Abstract
This paper describes a new approach for graft copolymerization of 2,2′-bithiophene onto a polystyrene (PSt) backbone by a multi-step process. For this purpose, first poly(styrene-co-4-chloromethyl styrene) [P(St-co-CMSt)] was synthesized via a free radical polymerization, and then the chlorine groups of P(St-co-CMSt) copolymer were converted to thiophene groups by a substitution nucleophilic reaction in the presence of a solvent composed of 2-hydroxymethylthiophene, sodium hydride, and dry tetrahydrofuran. The thiophene-functionalized polystyrene multicenter macromonomer obtained was used in oxidative copolymerization with 2,2′-bithiophene to yield a graft copolymer [polystyrene-graft-poly(2,2′-bithiophene) (PSt-g-PBT)]. The chemical structures of all samples as representatives were characterized by 1H nuclear magnetic resonance, and Fourier transform infrared spectroscopy. The thermal behaviors of the synthesized polymers were investigated by means of thermogravimetric analysis. Moreover, the conductivity of the PSt-g-PBT copolymer was measured by four-point probe method, and its electroactivity behavior was verified under cyclic voltammetric conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Intrinsically conductive polymers (ICPs) have attracted significant attention in recent decades because of their wide range of potential applications in various fields such as chemistry, physics, electronics, optics, materials, and biomedical sciences [1–4]. On the other hand, since the discovery of doped polyacetylene as a conducting polymer in 1977 it is well demonstrated that only a few polymers are stable enough under normal processing conditions to be incorporated in various practical and technological applications. Among leading candidates are polythiophene (PTh), polyaniline (PANI), and polypyrrole (PPy) [5–9]. In this respect, poly(2,2′-bithiophene) (PBT) and its derivatives stands out as the most promising members of the thiophene-based conducting polymers family because of their a high degree of π-conjugation due to their rigidified, and planar structure which intrinsically affords smaller HOMO–LUMO gaps [10–12]. Moreover, 2,2′-bithiophene can be polymerized under mild conditions due to its lower oxidation potential in comparison with thiophene, and predominantly yields higher quality α-linked polymer compared to that prepared from thiophene monomer by inhibiting the formation of α,β-defects [13, 14].
PBT and its derivatives have attracted significant attention in recent years because of their wide range of potential applications in various fields including electronic devices such as organic or polymer based light-emitting diodes (OLEDs/PLEDs), photovoltaic cells, field-effect transistors (FETs), electrochromic or smart windows, photoresists, nonlinear optical (NLO) devices, and polymeric sensors [11, 14, 15]. However, the main drawback of poly(2,2′-bithiophene) similar to other π-conjugated polymers is lack of solubility which explains its limited processability due to its rigid backbone. In order to overcome these major deficiencies, the most commonly used approach is side chain functionalization [16, 17], and to the best of our knowledge, the synthesis of poly(2,2′-bithiophene) copolymers with conventional thermoplastics was neglected.
In previous studies, the synthesis and characterization of PSt-g-PTh by two different methodologies have been investigated [18, 19]. The present work, designed an effective route for the graft copolymerization of 2,2′-bithiophene onto thiophene-functionalized polystyrene multicenter macromonomer (ThPStM) via the oxidation polymerization method. For this purpose, a thiophene-functionalized polystyrene macromonomer (ThPStM) was synthesized by a substitution nucleophilic reaction between poly(styrene-co-4-chloromethyl styrene), and 2-hydroxymethylthiophene in the superbasic medium (NaH/THF). The synthesized macromonomer (ThPStM) was subsequently used in the graft copolymerization of 2,2′-bithiophene monomers onto polystyrene backbone via chemical oxidative polymerization in the presence of anhydrous FeCl3 to produce a PSt-g-PBT graft copolymer.
2 Experimental
2.1 Materials
Styrene and 4-chloromethyl styrene monomers from Merck (Darmstadt, Germany) were distilled under reduced pressure before use. 2,2′-Azobis(isobutyronitrile) (AIBN) from Merck was recrystallized in ethanol at 50 °C. Tetrahydrofuran (THF), and toluene from Merck were dried by refluxing over sodium and distilled under argon prior to use. Tetrabutylammonium perchlorate (Bu4NClO4), 2-bromothiophene, dichloro[1,3-bis(diphenylphosphino)propane]nickel(II) [Ni(dppp)Cl2], ferric chloride (FeCl3), and thiophene-2-carbaldhyde were purchased from Merck, and were used as received. All solvents (Merck) were purified with the literature methods and distilled right before used.
2.2 Synthesis of poly(styrene-co-4-chloromethyl styrene)
In a two-neck round-bottom flask equipped with condenser, gas inlet/outlet, and a magnetic stirrer, styrene and 4-chloromethyl styrene monomers with different feed ratios of monomers (styrene:4-chloromethyl styrene ≈ 1:1 and 3:1) were dissolved in anhydrous toluene. The initiator (AIBN) was added to the solution, and the mixture was de-aerated by bubbling highly pure argon for 20 min. The mixture was heated to 70 °C (external temperature) in an oil bath and stirred for 48 h, under argon atmosphere to complete the polymerization. At the end of this period, the polymer was precipitated in excess methanol. The product was dried overnight under vacuum at room temperature (Scheme 1).
2.3 Reduction of thiophene-2-carbaldhyde to 2-hydroxymethylthiophene
To a well-stirred solution of 0.89 g (15.8 mmol) KOH, and 1.46 g (38.6 mmol) NaBH4 in 8 ml H2O, 5.84 ml (63.6 mmol) thiophene-2-carbaldhyde solution in 100 ml ethanol was added. After the addition was complete, the mixture was stirred at room temperature for about 2 h. The mixture was then washed with 100 ml of water and the aqueous layer was extracted with an additional 50 ml CHCl3. The combined organic layers were dried (using MgSO4), filtered, and concentrated in a rotary evaporator to afford of yellow liquid product (Scheme 2).
2.4 Synthesis of thiophene-functionalized polystyrene multicenter macromonomer (ThPStM)
In a three-neck round-bottom flask equipped with condenser, dropping funnel, gas inlet/outlet, and a magnetic stirrer, 0.50 g (4.4 mmol) of 2-hydroxymethylthiophene was dissolved in anhydrous THF (30 ml), and added under argon atmosphere to 0.184 g (7.2 mmol) of n-hexane-washed NaH (60 % suspension in oil). The mixture was stirred for about 30 min, and 1.50 g of P(St-co-CMSt) was then added under an argon atmosphere, and refluxed for about 24 h at 65 °C. The thiophene-functionalized polystyrene macromonomer (ThPStM) was separated by precipitation in excess methanol, filtered, washed several times with methanol, and dried in vacuum at room temperature (Scheme 3).
2.5 Synthesis of 2,2′-bithiophene (BT)
In a flame-dried three-neck round-bottom flask (flask 1) equipped with condenser, dropping funnel, gas inlet/outlet, and a magnetic stirrer, 1.38 g (57 mmol) magnesium and 0.05 g (0.2 mmol) of iodine was dissolved in anhydrous diethyl ether (50 ml). This mixture was refluxed under an argon atmosphere for about 30 min. At the end of this period, 3.67 ml (38 mmol) of 2-bromothiophene was added to the mixture, and then the mixture was refluxed for about 3 h at room temperature.
In a separate flame-dried three-neck round-bottom flask (flask 2) equipped with condenser, septum, gas inlet/outlet, and a magnetic stirrer, 3.67 ml, (38 mmol) 2-bromothiophene, and 0.15 g (0.285 mmol) of Ni(dppp)Cl2 was dissolved in 30 ml anhydrous diethyl ether. The solution was de-aerated by bubbling highly pure argon for 20 min, and then the Grignard reagent (2-thienylmagnesium bromide; flask 1) was introduced with a syringe through the septum. The mixture was refluxed at room temperature. The reaction was quenched by dilute HCl after 20 h. The organic phase was separated, and combined with the diethyl ether extraction from the aqueous phase. Thereafter, the ether extracts were washed with water and brine, dried (using MgSO4) and evaporated under reduced pressure to produce a crude product. The crude product was further purified by flash column chromatography using n-hexane as the eluent (Scheme 4).
2.6 Graft copolymerization of 2,2′-bithiophene onto thiophene-functionalized polystyrene
In a three-necked round-bottom flask equipped with condenser, dropping funnel, gas inlet/outlet, and a magnetic stirrer, 1 g of macromonomer (ThPStM), and 2,2′-bithiophene monomer (1 g, 6 mmol) were dissolved in 60 ml of dried carbon tetrachloride (CCl4). The solution was de-aerated by bubbling highly pure argon for 20 min. In a separate container 3.9 g (24 mmol) of ferric chloride (FeCl3) was dissolved in 20 ml of dried acetonitrile (CH3CN). The solution was de-aerated by bubbling highly pure argon for 20 min and was slowly added to the mixture under an argon atmosphere. The mixture was refluxed for 24 h at room temperature under inert atmosphere. The reaction was terminated by pouring the contents of the flask into a large amount of methanol. The crude product was filtered and washed several times with methanol (Scheme 5).
The crude product was extracted with THF in a Soxhlet apparatus for 24 h in order to remove pure poly(2,2′-bithiophene) (PBT is not soluble in THF, see Table 1). The polymer solution was filtered and precipitated into excess methanol. Afterwards, the product was added into cyclohexane and refluxed at 40 °C to remove any residual ungrafted polystyrene chains. The synthesized PSt-g-PBT is partially soluble in cyclohexane, while the ungrafted polystyrene is completely soluble in cyclohexane at 40 °C. The dark red solid was filtered and dried in vacuum at room temperature.
2.7 Characterization
Fourier transform infrared (FTIR) spectra of the samples were recorded on a Shimadzu 8101M FTIR (Shimadzu, Kyoto, Japan). The samples were prepared by grinding the dry powders with potassium bromide (KBr) and compressing the mixture into disks. The disks were stored in a desiccator to avoid moisture absorption. The spectra were recorded at room temperature. 1H nuclear magnetic resonance (NMR) spectra were recorded at 25 °C using an FT-NMR (400 MHz) Bruker spectrometer (Bruker, Ettlingen, Germany). The sample for NMR spectroscopy was prepared by dissolving about 10 mg of samples in 1 ml of deuterated chloroform (CDCl3), and chemical shifts were reported in ppm units with tetramethylsilane as internal standard. Thermal properties of the samples were examined by thermogravimetric analyzer [TGA-PL STA 1640 equipment (Polymer Laboratories, Shropshire, UK)]. The TGA experiments were conducted under nitrogen atmosphere in a temperature range of 25–700 °C with heating rate of 10 °C min−1. The electrochemical studies were carried out with Auto-Lab PGSTA T302N. The electrochemical cell contained five openings: three of them were used for the electrodes and two for argon bubbling in the solutions during all experiments. The four probe technique (Azar Electrod, Urmia, Iran) was used to measure the conductivity of the PSt-g-PBT and pure PBT samples at room temperature. Electrical conductivity measurements were carried out on disk shaped test prepared by compression molding of the synthesized materials between two Ni foils by a laboratory press.
3 Results and discussion
Creative design and developmental strategies of new polythiophenes has led to interesting new materials with improved physicochemical properties and potential for various technological applications. In order to overcome some drawback of unsubstituted poly(2,2′-bithiophene) such as insolubility and inprocessability an efficient method is synthesis of its copolymers with conventional thermoplastics. As illustrated in Scheme 5, in this study a thiophene-functionalized polystyrene was used as the macromonomer for the 2,2′-bithiophene monomer grafts via the chemical oxidation polymerization approach.
3.1 Synthesis of poly(styrene-co-4-chloromethyl styrene) copolymers
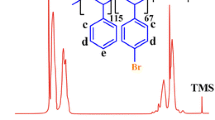
The 1H NMR spectra of the P(St-co-CMSt) copolymers with mole ratios of 1:1 and 3:1 are shown in Fig. 1. The 1H NMR spectra of the P(St-co-CMSt) copolymers indicate the chemical shifts at 0.80–2.20 and 6.20–7.40 ppm represent the aliphatic (a and b) and aromatic (d, e, and f) protons, respectively. The chemical shift at 4.50 ppm shows the –CH2Cl protons (c) in the P(St-co-CMSt) copolymers. Moreover, the 4-chloromethyl styrene content in the copolymers was calculated to be 43 and 23 % (by mole) based on peak at 4.50 ppm (–CH2Cl) in the 1H NMR spectra for copolymers with experimental mole ratios of 1:1 and 3:1, respectively [19]. Thus, hereinafter the mole ratios of copolymers are corrected to 1.3:1 and 3.4:1.
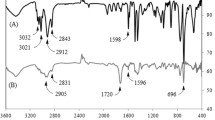
The FTIR spectra of the P(St-co-CMSt) copolymers with mole ratios of 1.3:1 and 3.4:1 are shown in Fig. 2. The FTIR spectra of the P(St-co-CMSt) copolymers shows the characteristic absorption bands due to the stretching vibrations of C–H (3,100–2,800 cm−1), weak aromatic overtone and combination bands in the 2,000–1,650 cm−1 region, CH2 bending vibrations (1,447 and 1,376 cm−1), C=C stretching vibrations at 1,603 and 1,492 cm−1, and γ (C–H) in the aromatic ring (845 and 768 cm−1). The strong peak at 702 cm−1 is attributed to the C–Cl group in the FTIR spectra of the P(St-co-CMSt) copolymers. In addition, the bending vibration from the CH2-Cl group can be seen at around 1,274 cm−1.
3.2 Synthesis of 2-hydroxymethylthiophene and 2,2′-bithiophene
The FTIR spectra of the 2-hydroxymethylthiophene and 2,2′-bithiophene are shown in Fig. 3. The FTIR spectrum of the 2-hydroxymethylthiophene shows the characteristic absorption bands due to the stretching vibrations of C–H appear in the 3,000–2,800 cm−1 region, the stretching vibration of C=C at 1,626 cm−1, C–O at 1,042 cm−1 and the C–S bending band at 826 cm−1. Moreover, the broad band at 3,417 cm−1 is due to the hydroxyl stretching vibration. The FTIR spectrum of the 2,2′-bithiophene shows the characteristic absorption bands due to the stretching vibration of C–H (3,150–3,020 cm−1), and C–S stretching vibrations (683 and 841 cm−1). The peak absorptions at 1,218 and 1,447 cm−1 can be attributed to the stretching of the thiophene ring.
The 1H NMR spectra of the 2-hydroxymethylthiophene and 2,2′-bithiophene are shown in Fig. 4. The 1H NMR spectrum of the 2-hydroxymethylthiophene indicate the chemical shifts at 2.84 and 4.77 ppm represent the hydroxyl (a), and aliphatic –CH2 protons (b), respectively. The chemical shifts at 6.90–7.30 ppm shows the thiophene ring protons. The 1H NMR spectrum of the 2,2′-bithiophene indicate the chemical shifts at 7.00–7.20 represent the aromatic protons of the 2,2′-bithiophene.
3.3 Synthesis of macromonomer
The development of novel polymeric materials usually requires the use of highly selective and efficient functionalization reactions. In this respect, substitution nucleophilic reaction has been proposed as one of the most powerful tool for functionalization of polymeric materials. At first in order to produce the active sites on poly(styrene-co-4-chloromethyl styrene) copolymers, thiophene moiety was added to the P(St-co-CMSt) copolymers. So, 2-hydroxymethylthiophene reacts with P(St-co-CMSt) copolymer by a substitution nucleophilic reaction to give a thiophene-functionalized polystyrene multicenter macromonomers (ThPStM).
The 1H NMR spectra of the macromonomers (ThPStM) with different feed ratios of monomers (styrene:4-chloromethyl styrene ≈ 1.3:1 and 3.4:1) are shown in Fig. 5. In comparison with 1H NMR spectra of the P(St-co-CMSt) copolymers these 1H NMR spectra show similar peaks with minor differences. The most distinctive features in the 1H NMR spectra of macromonomers and P(St-co-CMSt) copolymers are the appearance of new peak at 4.65 ppm, corresponding to four aliphatic protons of Th-CH2-O-CH2-Ph (d and e) groups. Furthermore, before introducing 2-oxymethylthiophene moiety to the P(St-co-CMSt) copolymers, the molar compositions of 4-chloromethyl styrene in the copolymers were 43 and 23 % for copolymers with mole ratios of 1.3:1 and 3.4:1, respectively. After introducing 2-oxymethylthiophene moiety a decrease (to 35 and 18 %) in the molar compositions of 4-chloromethyl styrene in the macromonomers, can be attributed to the fact that the 2-oxymethylthiophene moiety were introduced into the P(St-co-CMSt) copolymers, and that the ThPStM macromonomers were successfully synthesized. Therefore, the extent of 2-oxymethylthiophene groups in the macromonomers are 43 − 35 % = 8 % and 23 − 18 % = 5 %. Additionally, we can report that in P(St-co-CMSt) copolymers with mole ratios of 1.3:1 and 3.4:1, the conversion of chlorine groups to the 2-oxymethylthiophene moiety were 19 and 22 %, respectively.
The FTIR spectra of the ThPStM macromonomers are shown in Fig. 6. In comparison with the FTIR spectra of the P(St-co-CMSt) copolymers the FTIR spectra of the macromonomers does not exhibit any distinct bands due to the overlapping.
3.4 Synthesis of PSt-g-PBT
The generally accepted mechanism for the oxidative polymerization of thiophene and its derivatives involves the formation of radical cations. In this study, two kinds of cation radicals initiate 2,2′-bithiophene polymerization after addition of ferric chloride. One is oxidized thiophene moieties coupled to the polystyrenic chains; the other is oxidized 2,2′-bithiophene cation radicals. As the reaction time is increased, more 2,2′-bithiophene monomers join in the polymerization. Some are entrapped into the polymer initiated by oxidized thiophene moieties coupled to the PSt chains; others incorporate polymer chains initiated by oxidized 2,2′-bithiophene cation radicals. These polymer chains could not be linked chemically to the polystyrene backbone. To remove the ungrafted PBT chains, the crude product was purified as described in the Sect. 2.
The FTIR spectra of the P(St-co-CMSt)(1.3:1)-g-PBT, and P(St-co-CMSt)(3.4:1)-g-PBT are shown in Fig. 7. These FTIR spectra show similar bands with minor differences. The FTIR spectra shows the characteristic absorption bands due to the stretching vibrations of C–H (3,050–2,900 cm−1), S=C stretching vibrations at 824 and 1,063 cm−1, and C=C stretching vibrations at 1,602 and 1,468 cm−1. The peak absorptions at 1,358 and 1,502 cm−1 can be attributed to the stretching of the thiophene ring. The absorption band from free charge carriers created from polymerization reaction is observed at 2,357 cm−1. Moreover, the peaks at 702 and 1,274 cm−1 (stretching and bending vibrations of CH2–Cl group, respectively) indicate that some of the chlorine group’s does not react with the 2-hydroxymethylthiophene moiety.
Additional evidence for the synthesis of the PSt-g-PBT graft copolymers was also obtained from 1H nuclear magnetic resonance (NMR) spectroscopy. The 1H NMR spectra of the P(St-co-CMSt)(1.3:1)-g-PBT, and P(St-co-CMSt)(3.4:1)-g-PBT graft copolymers are shown in Fig. 8. To calculate the extent of the PSt chains and PBT in the graft copolymers the following method was adopted. The ratio of aliphatic hydrogens to aromatic hydrogens in the P(St-co-CMSt)(1.3:1) is 3:(0.43 × 4) + (0.57 × 5) ≈ 3.05:5.03; after graft copolymerization of bithiophene onto PSt chains, this ratio changed to 3:(0.43 × 4) + (0.57 × 5) + 4X ≈ 4.99:10.9, where X is the percentage of PBT in the graft copolymer, 0.43 and 4 are the percentage of PCMSt and the number of its aromatic hydrogens in the P(St-co-CMSt)(1.3:1) part, 0.57 and 5 are the percentage of PSt and the number of its aromatic hydrogens in the P(St-co- CMSt)(1.3:1) part, and 4 is the number of aromatic hydrogens in the PBT segments. In Fig. 8, by solving the equations, it can be seen that X = 49.7 % and X = 18.6 % (by mole) for P(St-co-CMSt)(1.3:1)-g-PBT, and P(St-co-CMSt)(3.4:1)-g-PBT graft copolymers, respectively [18, 19].
3.5 Thermal property study
The thermal behaviors of the P(St-co-CMSt) copolymers (a and b), and PSt-g-PBT graft copolymers (c and d) upon heating in nitrogen atmosphere were investigated by means of thermogravimetric analysis (TGA). As shown in Fig. 9 the thermal decomposition of the P(St-co-CMSt) copolymers are started from 250 °C and the weight loss increases rapidly from this temperature to about 380 °C, after which the loss rate slows down. However, the TGA curve of the P(St-co-CMSt)-g-PBT graft copolymers exhibits a two-step weight loss process; the first step corresponds to the decomposition of the polystyrene chains (280–390 °C), whereas the second step is associated with the poly(2,2′-bithiophene) chains scission (390–680 °C). Moreover, in compression with P(St-co-CMSt)(3.4:1)-g-PBT the P(St-co-CMSt)(1.3:1)-g-PBT graft copolymer showed higher thermal stability due to its higher weight percent of PBT (49.7 %).
3.6 Solubility test
The solubility of the pure PBT, and P(St-co-CMSt)-g-PBT graft copolymers in common organic solvents are summarized in Table 1. Polystyrene has excellent solubility in non-polar solvents. The solubility of P(St-co-CMSt)-g-PBT graft copolymers in common organic solvents improved compared to pure PBT, because 2,2′-bithiophene has grown onto polystyrene backbone.
3.7 Electrical conductivity and electroactivity measurements
Unlike to other type of polymers, π-conjugated polymers exhibit conducting and/or semiconducting behaviors and thus serve as potential candidates for both optical and electronic applications [20–22]. The electrical resistivity (R) of the synthesized samples, was measured at room temperature and then converted to volume specific resistivity (ρ) using the equation R = ρL/A. In this equation, A is the test sample cross-section area and L the sample thickness. Finally, the electrical conductivity (σ) is the reciprocal of ρ(σ = 1/ρ). Table 2 summarizes the results obtained for pure PBT and P(St-co-CMSt)-g-PBT graft copolymers.
The cyclic voltammograms (CVs) of the synthesized samples in the acetonitrile-tetrabutylammonium perchlorate, solvent-electrolyte couple (0.1 mol l−1), at a scan rate of 100 mV s−1 between 0.0 and +1.60 V versus the Ag/AgCl electrode are shown in Fig. 10. In cyclic voltammograms (CVs) of the pure PBT the oxidation and reduction peak currents are observed at approximately 1.14 and 0.72 V versus Ag/AgCl, respectively. The CVs of the P(St-co-CMSt) copolymer does not exhibit any detectable redox peak. Thus this sample is a non-electroactive polymer. As shown in Fig. 10, when cyclic voltammograms (CVs) of the P(St-co-CMSt)(1.3:1)-g-PBT, and P(St-co-CMSt)(3.4:1)-g-PBT graft copolymers are recorded between 0.0 and +1.60 V versus the Ag/AgCl electrode for both copolymers the oxidation and reduction peak currents are observed at approximately 1.17 and 0.75 V versus Ag/AgCl, respectively. Moreover, in compression with P(St-co-CMSt)(3.4:1)-g-PBT the P(St-co-CMSt)(1.3:1)-g-PBT copolymer showed higher electroactivity due to its higher weight percent of PBT (49.7 %).
4 Conclusion
In summary, we have demonstrated herein a novel, and efficient approach for the graft copolymerization of 2,2′-bithiophene onto a polystyrene backbone via the oxidative polymerization method. The synthesis of PSt-g-PBT graft copolymers were confirmed by FTIR and 1H NMR spectroscopy. The growth of 2,2′-bithiophene onto functionalized polystyrene backbone enhanced the solubility and processability compared with pure poly(2,2′-bithiophene). The electroactivity study under cyclic voltammetric conditions exhibited that the grafted PBT onto polystyrene backbone still remained a good redox activity in the resulting graft copolymers, and the synthesized graft copolymers were highly stable. The PSt-g-PBT graft copolymers exhibited lower electrical conductivity and electroactivity than those of the pure PBT due to the decreased conjugation length distribution. However, the lower electrical conductivity and electroactivity levels in the PSt-g-PBT copolymers can be improved at the price of solubility and processability. As a result, we envision that the synthesized conducting polystyrene-graft-poly(2,2′-bithiophene) could find applications in organic light-emitting diodes (OLEDs), photovoltaic cells, field-effect transistors (FETs), electrochromic or smart windows, photoresists, and nonlinear optical (NLO) devices, due to their good redox, conductivity, solubility, and processability properties.
References
S. Hunter, J. Chen, T.D. Anthopoulos, Adv. Funct. Mater. 24, 5969 (2014)
M. Jaymand, M. Hatamzadeh, Y. Omidi, Prog. Polym. Sci. (in press). doi:10.1016/j.progpolymsci.2014.11.004
M. Jaymand, Prog. Polym. Sci. 38, 1287 (2013)
B. Kumar, B.K. Kaushik, Y.S. Negi, J. Mater. Sci.: Mater. Electron. 25, 1 (2014)
G. Samsonidze, F.J. Ribeiro, M.L. Cohen, S.G. Louie, Phys. Rev. B. 90, 035123 (2014)
M.M. Pérez-Madrigal, M.I. Giannotti, E. Armelin, F. Sanz, C. Alemán, Polym. Chem. 5, 1248 (2014)
M. Jaymand, Design. Monomer. Polym. 14, 433 (2011)
B. Massoumi, M. Jaymand, R. Samadi, A.A. Entezami, J. Polym. Res. 21, 442 (2014)
P. Sehgal, A.K. Narula, J. Mater. Sci.: Mater. Electron. 25, 4793 (2014)
X. Zhang, A.J. Matzger, J. Org. Chem. 68, 9813 (2003)
T. Baumgartner, J. Inorg. Organometal. Polym. Mater. 5, 389 (2005)
R.V. Solomon, R. Jagadeesan, S.A. Vedha, P. Venuvanalingam, Dye. Pigment. 100, 261 (2014)
H. Tohma, M. Iwata, T. Maegawa, Y. Kiyono, A. Maruyama, Y. Kita, Org. Biomol. Chem. 1, 1647 (2003)
A.G. Macedo, D.C. Silva, N.A.D. Yamamoto, L. Micaroni, R.M.Q. Mello, L.S. Roman, Synthetic. Met. 170, 63 (2013)
T.W. Kelley, P.F. Baude, C. Gerlach, D.E. Ender, D. Muyres, M.A. Haase, D.E. Vogel, S.D. Theiss, Chem. Mater. 16, 4413 (2004)
Y. Fujinami, J. Kuwabara, W. Lu, H. Hayashi, T. Kanbara, ACS. Macro Lett. 1, 67 (2012)
L. Haiyan, F. Jäkle, Polym. Chem. 2, 897 (2011)
M. Hatamzadeh, M. Jaymand, RSC Adv. 4, 16792 (2014)
M. Hatamzadeh, M. Jaymand, B. Massoumi, Polym. Int. 63, 402 (2014)
S. Song, T. Kim, S.Y. Bang, M. Heo, J. Kim, Y. Jin, I. Kim, J.Y. Kim, H. Suh Synthetic. Met. 177, 65 (2013)
Y.A. Getmanenko, S.W. Kang, N. Shakya, C. Pokhrel, S.D. Bunge, S. Kumar, B.D. Ellman, R.J. Twieg, J. Mater. Chem. C. 2, 256 (2014)
J.H. Kim, Y.H. Seo, W.H. Lee, Y. Hong, S.K. Lee, W.S. Shin, S.J. Moon, I.N. Kang, Synthetic. Met. 161, 72 (2011)
Acknowledgments
We express our gratitude to the Payame Noor University for supporting this project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Massoumi, B., Javid, M., Hatamzadeh, M. et al. Polystyrene-graft-poly(2,2′-bithiophene): synthesis, characterization, and properties. J Mater Sci: Mater Electron 26, 2887–2896 (2015). https://doi.org/10.1007/s10854-015-2774-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2774-0