Abstract
A two-step sol–gel process is used for the preparation of undoped, Mn and V-doped Zn2SiO4 nanoparticles embedded in silica host matrix. The formation of a crystalline phase of willemite structure in the samples was confirmed by XRD measurements. Undoped Zn2SiO4 sample exhibits an intense emission band centered at 760 nm, can be attributed to the formation of non-bridging oxygen hole centers (NBOHcs) excited at the spectral region hν ≥ 5.4 eV. In the case of Zn2SiO4:V, a band centered at 540 nm has been detected. This emission is allocated to the V in the interfaces between Zn2SiO4 particles and SiO2 host matrix. In addition, the green emission peak centered around 525 nm of Zn2SiO4:Mn nanoparticles is attributed to the 4T1–6A1 transitions of Mn2+ ions. The crystallinity and luminescent property of nanoparticles were investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last decade, there has been a renaissance in the study of optical performances of inorganic materials in many technological challenges, such as in optical amplification [1], lasing [2, 3], optical data storage [4, 5], up-conversion and phosphors [6–8]. Nanoparticles have recently been recognized to hold tremendous potential in the area of photonic applications [9, 10]. These nanometer-sized inorganic materials can exhibit a wide range of potential optical and electrical applications [4, 5]. Novel properties would be obtained when the crystal structure of the host materials is changed. However, the synthesis and the luminescence properties of nanometer-sized silicate are rarely reported.
Up to now, the commercial Zn2SiO4 phosphors have been fabricated mainly by the solid reaction method [6]. However, this method has some disadvantages such as high firing temperature and difficulties to control the particles size and shape. Therefore, in order to overcome these problems, new fabrication methods such as the hydrothermal [7], sol–gel [8–12] and fume pyrolysis methods [13] have been investigated and produced an interesting photoluminescence. However, there is some difficulties to commercialize green phosphors due to the complexity of those new fabrication methods. At present, Taghavinia et al. [14] used porous silicon as one of the starting materials and impregnated porous silicon layers with luminescent Zn2SiO4:Mn2+ particles. Porous silicon was directly involved in the reaction responsible for the formation of luminescent Zn2SiO4 phosphors phase. These particles were obtained inside a transparent porous body, making it possible to activate porous silicon layers with highly efficient phosphors [14]. Kang and Park used the colloidal solution method to fabricate spherical Zn2SiO4 phosphors with an optimal emission intensity of about 112 % compared to that of commercially available materials [15]. Although these chemical methods can be applied to produce fine particles with good luminescence, they have the shortcomings related to complex processing, difficulties in achieving controllability and mass production, and high cost [16–18]. The sol–gel method has been confirmed to have more advantages in lowering the firing temperature, distributing the activator ions homogeneously and improving the emission efficiency for the phosphors centres [19, 20]. Additionally, the sol–gel technology is suitable for deposition of thin amorphous and crystalline films, which have found extensive applications in many fields [21].
Important for color rendering, the host lattice of Zn2SiO4 containing Eu3+, V5+, Mn2+ or Ce3+ dopant ions covers the red, yellow, green, and blue portions of the visible spectrum, respectively [22–28]. Nanophosphors based on the rare-earth and transition metals are receiving considerable attention as potential coatings in lamps, cathode ray tubes [4], flat panel displays, electroluminescent and optoelectronic devices [6] and more recently, radiation detectors in medical imaging systems [8]. The long decay time of Zn2SiO4 is an obstacle for the PDP application. Thus, a lot of efforts have been dedicated to shorten the decay time of Zn2SiO4:Mn phosphor particles without losing the higher efficiency [25, 26].
In this study, the sol–gel method combined with a furnace firing at higher temperature [29, 30] is applied to prepare Zn2SiO4, Zn2SiO4:V and Zn2SiO4:Mn particles embedded in silica monolith. The crystalline properties, optical emission, excitation spectra and decay time of nanocomposites were investigated.
2 Experimental
2.1 Preparation of nanoparticles
The preparation of colloid suspension particles in silicate host matrix has been done in three steps. In the first one, undoped, V and Mn doped ZnO aerogels were prepared by a sol–gel method under supercritical conditions of ethyl alcohol (EtOH) based on El Ghoul et al. protocol [29, 30], where the water for hydrolysis was slowly released by esterification reaction to control the size of the formed nanoparticles. In the second step, we have prepared undoped, Mn and V doped ZnO confined in silica aerogel according to the following process: 0.5 ml of TEOS was first dissolved in EtOH. Then, with constant stirring of the mixture of TEOS and EtOH, 0.44 ml of water and 30 mg of nanoparticles powder prepared in the first step were added. The whole solution was stirred for about 30 min, resulting in the formation of a uniform sol. The sol was transferred to tube in ultrasonic bath where 100 ml of fluoride acid was added. The wet gel formed in few seconds. Monolithic and white aerogel was obtained by supercritical drying in EtOH as described in the first step. Finally, silica glasses containing undoped Zn2SiO4 and Mn and V doped Zn2SiO4 nanoparticles were obtained after firing aerogel at 1200 °C for 2 h.
2.2 Analyses
The crystalline phases of annealed samples were identified by X-ray diffraction (XRD) using a Bruker D5005 powder X-ray diffractometer using a CuKα source (1.5418 Å radiation). Crystallite sizes were estimated from the Scherrer’s equation [31].
where λ is the X-ray wavelength (λ = 1.5418 Å), θB is the maximum of the Bragg diffraction peak (rad) and Β is the line width at half maximum.
Transmission electron microscopy (TEM, JEM-200CX) was used to study the morphology and particle size of the phosphor powders. The specimens for TEM were prepared by putting the as-grown products in EtOH and immersing them in an ultrasonic bath for 15 min, then dropping a few drops of the resulting suspension containing the synthesized materials onto TEM grid. For photoluminescence (PL) measurements, the 450-W Xenon lamp was used as an excitation source. The emitted light from the sample collected by an optical fiber on the same side as that of excitation was analyzed with a Jobin–Yvon spectrometer HR460 and a multichannel CCD detector (2000 pixels). The photoluminescence excitation (PLE) measurements were performed on a Jobin–Yvon Fluorolog 3-2 spectrometer. The decays were analyzed by a PM Hamamatsu R928 and a scope Nicolet 400 with a time constant on the order of 1 ns. The low temperature experiments were carried out in a Janis VPF-600 Dewar with variable temperature controlled between 78 and 300 K.
3 Results and discussion
3.1 Structural studies
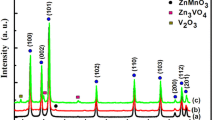
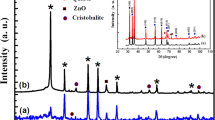
The formation of crystalline phases in the synthesized samples was confirmed by XRD measurements. Figure 1 shows X-ray diffraction spectra obtained from the Zn2SiO4 (a), Zn2SiO4:V (b) and Zn2SiO4:Mn (c) nanoparticles treated at 1200 °C for 2 h in air. These spectra show the X-ray diffraction pattern corresponding to a well-developed willemite structure (α-Zn2SiO4, JCPDS No. 37-1485) [29, 32]. The lattice constants calculated from the XRD pattern for the three samples are about a ≈ 13.939 Å and c ≈ 9.301 Å, which are very close to willemite α-Zn2SiO4 phase [32, 33]. These results are in a good agreement with those obtained by El Mir et al. [34]. At high temperature, the surface mobility of Zn and Si species is high enough, so they move and diffuse inside the porous body and contribute to the formation of zinc silica phase. This result indicates that α-Zn2SiO4 has a rhombohedral structure [30]. It is clear that the crystalline phase is the most dominant one corresponding to the α-Zn2SiO4 phase, in parallel we note the appearance of three other phases corresponding to ZnO, cristobalite and quartz [30]. Therefore, the hexagonal zinc oxide and willemite Zn2SiO4 coexist in the composite, this means that the solid reaction at higher temperature is not completely achieved and the obtained composite is formed by very small ZnO nanoparticles covered by willemite Zn2SiO4 as a shell supported by SiO2 as a host matrix. The average of the grain size (G) of the crystallites Zn2SiO4 varies from 50 to 80 nm, estimated by Scherrer’s formula (1).
The SEM micrographs of Zn2SiO4:V and Zn2SiO4:Mn samples are shown in Fig. 2. The surface morphology indicates a good grain growth with anisotropic shape with the presence of two phases in the structure. The grains of unequal size are distributed throughout the sample. The average grain size (d ~ 1–4 µm) has been evaluated by using the linear intercept method by the relation:
where L is the random line length, M the magnification of image and N the number of grain-boundary intercepts by the line. However, the obtained sizes of the particles are larger than those obtained by XRD analysis.
The TEM micrographs, HRTEM image and energy dispersive spectroscopy (EDX) analyzes of the Zn2SiO4 nanoparticles treated at 1200 °C for 2 h in air are shown in Fig. 3. The morphology of the sample is found to be nearly spherical in nature with the diameters ranging from 40 to 90 nm. So it’s clearly shown that the average particle size is nanoscale range and it is in accordance with the results of the XRD. EDX analysis shows the presence of the elements Zn and Si. At high temperature 1200 °C, Zn and Si species, move and diffuse inside the porous body to form Zn2SiO4 phase. In parallel, the HRTM image and the corresponding EDX analysis showed the presence of ZnO nanoparticles in the sample, such results confirm XRD results.
The TEM images corresponding to Zn2SiO4:V and Zn2SiO4:Mn nanoparticles are displayed in Fig. 4a, b, respectively. The Zn2SiO4 colloid suspension is formed in silica host matrix with a particle size of about 70 nm. The EDX analysis are shown in Fig. 4a, b. The concentrations of Zn, Si, and O are illustrated in the corresponding tables. The excess of Zn ions over the stoichiometric ratio of Zn2SiO4 crystals exist at the near-surface side due to low Si concentrations at a distance far from the quartz surface, and they are also textured with some preferred orientations of crystals as seen in the XRD results. The other peaks such as Cu and C come from copper grid and carbonisation.
3.2 Photoluminescence properties
To further characterize the optical responses of our samples, we present in the following, the different luminescence properties of the nanoparticles in the silica host matrix. The PL spectra of Zn2SiO4, Zn2SiO4:V and Zn2SiO4:Mn nanoparticles at different temperatures are shown in Figs. 5, 6 and 7, respectively. In the case of Zn2SiO4, the striking feature is the absence of almost any of the usually reported visible emission bands in the range of 450–650 nm (2.4–2.75 eV), and the presence of a strong and wide near infrared (NIR) emission band centered around 760 nm, besides a near band edge emission including the bound exciton line [34] (Fig. 5). The observed UV–visible emission band is also quite different from what is usually seen [34].
The PL spectrum of the Zn2SiO4:V nanoparticles consists of a very intensive emission band located in the green–yellow spectral range centred at 540 nm (Fig. 6). We have studied the intensity and the peak energy dependence of this PL band versus the measurement temperature. The intensity of this luminescence band decreases as the temperature increases. Furthermore, no change in the PL peak position was observed with the measurement temperature. The shape and the energy location of the PL emission bands are characteristic of a deep level emission. Nine very broad emission bands appear between 450 and 690 nm with vibrational progressing of Δγ = 970 cm−1corresponds to the transitions from the lowest vibrational level of the excited triplet state T 1(V 4+ − O −) to the various vibration levels of the ground state S 0(V 5+ = O 2−) [35]. In our previous works, using the same protocol, we have seen a similar behavior after incorporation of Al2O3 in silica but not with the incorporation of ZnO in silica [34]. This result is in agreement with the studies of Dzwigaj et al. [36], i.e., that the vanadium-related species surrounded by silanol groups improve the luminescence intensity of these broad emission bands in the visible range.
On the other hand, in the case of Zn2SiO4:Mn nanoparticles, the green emission has been assigned to an electronic transition of 4T1(4G) → 6A1(6S) peaking at the wavelength 525 nm and which is a parity forbidden emission transition of Mn2+ ions [33]. The graph shows that the relative PL intensity of the nanocomposites decreases as the measurement temperature increases (Fig. 7). It is generally recognized that the luminescence of the Mn2+ ion depends on the Zn2SiO4 host crystal field. Mn2+ ions in the Zn2SiO4 host with higher crystallinity feel a stronger crystal field which corresponds to the reduction of the energy difference of the ground and first excited state, resulting in peak broadening and red-shift of the emission peak [33]. This emission centered at 525 nm, corresponds to the d–d transition of Mn2+ through energy transfer from Zn2SiO4 [32, 33], in this case Mn2+ occupies part of the Zn2+ sites, which is coordinated by four oxygen atoms [33]. The weak crystal field around Mn2+ results in the low splitting width of its 3d energy levels, in accordance with the observations of Stevels et al. [37].
3.3 Photoluminescence excitation properties
PLE spectra of our samples at the measurement temperature T = 78 K are shown in Fig. 8. In the case of undoped Zn2SiO4 nanoparticles (inset of Fig. 8), the PLE spectrum detected at 760 nm shows the presence of a very weak peak at 375 nm (3.3 eV). This energy excitation band is due to carrier excitation in the near band edge of ZnO nanoparticles. Indeed, as it has been shown by Chakrabarti et al. [38], a high annealing temperature (1073 K) results in a rapid grain growth and when the radii of the nanoparticles increases to 8.2 nm, a bulk ZnO like band gap is obtained. However, the most efficient excitation process is with photon energies of about 5.4 eV (230 nm), which are much higher than the ZnO bulk band gap [34]. Unfortunately, the high energy peak position of the PLE spectrum cannot be clearly determined due to the high energy range limit of our setup. The shape and the structured nature of the PL emission band and the large shift between the PL and the PLE energy peaks of the 760 nm PL emission are in principle a signature of a deep level emission with an electron–phonon coupling [34]. We suggest that this luminescence band (760 nm) is attributed to the contribution at the formation of NBOHcs excited at the spectral region hν ≥ 5.4 eV, such band arises from the absorption of Zn2SiO4 particles. On the other hand, the PLE spectrum of the Zn2SiO4:V nanoparticles detected at 540 nm shows the appearance of an excitation band ranging from 240 to 350 nm with a maximum at about 250 nm. This excitation band was attributed to the charge transfer (CT) in Zn2SiO4 lattice [32, 39]. The large shift between the PL and PLE energy peaks, the shape and the structured nature of the PL emission bands are very close to those of hydroxyl-related species [40] with small shift, probably due to the change of the environment. Recently, the excitation spectra of Zn2SiO4:Mn nanoparticles of the band detected at 525 nm show an intense and a broadband peak (Fig. 8b), which is ascribed to the CT band of Mn2+ in the Zn2SiO4 system as reported by Mishra et al. [41]. In addition to the CT band, other bands of Mn2+ (d–d) transition are also observed at higher wavelengths; these are caused by the splitting of the 4D and 4G levels because of the crystal field, as shown by the Orgel diagram for Mn2+ [25, 39, 42]. The electrons at the 6A1(6S) ground state of Mn2+ ions, which originate from the photoexcited ionization of Mn2+, are excited to the conduction band of Zn2SiO4 by photons, and the free electrons in the conduction band relax back to the 4T1(4G) excited state through a non-radiative process [43]. Finally, this is followed by radiative transition from the 4T1(4G) excited state to the 6A1(6S) ground state, emitting green light (525 nm). This happens when Mn ions occupy the tetrahedral sites of the willemite structure.
3.4 Decay time
The photoluminescence decay curve of our nanocomposites at 78 K is shown in Fig. 9. The curves show biexponential decay, indicating that the lifetimes of excited luminescent centers are composed of two components: a very fast recombination and a fast one. By fitting, in the case of the Zn2SiO4:V nanocomposite corresponding to the emission band at 540 nm (Fig. 9a), we find that the very fast component lifetime is 2 ns comparable to time constant of our setup while the slower one is 13 ns. The obtained lifetimes of some nanoseconds are much shorter than the ∼1 ms of bulk silica [44]. Thus, the obtained values confirm our assertion that the band in the PL spectra of the composite is due to interface defects [44]. In the case of Zn2SiO4:Mn nanocomposite (Fig. 9b), the curve presents a bi-exponential decay, indicating that the lifetimes of Mn2+-excited states are composed of two components [25]. After fitting, we obtained a fast component is 2 ms and a slow component is 11 ms. This result is in agreement with the previous observations [25]. This result suggests that this emission decay is due to isolated manganese ions. It is possible to reduce the decay time of a phosphor by increasing the manganese doping content. Although there is not yet a sound theoretical model to explain this phenomenon, Ronda and Amrein [45] associate the effect with an exchange interaction between Mn2+ ions forming pairs at higher Mn concentration, and show that this makes the spin-forbidden transition partially allowed and as a result the transition oscillator strength larger. This model, however, fails to explain the temperature dependence of the decay time [25].
4 Conclusion
In conclusion, the photoluminescence characteristics and the behavior of the phosphor formation of the phosphor particles with fine size were investigated. The PL spectra of the Zn2SiO4 nanoparticles showed a strong near-infrared luminescence band and the absence of the commonly reported visible emission bands. From the analysis of the PL and PLE spectra, it can be concluded that the excitation peak near 760 nm can be attributed to the formation of NBOHcs excited at the spectral region hν ≥ 5.4 eV, such band arises from the absorption of Zn2SiO4 particles. In the case of Zn2SiO4:V nanoparticles, the PL spectra showed that a dominant peak at 540 nm appeared, which originated from the V in the interfaces between Zn2SiO4 particles and SiO2 host matrix. From the analysis of the PL and PLE spectra of this sample, it can be concluded that the luminescence band at 525 nm can be attributed to Mn2+ in Zn2SiO4 particle. In the present works, nanosized Zn2SiO4 nanoparticles can be obtained by the sol–gel method with decay time varying from ms to ns range adjustable by the doping element. This sol–gel process should be improved to obtain well-crystallized Zn2SiO4 phosphors with excellent luminescence properties without following high heat treatment.
References
C. Yoon, S. Kang, J. Mater. Res. 16, 1210 (2001)
X. Li, F. Chen, Mater. Res. Bull. 48, 2304–2307 (2013)
A. Polman, J. Appl. Phys. 82, 1–39 (1999)
S. Heer, K. Petermann, H.U. Gudel, J. Lumin. 102, 144–150 (2003)
A. Patra, C.S. Friend, R. Kapoor, P.N. Prasad, J. Phys. Chem. B 106, 1909–1912 (2002)
J. Silver, M.I. Martinez-Rubio, T.G. Ireland, G.R. Fern, R.J. Withnall, Phys. Chem. B. 105, 948–953 (2001)
A. Patra, C.S. Friend, R. Kapoor, P.N. Prasad, Chem. Mater. 15, 3650–3655 (2003)
A. Patra, D. Kundu, D. Ganguli, Mater. Lett. 32, 43–47 (1997)
A. Patra, E. Sominska, S. Ramesh, Yu. Kolypin, Z. Zhong, H. Minti, R. Reisfeld, A. Gedanken, J. Phys. Chem. B 103, 3361–3365 (1999)
J. El Ghoul, C. Barthou, M. Saadoun, L. El Mir, Phys. B 405, 597 (2010)
J. El Ghoul, C. Barthou, L. El Mir, Superlattices Microstruct. 51, 942 (2012)
J. El Ghoul, N. Bouguila, S.A. Gómez-Lopera, L. El Mir, Superlattices Microstruct. 64, 451 (2013)
R.N. Bhargava, D. Gallaghar, X. Hong, A. Nurmikko, Phys. Rev. Lett. 72, 416–419 (1994)
N. Taghavinia, G. Lerondel, H. Makino, A. Parisini, A. Yamamoto, T. Yao, Y. Kawazoe, T. Goto, J. Lumin. 96, 171–175 (2002)
E.S. Park, T.H. Cho, H.J. Chang, Appl. Chem. 1, 406–409 (1997)
M. Abdullah, S. Shibamoto, K. Okuyama, Opt. Mater. 26, 95–100 (2004)
T.H. Cho, H.J. Chang, Ceram. Int. 29, 611–618 (2003)
A. Shila, D.G. Jeannette, C. Brigitte, Mater. Res. Bull. 43, 2751–2762 (2008)
Q. Lu, G. Yun, Ceram. Int. 39, 3533–3538 (2013)
J. Lin, Q. Su, J. Mater. Chem. 5, 603–606 (1995)
W.C. Wang, Y.T. Tian, K. Li, E.Y. Lu, D.S. Gong, X.J. Li, Appl. Surf. Sci. 273, 372–377 (2013)
J. El Ghoul, K. Omri, S.A. Gómez-Lopera, L. El Mir, Opt. Mater. 36, 1034 (2014)
J. El Ghoul, C. Barthou, L. El Mir, Phys. E 44, 1910 (2012)
C. Barthou, J. Benoit, P. Benalloul, A. Morell, J. Electrochem. Soc. 141, 524–528 (1994)
J. El Ghoul, K. Omri, A. Alyamani, C. Barthou, L. El Mir, J. Lumin. 138, 218 (2013)
Q.Y. Zhang, K. Pita, W. Ye, W.X. Que, Chem. Phys. Lett. 351, 163 (2002)
J. El Ghoul, L. El Mir, J. Lumin. 148, 82 (2014)
J. El Ghoul, K. Omri, L. El Mir, C. Barthou, S. Alaya, J. Lumin. 132, 2288 (2012)
J. El Ghoul, C. Barthou, M. Saadoun, L. El Mir, J. Phys. Chem. Solids 71, 194–198 (2010)
J. El Ghoul, C. Barthou, M. Saadoun, L. El Mir, J. Phys. B 405, 597–601 (2010)
B.D. Cullity, Elements of X-Ray Diffraction (Addison-Wesley, Reading, 1978), p. 102
Y. Jiang, J. Chen, Z. Xie, L. Zheng, J. Mater. Chem. Phys. 120, 313–318 (2010)
M. Takesue, H. Hayashi, R.L. Smith Jr., A review. J. Prog. Cryst. Growth Charact. Mater. 55, 98–124 (2009)
L. El Mir, A. Amlouk, C. Barthou, J. Phys. Chem. Solids 67, 2395–2399 (2006)
M. Anpo, S. Higashimoto, M. Matsuoka, N. Zhanpeisov, Y. Shioya, S. Dzwigaj, M. Che, Catal. Today 78, 211–217 (2003)
S. Dzwigaj, M.M. Che, J. Phys. Chem. B 109, 22167–22174 (2005)
A.L.N. Stevels, A.T. Vink, J. Lumin. 8, 443–446 (1974)
S. Chakrabarti, D. Das, D. Ganguli, S. Chaudhuri, Thin Solid Films 441, 228–237 (2003)
L.E. Orgel, J. Chem. Phys. 23, 1819–1823 (1955)
G. Gao, R. Meszaros, M. Peng, L. Wondraczek, Opt. Express 19, A312–A318 (2011)
K.C. Mishra, K.H. Johnson, B.G. DeBoer, J.K. Berkowitz, J. Olsen, E.A. Dale, J. Lumin. 47, 197–206 (1991)
A. Morell, N. El Khiati, J. Electrochem. Soc. 140, 2019–2022 (1993)
L. Xiong, J. Shi, J. Gu, L. Li, W. Huang, J. Gao, M. Ruan, J. Phys. Chem. B 109, 731–736 (2005)
M. Goldberg, H.-J. Fitting, A. Trukhin, Non-Cryst. Solids 220, 69–77 (1997)
C.R. Ronda, T. Amrein, J. Lumin. 69, 245–248 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Ghoul, J., El Mir, L. Synthesis by sol–gel process, structural and luminescence of V and Mn doped α-Zn2SiO4 . J Mater Sci: Mater Electron 26, 3550–3557 (2015). https://doi.org/10.1007/s10854-015-2868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2868-8