Abstract
Titanium dioxide nanoparticles (NPs) were modified by alkyl methacrylate (alkyl = methyl, butyl, ethylhexyl, lauryl and octadecyl) through graft copolymerization at its surface, respectively. The characteristics of these products were analysed by Fourier transform infrared, thermogravimetric analysis and transmission electron microscopy. The average particle size, zeta potential and electrophoretic mobility of modified NPs were tested in Isopar dispersion system and the water contact angle was also measured. The average particle size of NPs decreased; zeta potential, hydrophobicity and the dispersion stability enhanced with the increase of alkyl chain length. The maximum values of the zeta potential of NPs modified by poly(octadecyl methacrylate) (PSMA/TiO2) electrophoretic particles reached −72.64 mV. The obtained NPs associated with carbon black were employed to prepare the display inks. An electrophoretic display prototype device based on the white–black inks was prepared. The results gave that the device based on NPs treated with poly(lauryl methacrylate) (PLMA/TiO2) presented 357 ms of quick response under an applied 5 V (DC) field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Inorganic nanoparticles modified with polymer have received widespread attentions due to its excellent optical, electrical, thermal, mechanical and catalytic properties [1–4]. In addition, Polymers also improve the surface properties of inorganic nanoparticles. For example, polymers could strengthen the resistance to chemical corrosion and the electrostatic interaction between particles, Creutz and Jérôme [5]. At the same time, the dispersion stability of inorganic nanoparticles coated by polymers in the dispersion medium was improved. The polymer cladding layer of the inorganic nanoparticles can reduce the density of nanoparticles, which makes the density of particles and the dispersion medium match each other and enhances the dispersion stability of nanoparticles in the dispersion medium.
Recently, titanium dioxide nanoparticle as the white particle is applied to electrophoretic display because of its excellent optical, electrical and chemical properties [6]. Although titanium dioxide nanoparticles has the advantages of high whiteness and light refractive, it is very easy to aggregate due to the large density of particle and the strong interaction force between particles and electrode, resulting in an intense impact on the electrophoretic display response time and contrast ratio [7–9]. Some researchers use polymers to modify the surface of particles in order to improve the stability of titanium dioxide nanoparticles dispersed in the medium. Park et al. [10] reported that TiO2 nanoparticles which were encapsulated with poly(methyl methacrylate-co-methacrylic acid) via the method of coacervation could improve the dispersion stability of nanoparticles in a dielectric medium. Lee et al. [11] studied that TiO2 nanoparticles modified with poly(vinyl alcohol) presented good electrophoresis motility and dispersion stability in the dispersion medium. Park et al. [12] researched that TiO2 nanoparticles modified with PMMA-co-EGMEA could enhance the dispersion stability in the dielectric medium and improve surface characteristics.
In this article, TiO2 nanoparticles were modified by methyl methacrylate, butyl methacrylate, ethylhexyl methacrylate, lauryl methacrylate and octadecyl methacrylate, respectively. The surface and photoelectric properties of modified particles were tested. TiO2 nanoparticles modified with methacrylate as a white electrophoretic particle were used to in the electrophoretic display, which could significantly shorten the response time of the display device.
2 Experimental
2.1 Materials
Ethanol, glacial acetic acid, N,N-dimethyl formamide (DMF), tetrahydrofuran and azobisisobutyronitrile (AIBN) were analytical grade and purchased from Guangfu Chemical Regent Co., Ltd., China. Anatase-type titanium dioxide (mean diameter 50 nm) was obtained from Beijing Mountain Technical Development Center. γ-methacryloxypropyltrimethoxysilane (KH570) was purchased from Liyang Kaituozhe Chemical Technical Service Center and Tianyang Additives Co., Ltd.,. Isoparaffin with a density of 0.76 g/cm3, dielectric constant of 2.011, and viscosity of 1.336 mPa s at 20 °C was purchased from Shanghai Huishuo Chemical Development Co., Ltd., China. Methyl methacrylate (MMA), butyl methacrylate (BMA), ethylhexyl methacrylate (EHMA), lauryl methacrylate (LMA) and octadecyl methacrylate (SMA) were bought from Aladdin Industrial Co., Ltd., China. Carbon black (mean diameter 50 nm) was obtained from Evonik Degussa. CH-5 and T151 were purchased from Shanghai Sanzheng Macromolecule material Co., Ltd., China and Wuxi South Petroleum Additive Co., Ltd., China, respectively.
2.2 Modification of TiO2 with a silane coupling agent
In a typical synthesis, 93.06 g of ethanol and 6.94 g of H2O were added into a 250 mL flask under gentle stirring, and then the pH value was adjusted to 4.5 with glacial acetic acid. After 20.00 g of TiO2 and 5.00 g of KH570 were added, the temperature increased gradually to 60 °C for 8 h. 19.46 g of titanium dioxide modified with KH570 (KH570/TiO2) was obtained by centrifugation and washing with ethanol for several times until removing completely the unreacted silane coupling agents.
2.3 Modification of KH570/TiO2 with methacrylate
Titanium dioxide modified with poly(methyl methacrylate) (PMMA/TiO2), poly(butyl methacrylate) (PBMA/TiO2), poly(ethylhexyl methacrylate) (PEHA/TiO2), poly(lauryl methacrylate) (PLMA/TiO2) and poly(octadecyl methacrylate) (PSMA/TiO2) were prepared via graft copolymerization, respectively. 5.72 g of methacrylate (MMA, BMA, EHMA, LMA and SMA) and 100 mL DMF were put into a 250 mL flask with nitrogen gas inlet, and then 5.00 g of TiO2 coated KH570 and 0.10 g of AIBN were added under stirring at 300 rpm. The temperature increased to 80 °C and maintained for 8 h. Titanium dioxide modified polymethacrylate particles were collected by centrifugation and washed with tetrahydrofuran until the unreacted monomer and oligomer removing completely, followed by drying in a vacuum oven for 24 h.
2.4 Preparation of electrophoretic display liquid with black and white particles
Carbon black and titanium dioxide modified polymethacrylate particles were dehydrated for 3 h at 120 °C in vacuum oven. 10 mL isoparaffin was put into a 50 mL ball bottle and 3.0 g of PMMA/TiO2, PBMA/TiO2, PEHMA/TiO2, PLMA/TiO2 and PSMA/TiO2 particles was respectively dispersed in isoparaffin added 0.2 g of CH-5 and 0.1 g of T151. After ball-milling 48 h, white electrophoresis liquid was prepared. 0.5 g of Carbon black was dispersed in isoparaffin added 0.2 g of CH-5 and 0.1 g of T151. After ball-milling 36 h, black electrophoresis liquid was prepared. Electrophoretic display liquid was prepared by mixing the white and black electrophoresis liquid according to a certain proportion.
2.5 Characterization methods and instruments
FT-IR spectroscopy of titanium dioxide modified polymethacrylate particles was performed in potassium bromide pellet on a NICOLET 380 Fourier Transform Infrared Spectroscopy. TEM photos were collected utilizing JEM2100F with an accelerating voltage of 200 kV. TGA was performed on a TGA-50 thermal analyzer in nitrogen atmosphere from 20 to 650 °C at a heating rate of 10 °C/min. The average particle size distribution, zeta potential and electrophoretic mobility were tested by Delsa Nano C zeta potential and particle size analyzer. Contact Angle was measured by JC2000D Contact Angle Measurement Instrument. Electrophoretic display contrast and response time were tested by the key parameter tester for electronic ink display device.
3 Results and discussion
3.1 IR of titanium dioxide modified with polymethacrylate
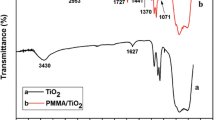
Figure 1 shows the infrared spectra of pristine TiO2 particles and TiO2 grafted with polymethacrylate particles. For pristine TiO2 (curve a), the broad peak at 3430 cm−1 corresponds to stretching vibration of surface hydroxyl and the peak at 1629 cm−1 corresponds to bending vibration of O–H bonds on the TiO2 particles’ surface. The infrared absorption band between 500 and 800 cm−1 is due to the framework vibrations of TiO2 [13, 14]. For TiO2 grafted with polymethacrylate (curve b–f), Two sharp peaks can be seen at 2926–2950 and 2853–2861 cm−1, which are attributed to stretching vibration of C–H. The peak at 1445–1485 cm−1 is due to the bending vibration of C–H. The absorption band around 1730 cm−1 is assigned to the carbonyl group stretching vibration of polymethacrylate on the surface of TiO2. Comparing with the FTIR spectra of pristine TiO2 particles and the treated TiO2 particles, it preliminarily reveals that the surface of TiO2 particles was modified with polymethacrylate.
3.2 TGA of titanium dioxide modified with polymethacrylate
The TGA of pristine TiO2 particles, TiO2 modified by KH570 and TiO2 grafted with polymethacrylate are revealed by Fig. 2. As curve a shown, except for the evaporation of the physically absorbed water, no obvious weight loss of TiO2 particles could be noticed. Curve b presets the TGA of KH570/TiO2, the main weight loss is occurred in the range of about 200–500 °C could be attributed to thermal decomposition of KH570 on the surface of TiO2 particles and the weight losses 0.22 %. The less weight loss and no signal of bending vibration of bonds Si–O–C in IR spectra indicates KH570 is monolayer modified on the surface of TiO2. For TiO2 grafted with polymethacrylate particles (curve c–g), the weight loss is occurred in the range of about 50–600 °C. The decrease of weight before 200 °C is due to thermal decomposition of a small amount of modification agent and water physical adsorption on the surface of TiO2 particles. The mainly weight loss in the range of 200–600 °C is attributed to thermal decomposition of polymethacrylate forming strong chemical bonds with TiO2. The weight loss of PMMA/TiO2, PBMA/TiO2, PEHMA/TiO2, PLMA/TiO2 and PSMA/TiO2 is 2.42, 2.53, 1.81, 3.14 and 2.03 %, respectively. The FTIR and TGA results showed that the surface of pristine TiO2 particles was successfully modified with polymethacrylate.
3.3 Morphology and topography analysis of titanium dioxide modified with polymethacrylate
The TEM image of pristine TiO2 particles is shown by Fig. 3a, which can clearly exhibit typical lattice fringes of TiO2. The TEM images of TiO2 particles grafted with polymethacrylate are revealed by Fig. 3b–f. As Fig. 3b–f shown, the surface of TiO2 particle is uniformly coated with a layer of poly methacrylate without lattice fringes and the thickness of the cladding layer is about 1–3 nm.
3.4 The average diameter distribution analysis of titanium dioxide modified with polymethacrylate
TiO2 nanoparticles modified with polymethacrylate were dispersed in Isopar under ultrasonic and then the average diameter of the modified particles was measured by zeta potential and particle size analyzer from 0 to 9 h, which were shown in Fig. 4. Without any addition, the pristine TiO2 nanoparticles obtain the largest average diameter of 1131.6 nm. Meanwhile, TiO2 nanoparticles modified with polymethacrylate has relatively smaller average diameter and the size decreases with the increase of the number of carbon atoms in alkyl chain of methacrylate modifiers. When the carbon atoms of alkyl chain are >8, the average diameter of the nanoparticles decreases obviously among the range of 200–250 nm. After standing for 1 h, both TiO2 nanoparticles with modification or not suffer aggregation resulting in the increase of the average diameter. The pristine TiO2, TiO2 nanoparticles modified with MMA and BMA experience drastically increases with average diameter of 1400–2500 nm. However, the TiO2 nanoparticles modified with EHMA, LMA and SMA exhibit minor increase with diameter of 200–300 nm. After standing for 3 h, the pristine TiO2 nanoparticles cannot be captures by the analyzer due to the severe aggregation thus the result gives 0. The average diameter of TiO2 nanoparticles modified with MMA and BMA are added up to 3500–5000 nm while the diameter of nanoparticles with the rest modifiers isn’t change. After standing for 5 h, TiO2 nanoparticles modified with MMA and BMA cannot be captures by the analyzer due to the severe aggregation while the diameter of nanoparticles modified with the EHMA, LMA and SMA still show steady distribution between 200 and 300 nm. It is the Brownian movement that causes the aggregation of the particles. The polymethacrylate modifier could provide steric effect which may contribute to the stability of the dispersion. Moreover, with the increase of carbon atoms of alkyl chain the steric effect grow strong which may lead to more stable dispersion.
3.5 The contact angle analysis of titanium dioxide modified with polymethacrylate
It can be seen from Table 1 that the contact angle of water on the surface of pristine TiO2 particles is 0° because TiO2 particles are high-energy solid and possess higher surface free energy. The hydrophilic of TiO2 particle’s surface is very strong leading to the poor wettability of TiO2 particles in organic solutions. However, organic polymer belongs to low-energy solid and has relatively lower surface free energy. Polymethacrylate coated on the surface of TiO2 particles could reduce the surface free energy of particles resulting in the increase of the contact angle of water on the surface of particles and reducing the hydrophilic. Modifier monomer reacts with active group on the surface of TiO2 nanoparticles making the nonpolar alkyl chain outwards and forming a non-polar organic molecular layer on the surface of TiO2 nanoparticles, which changes the surface properties of TiO2 nanoparticles. As the number of carbon atoms in alkyl chain of methacrylate modifiers increased, the contact angle of water on the surface of TiO2 modified with polymethacrylate particles increases from 74.8° to 136.0° and the surface of nanoparticles changes from hydrophilic to hydrophobic, which enhances the dispersion stability of nanoparticles in organic solvents.
3.6 Zeta potential analysis of titanium dioxide modified with polymethacrylate
Zeta potential is the measurement of the strength of the repulsive or attractive forces between particles whose value has close relationship with the stability of the dispersion system. The more the zeta potential is, the more steady the disperse system will be. Otherwise, the dispersion system is prone to agglomerate. From Table 2, we could get the conclusion that the dispersion system is not stable since the zeta potential is −23.14 mV. After modified by polymethacrylate, the zeta potential and electrophoretic mobility of TiO2 nanoparticles increase gradually which indicate that the dispersion system is turning to stability accordingly. From the literature [15–17], the hydrophily of the surface of the nanoparticles has major effect on the zeta potential and electrophoretic mobility. The surface charge of the nanoparticles is surrounded by the hydrophilic layer, which may lead to an increase of the surface conductivity due to the addition of the ionic bonding. Subsequently, the shear plane is stretched which may lower the zeta potential and electrophoretic mobility of the hydrophilic surface. The pristine TiO2 nanoparticles possess very strong hydrophily, while after modified by polymethacrylate the surface switches to hydrophobic. Consequently, the zeta potential and electrophoretic mobility increase and the stability of the system have been reinforced.
3.7 The response time analysis of electrophoretic display prototype device
Electrophoretic medium is consisted of the white and black electrophoresis liquid according to a certain proportion. First, two conductive glasses are conductive surface disposed in parallel. And then a 0.1 mm thick polyester film is placed into the middle of the conductive glasses and the display inks prepared according to the Sect. 2.5 are also added into it. Finally, two glasses are bonded by glass resin adhesive to make a prototype device. The response time of prototype devices was tested by the key parameter tester for electronic ink display device at dc voltage U = 5 V and the results were shown in Table 3. The response time of the prototype device with pristine TiO2 as the white electrophoretic particles is 1930 ms, which has the longest response time, as shown in Table 3. However, when the white electrophoretic particles turn into TiO2 modified with polymethacrylate particles, the response time of the prototype devices has an obvious decrease. The response time of the prototype device with TiO2 modified with LMA as the white electrophoretic particles is 357 ms, which has the shortest response time. Figure 5 shows black/white change due to the electrophoretic migration of pristine TiO2 and PLMA/TiO2 particles of the display prototype device, respectively. As the number of carbon atoms in alkyl chain of methacrylate modifiers increased, the response time of the prototype devices presents the trend of shorten first, and then growth. Compared with pristine of TiO2 nanoparticles, the surface of TiO2 modified with polymethacrylate is hydrophobic leading to particles well dispersing in Isopar. Meanwhile, TiO2 modified with polymethacrylate particles possess higher zeta potential and the electrophoretic mobility value, which improves the velocity of particles in the dispersion medium under the action of electric field. Therefore, the response time of the prototype device shortens. Although the zeta potential and the electrophoretic mobility value of TiO2 modified with SMA is high, the longer carbon chain on the surface of TiO2 particles hinders the movement of the particles. Thereby, the response time of the prototype device becomes increase.
4 Conclusions
In summary, TiO2 nanoparticles modified with alkyl methacrylate (alkyl = methyl, butyl, ethylhexyl, lauryl and octadecyl) were obtained. Owing to the repulsive forces among the treated TiO2 NPs resulting from the extending alkyl chain, the surface modification hinders the aggregation of the treated particles and increases the dispersion and suspension stability of the particles in Isopar G. The maximum values of the zeta potential of PSMA/TiO2 electrophoretic particles reached −72.64 mV. The modified TiO2 NPs can be applied in the white/black electronic ink together with carbon black. Under the DC field of 5 V, the device based on PLMA/TiO2 presented 357 ms of quick response.
References
B. Peng, F. Tang, D. Chen, X. Ren, X. Meng, J. Ren, J. Colloid Interface Sci. 329, 62–66 (2009)
C. Barthet, A.J. Hickey, D.B. Cairns, Adv. Mater. 11, 408–410 (1999)
M. Badila, C. Brochon, A. Hebraud, G. Hadziioannou, Polymer 49, 4529–4533 (2008)
M.P.L. Werts, M. Badila, C. Brochon, A. Hébraud, G. Hadziioannou, Chem. Mater. 20, 1292–1298 (2008)
S. Creutz, R. Jérôme, Prog. Org. Coat. 40, 21–29 (2000)
L.S. Park, J.W. Park, H.Y. Choi, Y.S. Han, Y. Kwon, H.S. Choi, Curr. Appl. Phys. 6, 644–648 (2006)
J.Y. Lee, J.H. Sung, I.B. Jang, B.J. Park, H.J. Choi, Synth. Met. 153, 221–224 (2005)
B. Comiskey, J.D. Albert, H. Yoshizawa, J. Jacobson, Nature 394, 253–255 (1998)
I.B. Jang, J.H. Sung, H.J. Choi, I. Chin, Synth. Met. 152, 9–12 (2005)
J.H. Park, M.A. Lee, B.J. Park, H.J. Choi, Curr. Appl. Phys. 7, 349–351 (2007)
J. Lee, J. Hong, D.W. Park, S.E. Shim, Opt. Mater. 32, 530–534 (2010)
B.J. Park, S.Y. Hong, H.H. Sim, H.J. Choi, Y.S. Yoon, Mater. Chem. Phys. 135, 259–263 (2012)
A.A. Haroun, A.M. Youssef, Synth. Met. 161, 2063–2069 (2011)
T. Ivanova, A. Harizanova, Solid State Ion. 138, 227–232 (2001)
O.E. Gholabzouri, M.A. Cabrerizo, R.H. Alvarea, Colloids Surf. 214, 243–250 (1999)
F.M. Watson, W. Tscharnuter, J. Miller, Colloids Surf. 140, 53–57 (1998)
M.T. Roy, M. Gallardo, J. Estelrich, J. Colloid Interface Sci. 206, 512–517 (1998)
Acknowledgments
This work was financially supported by the National High Technology Research and Development Program of China (No. 2015AA033402) and the Science and Technology Planning Project of Tianjin Province, China (No. 14TXGCCX00017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Jiang, Y., Li, X. et al. Preparation of titanium dioxide nanoparticles modified with methacrylate and their electrophoretic properties. J Mater Sci: Mater Electron 26, 5263–5269 (2015). https://doi.org/10.1007/s10854-015-3062-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3062-8