b, the surface of obtained SrAl2O4:Eu2+ film is smooth and has some micro–pinholes. This is further confirmed by the AFM measurements (Fig. 3d). The AFM results show that the SrAl2O4:Eu2+ films exhibit homogeneous microstructures consisting of small and large grains with a statistical roughness, root mean square of 28 nm approximately. Figure 3c shows the cross-sectional view, suggesting that the compact SrAl2O4:Eu2+ films have the depth about 2 µm.
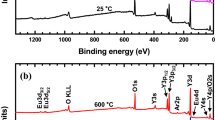
Further evidence of the composition is obtained by the XPS. Figure 4 shows the XPS spectra of SrAl2O4:Eu2+ films annealed at 1100 °C for 2 h. As shown in Fig. 4a, peaks corresponding to O 2s and O 1s, Al 2p and Al 2s, Sr 3d and Sr 3p 3/2 can be observed. Figure 4b gives the peaks corresponding to Eu2+ 3d 5/2 and Eu2+ 3d 3/2, indicating that Eu3+ has been reduced to be Eu2+ successfully. There is no the XPS peaks corresponding to the Eu3+, which demonstrates to the complete reduction of Eu3+.
In the SrAl2O4 host, there are two nonequivalent strontium sites with the same coordination number and similar average Sr–O distance, and the difference of these two sites is only the slight distortion of their square planes [19]. As a result, the Eu2+ centers are expected to show similar luminescence properties because of the similar local environments of Eu2+ ions substituting different Sr2+ ions. Figure 5 shows the excitation and emission spectra of SrAl2O4:Eu2+ films. Figure 5a shows the excitation spectrum of SrAl2O4:Eu2+ film annealed at 1100 °C for 2 h by monitoring 528 nm emission. As seen from the excitation spectrum, a relatively wide absorption in the range of 200–450 nm appears. Furthermore, four distinguished absorption peaks at 254, 308, 349 and 410 nm emerge in the broad excitation bands, which correspond to the crystal field splitting of the Eu2+ d-orbital. In SrAl2O4, the 5d levels of the Eu2+ ions are located below the 6PJ state of the 4f 7* configuration due to the strong crystal field strength, thus creating a broadband luminescence owing to the allowed 4f 65d → 4f 7 transition [20]. Upon the excitation of 254 nm, spectra of SrAl2O4:Eu2+ films annealed at 900, 1000 and 1100 °C for 2 h consist of the single green emitting band with the maximum at 528 nm, which originates from the electric-dipole allowed 4f–5d transition of the Eu2+ ions [21]. All spectra have the same peak position but different emission intensity, by comparison, between the films obtained from various annealed temperatures, indicating the same Eu2+ surroundings and different crystallinity. This is according with the XRD results. As shown in Fig. 2, with the increases of annealed temperature, the crystallinity increases, but the films maintain the pure monoclinic SrAl2O4 phase, which induces the increases of emission intensity and the same peak positions. In addition, it should be noted that there is no emission bands coming from the Eu3+, suggesting the Eu3+ ions have been reduced to be Eu2+ ions completely. This is according with the XRS measurements.
In Fig. 6, the temperature dependence of the intensities of the 4f 65d emission for SrAl2O4:Eu2+ films annealed at 1100 °C is plotted. As the 4f 65d level is thermally populated, the fast parity allowed emission from this state takes over as is typically observed for Eu2+ in host lattices where the 4f 65d is situated just above the 6P7/2 state. In the temperature range of 285–360 K, the emission intensity is nearly constant. Above 360 K, temperature quenching sets in and the quenching temperature of the emission is about 400 K. The quenching temperature is defined as the temperature at which the emission intensity is reduced to be 50 % of the initial intensity. The decreases of the emission intensity are due to the electron–phonon interaction between both the ground state and excited states of the luminescence center at high temperature [22].
Figure 7 shows the decay curve of SrAl2O4:Eu2+ films annealed at 1100 °C. It can be seen that the films show the long persistence. The decay curve indicates that the decay process involves two regimes of an initial rapid-decaying process and a subsequent slow-decaying process. The initial rapid decay corresponds to the intrinsic lifetime of Eu2+, and the long decay of the afterglow due to thermal trapping–detrapping of charge carriers [23]. The decay curve well fits the bio-exponential decay function as follows: I = I0 + A1 exp(−t/τ1) + A2 exp(−t/τ2), where I is the photoluminescence intensity, I0, A1, A2 are the constants, t is the time, τ1 and τ2 are the decay constants for exponential components [24]. The decay lifetimes (τ) can be calculated as τ = (A1τ 21 + A2τ 22 )/(A1τ1 + A2τ2) [25]. The calculated lifetime is about 236 s.
4 Conclusions
In summary, we synthesized SrAl2O4:Eu2+ films successfully on the Si substrates by metal–organic solution deposition and subsequent treatment up to 1100 °C. It showed that the films had the pure monoclinic phase and the depth about 2 µm. Under the excitation of 254 nm, SrAl2O4:Eu2+ films exhibited an intense green emission band peaking at 528 nm originating from the 4f–5d transition of the Eu2+ ions. The quenching temperature of the d–f emission is about 400 K. The results indicated that the SrAl2O4:Eu2+ films have good potential for use as a green phosphor for displays and/or white light emitting diodes.
References
N. Thompson, P. Murugaraj, C. Rix, D.E. Mainwaring, J. Alloys. Compd. 537, 147 (2012)
H.R. Zhang, Z.P. Xue, B.F. Lei, H.W. Dong, H.M. Zhang, S.Q. Deng, M.T. Zheng, Y.L. Liu, Y. Xiao, Opt. Mater. 36, 1802 (2014)
Y.M. Tian, P. Zhang, Z.T. Zheng, Y.S. Chai, Mater. Lett. 73, 157 (2012)
S.J. Kim, H. Won, N. Hayk, C.W. Won, D.Y. Jeon, A.G. Kirakosyan, Mater. Sci. Eng. B 176, 1521 (2011)
B. Smets, J. Rutten, G. Hoeks, J. Verlijsdonk, J. Electrochem. Soc. 136, 2119 (1989)
F.C. Palilla, A.K. Levine, M.R. Tomkus, J. Electrochem. Soc. 115, 642 (1968)
S.Y. Kaya, E. Karacaoglu, B. Karasu, Ceram. Int. 38, 3701 (2012)
C. Zollfrank, S. Gruber, M. Batentschuk, A. Osvet, F. Goetz-Neunhoeffer, S. Dittrich, J. Grabow, H.-D. Kurland, F.A. Muller, Acta Mater. 61, 7133 (2013)
E. Shafia, M. Bodaghi, S. Esposito, A. Aghaei, Ceram. Int. 40, 4697 (2014)
D.S. Kshatri, A. Khare, J. Alloys. Compd. 588, 488 (2014)
Q. Man, W. Sun, F. Yang, C. Qiu, Y. Zhao, G. Hu, J. Mater. Sci. Mater. Electron. 25, 1269 (2014)
W. Wang, Q.X. Zhu, M.M. Yang, R.K. Zheng, X.M. Li, J. Mater. Sci. Mater. Electron. 25, 1908 (2014)
K. Muthukrishnan, M. Vanaraja, S. Boomadevi, R.K. Karn, J.B.B. Rayappan, V. Singh, K. Pandiyan, J. Mater. Sci. Mater. Electron. 26, 1908 (2015)
W.Z. He, T.S. Atabaev, H.K. Kim, Y.-H. Hwang, J. Phys. Chem. C 117, 17894 (2013)
D.P. Norton, Mater. Sci. Eng. R 43, 139 (2004)
B. Liu, X.P. Wang, Y.Y. Zhang, X.S. Lv, Y.G. Yang, Ionics 20, 1795 (2014)
Y.G. Yang, X.P. Wang, B. Liu, Surf. Interface Anal. 46, 109 (2014)
M. Ayvacıkli, Z. Kotan, E. Ekdai, Y. Karabulut, A. Canimoglu, J.G. Guinea, A. Khatab, M. Henini, N. Can, J. Lumin. 144, 128 (2013)
F. Clabau, X. Rocquefelte, S. Jobic, P. Deniard, M.-H. Whangbo, A. Garcia, T. Le Mercier, Chem. Mater. 17, 3904 (2005)
Y. Liu, C.-N. Xu, J. Phys. Chem. B 107, 3991 (2003)
X.Q. Xing, D.Y. Ling, L. Tan, J. Mater. Sci. Mater. Electron. 25, 4774 (2014)
B.F. Lei, K. Machida, T. Horikawa, H. Hanzawa, Chem. Lett. 40, 140 (2011)
P. Huang, Q.C. Zhang, C. Cui, J. Li, Opt. Mater. 33, 1252 (2011)
F.J. Wei, Q.L. Jia, J. Mater. Sci. Mater. Electron. 26, 262 (2015)
Y.X. Liu, A.Y. Lan, Y. Jin, G.Q. Chen, X. Zhang, Opt. Mater. 40, 122 (2015)
Acknowledgments
This work is supported financially by the National Science Foundation of China (Grant Nos. 51102158, 51202135 and 51302158), the Science and Technology Develop Project in Shandong Province (Grant No. 2013GGX10203), Natural Science Foundation of Shandong Province (Grant No. ZR2014EMQ004), and Youth Foundation of Shandong Academy of Sciences (Grant Nos. 2013QN011, 2014QN027 and 2014QN028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Liu, B., Zhang, Y. et al. Fabrication of SrAl2O4:Eu2+ films on Si substrates via metal–organic solution. J Mater Sci: Mater Electron 26, 8267–8271 (2015). https://doi.org/10.1007/s10854-015-3490-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3490-5