Abstract
This study systematically examined the thermoelectric (TE) properties of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) thin films by enhancing the electrical conductivity with D-sorbitol and by controlling the oxidation level with tetrakis (dimethylamino) ethylene (TDAE) vapor treatment. Mixing sorbitol into PEDOT:PSS changed the morphology of the films, increasing the electrical conductivity up to 722.06 S cm−1, which is over two orders of magnitude higher than that of pristine PEDOT:PSS (1.53 S cm−1). For even better TE properties, we subsequently exposed the films to TDAE vapor (which is a well-known reducing agent) for various treatment times to control their oxidation levels. The difference in the redox state as a function of the reduction time was easily identified by the naked eye owing to the electrochromic properties of PEDOT:PSS. As a result, we obtained a high power factor of 22.28 μW m−1 K−2 from the sorbitol-mixed PEDOT:PSS thin films that were treated via chemical reduction. This value is three times higher than that of devices without TDAE treatment (7.26 μW m−1 K−2). The properties and changes in the films fabricated with sorbitol and TDAE treatments were characterized by atomic force microscopy and UV–Vis-NIR absorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
For several years, researchers have tried to develop environmentally-friendly and renewable energy sources to replace fossil fuels, which are being depleted and cause environmental pollution. So far, many technologies have been reported for eco-friendly energy harvesting from natural sources including the sun, wind, waves, and so on. However, there are many constraints in terms of time, cost, and space for harnessing these natural energy sources. Meanwhile, semiconductor thermoelectric (TE) devices, which convert thermal energy into electricity, are considered to be a candidate for next-generation renewable energy systems because they can reuse waste heat and thermal energy from the nature to generate small amounts of power. TE devices were developed decades ago and are widely used for heating and cooling based on the Peltier effect. In their power generation mode, TE devices have the capability to directly convert heat to electricity via the Seebeck effect. Typical semiconductor materials used to fabricate TE devices are based on inorganic compounds, such as Bi2Te3, PbTe, and their complexes [1–4]. TE devices with these inorganic compounds typically show a high TE figure of merit (ZT), defined as ZT = S 2 σT/κ, where S, σ, T, and κ are the Seebeck coefficient, electrical conductivity, absolute temperature, and thermal conductivity, respectively. A high figure of merit implies high energy conversion efficiency. Nevertheless, these inorganic TE devices have not been widely utilized to generate TE power due to limits related to their size of fabrication, flat and rigid form factor, and high cost.
Recently, thin film TE devices based on organic molecules and conducting polymers have attracted attention for power generation. These materials are appealing due to their low cost and solution-processability, which allows for patterning on large areas [5–9]. Additionally, due to their mechanical flexibility/stretchability, they can be also applied to rounded surfaces that effuse heat (e.g., the external surfaces of exhaust pipes, engines, and turbines in a wide variety of applications ranging from automobiles to factory facilities). Moreover, organic materials and/or conducting polymers generally possess lower intrinsic thermal conductivity than inorganic compounds, indicating that a high ZT can be achieved. Researchers have reported the TE properties of conducting polymers using poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS), polyaniline, polyacetylene, polycarbazoles, polythiophene, and their derivatives [9–26]. The ZT of these organic semiconductors have been increased to ~10−1 [12, 24], meaning that the performance of organic TE devices are now quite close to inorganic-based TE devices. Among them, PEDOT:PSS is the most representative material for thin film TE devices due to its high electrical conductivity (>1,000 S cm−1) and good thermal stability [13, 14]. One easy way to improve the TE properties of PEDOT:PSS is to increase its electrical conductivity. There are many such techniques to do this, but the addition of a polar solvent with a higher dielectric constant, such as glycerol, ethylene glycol (EG), or dimethyl sulfoxide (DMSO), is one of the most widely-used methods [12, 16–19]. The electrical conductivity of PEDOT:PSS thin films treated with these solvent additives can be enhanced by two to three orders of magnitude due to the separation of PEDOT and PSS chains and/or selective doping effects [12, 16–19], which in turn improve the TE properties. For instance, several groups have demonstrated that by incorporating these solvents/additives into typical PEDOT:PSS, the TE performance can be enhanced, generating power factors (PFs) of 9.47–21.0 μW m−1 K−2 [20–23]. Several recent papers have introduced chemosynthetic methods to advance the electrical conductivity of PEDOT by adopting efficient counter-ions such as tosylate (Tos) and bis(trifluoromethylsulfonyl)imide (BTFMSI) instead of PSS [11, 24], and polymer–nanostructured material (e.g., carbon nanotubes and graphenes) composites [25–28].
However, high electrical conductivity does not always guarantee good TE properties because of the trade-off between electrical conductivity and the Seebeck coefficient. This is the case because the electrical conductivity is generally related to the carrier density. Increasing the carrier density tends to increase the thermal conductivity, resulting in a decrease of the Seebeck coefficient. Therefore, it is important to optimize the electrical conductivity and the Seebeck coefficient in order to improve the TE properties. For this purpose, a few methods have been reported to increase the Seebeck coefficient by tuning the oxidation level of PEDOT:PSS films using organic molecules or ionic liquids [11, 29–31].
In this work, we investigated the TE properties of PEDOT:PSS films by enhancing the electrical conductivity with D-sorbitol and by controlling the oxidation level with a tetrakis(dimethylamino)ethylene (TDAE) treatment. The effect of sorbitol on the characteristics, including the electrical conductivity of PEDOT:PSS, have been studied previously [32–34]. Also, in 2011, Park et al. [35] reported on organic photovoltaic devices using sorbitol-doped PEDOT:PSS as an interfacial layer . However, no further application of sorbitol-doped PEDOT:PSS films could be found. Sorbitol is a sugar alcohol as a nature product. It has high solubility in water, which is the solvent for PEDOT:PSS, and requires no additional annealing or treatment. Therefore, the TE performance of devices was observed as a function of their sorbitol concentration. We also optimized the electrical conductivity and Seebeck coefficient of the sorbitol-mixed PEDOT:PSS films by tuning the oxidation level with the TDAE vapor treatment. Using this method, the PF (S 2 σ) was increased up to 22.3 μW m−1 K−2, which is about three times higher than the films without TDAE treatment.
2 Experimental section
2.1 Materials
The PEDOT:PSS aqueous solution (Clevios PH 1000) was purchased from H. C. Starck GmbH. The concentration of PEDOT:PSS was 1.3 wt % dispersed in water, and the weight ratio of PEDOT to PSS was 2.5. D-sorbitol and TDAE were purchased from Sigma-Aldrich. All materials were used as-received. The chemical structures of PEDOT:PSS, D-sorbitol, and TDAE are shown in Fig. 1a.
2.2 Preparation of sorbitol-mixed PEDOT:PSS thin films
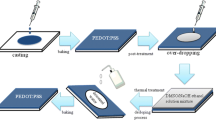
D-sorbitol was blended into PEDOT:PSS aqueous solutions at different concentrations (2,4, 6, 8, and 10 wt %) and then stirred for 12 h. The glass substrates (3 × 3 cm2) were sequentially cleaned with acetone, isopropyl alcohol, and deionized water in a series of ultrasonic baths. After drying, the substrates were treated with UV-ozone for 20 min to change the surface hydrophilicity. All mixed solutions were filtered through a 0.45-μm pore-size syringe filter and spin-coated onto the glass substrates at 2,000 rpm for 30 s. The thickness of the films was about 60–65 nm. Next, the samples were annealed on a hot plate at 150 °C for 30 min to remove any residual solvent.
2.3 TDAE vapor treatment of sorbitol-mixed PEDOT:PSS thin films
A small amount of the TDAE solution was dropped into a closed vessel to maintain the vapor atmosphere. After spin-coating the sorbitol-PEDOT:PSS blend solution on the substrates, the samples were placed in a vessel filled with TDAE vapor. We changed the oxidation level of the PEDOT:PSS films by varying the TDAE exposure time (10, 20, 30, and 40 min). After the reduction process, the films were annealed on a hot plate at 150 °C for 30 min.
2.4 Characterization of thermoelectric properties
Figure 1b presents the schematic diagram of the TE device measurement system for the PEDOT:PSS thin film TE devices used in this study. A sample was placed over two Peltier devices. By applying a different direct current to each Peltier module, a temperature gradient was created within the sample between the two electrodes and a thermovoltage was generated. To measure the temperature gradient, two type-K thermocouples were attached to the Au electrodes of the sample with thermal paste to ensure good thermal contact. The thermovoltage was measured with a digital multimeter (Agilent 3458A). The Seebeck coefficient was calculated from the measured data using the equation S = ΔV/ΔT, where ΔV and ΔT are the thermovoltage and temperature differences across the electrodes, respectively. All instruments were connected to a computer and controlled with LabView. To ensure accuracy in our experiments, each sample was measured three times. To characterize the electrical conductivity, the sheet resistance (R S ) of the films was measured using a four-point probe system (AIT CMT-SR2000 N) and the corresponding thicknesses (t) were measured using a non-contact 3D surface profiler (Nanosystem NV-E1000). Then, the electrical conductivity (σ) was calculated using the equation σ = 1/R S × t. The surface morphologies of the PEDOT:PSS films were characterized using an atomic force microscope (AFM, TopoMetrix Explorer). The absorption spectra were obtained with an UV–Vis–NIR spectrometer (Agilent Cary 5000) between 300 nm and 1,800 nm.
3 Result and discussion
3.1 Enhanced electrical conductivity of sorbitol-mixed PEDOT:PSS thin films
We investigated the electrical conductivity and TE properties of the sorbitol-mixed PEDOT:PSS thin films with different sorbitol concentrations (0, 2,4, 6, 8, and 10 wt %). Here, 0 wt % refers to the film without any sorbitol. As the sorbitol content was increased, the thickness of the films increased from 50 to 140 nm, while the sheet resistance of the samples measured at room temperature decreased gradually from 1.22 × 105 to 2.64 × 102 Ω sq−1. As plotted in Fig. 2a, the electrical conductivity calculated from the sheet resistance and the thickness was drastically improved by mixing sorbitol into PEDOT:PSS. The electrical conductivity of the pristine PEDOT:PSS thin film was 1.53 S cm−1; this was increased to 722.06 S cm−1 after the addition of 6 wt % sorbitol. When the sorbitol content was above 6 wt %, the electrical conductivity slightly decreased. It is well-known that sorbitol promotes the separation and aggregation of PEDOT and PSS, resulting in higher electrical conductivity [32, 33].
Figure 2b shows the Seebeck coefficients and PFs of the films depending on their sorbitol concentration. The Seebeck coefficient of the pristine PEDOT:PSS film was 10.3 μV K−1; this value was slightly decreased but maintained between 8.5 and 10.0 μV K−1 as sorbitol was mixed into PEDOT:PSS. The PF of the pristine PEDOT:PSS film was calculated to be 0.016 μW m−1 K−2, which reflects poor TE properties due to the low electrical conductivity. Meanwhile, the values of the sorbitol-mixed PEDOT:PSS films improved to 4.89, 6.27, and 7.26 μW m−1 K−2, respectively, as the sorbitol concentration increased to 2, 4, and 6 wt %. The PFs of the 8 and 10 wt % sorbitol-mixed films decreased, similar to the trend observed for the electrical conductivity.
In order to observe the morphological changes in the sorbitol-treated PEDOT:PSS films, we compared the AFM images of the pristine PEDOT:PSS film (Fig. 3a) and the 6 wt % sorbitol-mixed PEDOT:PSS film (Fig. 3b). Due to the rearrangement of PEDOT clusters and PSS lamellae [36], the root-mean-square (RMS) surface roughness decreased from 1.06 to 0.63 nm after the addition of 6 wt % sorbitol. Although the AFM images are insufficient for revealing the exact shape and mechanism of phase separation between PEDOT and PSS, the difference in morphology is plausible evidence for the fact that PEDOT and PSS rearrangement occurred.
3.2 Improved thermoelectric properties of sorbitol-mixed PEDOT:PSS thin films by TDAE vapor treatment
Adding sorbitol changed the morphology of the PEDOT:PSS thin films and improved their electrical conductivity. However, the Seebeck coefficient remained near 10 μV K−1 because the charge carrier density was not changed by blending sorbitol with PEDOT:PSS. In order to enhance the TE properties, we controlled the oxidation level of the films with a chemical reduction method. As reported previously, chemical reduction of PEDOT:PSS films decreases the hole carrier density and electrical conductivity, which has the potential to simultaneously increase the Seebeck coefficient [11, 31]. Here, we used TDAE vapor as the reducing agent, which is known as a strong electron-donor molecule [37], because we can easily and precisely control the reduction level of the sorbitol-mixed PEDOT:PSS films by changing the vapor exposure time.
We verified the chemical reduction of PEDOT:PSS by measuring the UV–Vis–NIR absorption spectra. It is known that PEDOT chains are generally in their bipolaron state (PEDOT2+), due to the presence of PSS, and can be reduced to their polaron state (PEDOT1+) or neutral state (PEDOT) [29, 30]. As shown in Fig. 4a, the pristine films with and without sorbitol show broad, strong absorption spectra in the NIR region above 1,250 nm. This originated from the absorption of the PEDOT dications (PEDOT2+). In this spectral region, the sorbitol-mixed sample shows slightly smaller absorption compared to the sample without sorbitol. Here, we assume that the number of the polymeric PEDOT dications decreased due to the phase-separation of PEDOT and PSS. Meanwhile, the TDAE-treated samples exhibit significantly reduced absorption in the spectral region above 1,250 nm and enhanced absorption at shorter wavelengths. The absorption bands around 900 and 600 nm indicate the formation of the polaron state (PEDOT1+) and neutral state (PEDOT), respectively. Thus, the shift of the absorption range caused by TDAE-treatment (shown in Fig. 4a) suggests that the redox state of the sorbitol-mixed PEDOT:PSS films was changed to a reduced state. Among the samples, the 20-min-treated sample showed the most highly reduced state. This can also be confirmed visually due to the electrochromism of PEDOT:PSS. As shown in Fig. 4b, the color of the film changed as a function of the TDAE treatment time, which corresponds to the absorption data. During the TDAE treatment, the film morphology seems to change slightly. Figure 3c shows the AFM image of the chemically-reduced PEDOT:PSS film with sorbitol. The RMS roughness decreased from 0.63 to 0.28 nm after 20 min of the TDAE treatment. Although we could not definitively elucidate the effect of TDAE on the film morphology with AFM, we believe that the smoother surface was caused by the additional rearrangement of PEDOT and PSS clusters by the TDAE vapors.
Figure 5 displays the electrical and TE properties of the sorbitol-treated (6 wt %) PEDOT:PSS films as a function of the TDAE vapor treatment time. Because the treatment chemically reduces the hole-conductive films, the electrical conductivity decreased, as plotted in Fig. 5a. The electrical conductivity of the sample without TDAE treatment was about 720 S cm−1. After 10 min of exposure to TDAE vapor, the value dropped to 337 S cm−1 and continuously decreased to 150 S cm−1 as the treatment time increased to 40 min. Meanwhile, the Seebeck coefficient of the films also varied with the TDAE treatment time (Fig. 5b). Without TDAE treatment, the device showed a low Seebeck coefficient of 10.03 μV K−1. When the TDAE treatment time increased to 10 min and 20 min, the value significantly increased to 24.78 and 27.47 μV K−1, respectively. These values are more than twice the value of the pristine device. Since the Seebeck coefficient began to decrease when the samples were exposed for more than 20 min, the optimal time exposure time for obtaining the highest Seebeck coefficient was determined to be 20 min. Based on these results, the PF was calculated and plotted (Fig. 5b). The highest PF of 22.28 μW m−1 K−2 was obtained in the 20-min sample; this value is three times higher than that of the pristine device (7.26 μW m−1 K−2). It is also superior than the values from recent reports that utilized typical PEDOT:PSS enhanced by solvents/additives (9.47–21.0 μW m−1 K−2) [20–23]. It is reported that thermal conductivities of PEDOT:PSS ranges 0.17–0.52 W m−1 K−1 depending on the treatment condition [6, 11, 12, 24, 29, 30] ). We estimated ZT of our devices using those values; the ZT value of the device with highest PF was ~10−2 (0.013–0.039 depending on κ) but that of the control device was ~10−3 (0.0042–0.013 depending on κ). The devices with TDAE treatment times of 10 min and 30 min also exhibited comparable PFs of around 20 μW m−1 K−2, but the device exposed to TDAE for 40 min showed a severely decreased PF (7.92 μW m−1 K−2). This reduction in performance was mainly due to the low electrical conductivity at this condition. Our results clearly show that investigating the optimum conditions via controlling both the electrical conductivity and Seebeck coefficient (which are, in general, inversely proportional) is an important procedure for achieving a high PF in TE devices.
4 Conclusion
In this work, we demonstrated the enhanced TE properties of PEDOT:PSS-based polymer TE devices by sequentially controlling the electrical conductivity and the Seebeck coefficient. Sorbitol mixing increased the electrical conductivity by more than two orders of magnitude, and the following TDAE treatment chemically tuned the oxidation level of the sorbitol-mixed PEDOT:PSS films. As a result, we obtained a PF that was three times higher than the PF in devices without the TDAE treatment. We believe that the systematic study presented here, which improved the properties of TE devices by examining various processing conditions, is meaningful for the advancement of polymer-based TE devices.
References
J.G. Snyder, E.S. Toberer, Nat. Mater. 7, 105 (2008)
B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich, B. Yu, X. Yan, D. Wang, A. Muto, D. Vashaee, X. Chen, J. Liu, M.S. Dresselhaus, G. Chen, Z. Ren, Science 320, 634 (2008)
J. Sootsman, D.Y. Chung, M.G. Kanatzidis, Angew. Chem. Int. Ed. 48, 8616 (2009)
Y. Pei, X. Shi, A. LaLonde, H. Wang, L. Chen, G.J. Snyder, Nature 473, 66 (2011)
B. Russ, M.J. Robb, F.G. Brunetti, P.L. Miller, E.E. Perry, S.N. Patel, V. Ho, W.B. Chang, J.J. Urban, M.L. Chabinyc, C.J. Hawker, R.A. Segalman, Adv. Mater. 26, 3473 (2014)
R. Yue, J. Xu, Synth. Met. 162, 912 (2012)
M. He, F. Qiu, Z. Lin, Energy Environ. Sci. 6, 1352 (2013)
Q. Zhang, Y. Sun, W. Xu, D. Zhu, Adv. Mater. 26, 6829 (2014)
N. Dubey, M. Leclerc, J. Polym. Sci. B Polym. Phys. 49, 467 (2011)
J. Sun, M.-L. Yeh, B.J. Jung, B. Zhang, J. Feser, A. Majumdar, H.E. Katz, Macromolecules 43, 2897 (2010)
O. Bubnova, Z.U. Khan, A. Malti, S. Braun, M. Fahlman, M. Berggren, X. Crispin, Nat. Mater. 10, 429 (2011)
G.-H. Kim, L. Shao, K. Zhang, K.P. Pipe, Nat. Mater. 12, 719 (2013)
Y.H. Kim, C. Sachse, M.L. Machala, C. May, L. Müller-Meskamp, K. Leo, Adv. Funct. Mater. 21, 1076 (2011)
Y. Xia, K. Sun, J. Ouyang, Adv. Mater. 24, 2436 (2012)
M. Culebras, C.M. Gómez, A. Cantarero, Materials 7, 6701 (2014)
A.J. Mäkinen, I.G. Hill, R. Shashidhar, N. Nikolov, Z.H. Kafafi, Appl. Phys. Lett. 79, 557 (2001)
J. Ouyang, Q. Xu, C.-W. Chu, Y. Yang, G. Li, J. Shinar, Polymer 45, 8443 (2004)
J.Y. Kim, J.H. Jung, D.E. Lee, J. Joo, Synth. Met. 126, 311 (2002)
J. Huang, P.F. Miller, J.S. Wilson, A.J. de Mello, J.C. de Mello, D.D.C. Bradley, Adv. Funct. Mater. 15, 290 (2005)
Q. Jiang, C. Liu, H. Song, H. Shi, Y. Yao, J. Xu, G. Zhang, B. Lu, J. Mater. Sci.: Mater. Electron. 24, 4240 (2013)
T.-C. Tsai, H.-C. Chang, C.-H. Chen, Y.-C. Huang, W.-T. Whang, Org. Electron. 15, 641 (2014)
J. Luo, D. Billep, T. Blaudeck, E. Sheremet, R.D. Rodriguez, D.R.T. Zahn, M. Toader, M. Hietschold, T. Otto, T. Gessner, J. Appl. Phys. 115, 054908 (2014)
M. Hokazono, H. Anno, N. Toshima, J. Electron. Mater. 43, 2196 (2014)
M. Culebras, C.M. Gómez, A. Cantarero, J. Mater. Chem. A 2, 10109 (2014)
D. Kim, Y. Kim, K. Choi, J.C. Grunlan, C. Yu, ACS Nano 1, 513 (2010)
G.P. Moriarty, K. Briggs, B. Stevens, C. Yu, J.C. Grunlan, Energy Technol. 1, 265 (2013)
G.H. Kim, D.H. Hwang, S.I. Woo, Phys. Chem. Chem. Phys. 14, 3530 (2012)
K. Xu, G. Chen, D. Qiu, J. Mater. Chem. A 1, 12395 (2013)
J. Luo, D. Billep, T. Waechtler, T. Otto, M. Toader, O. Gordan, E. Sheremet, J. Martin, M. Hietschold, D.R.T. Zahn, T. Gessner, J. Mater. Chem. A 1, 7576 (2013)
T. Park, C. Park, B. Kim, H. Shin, E. Kim, Energy Environ. Sci. 6, 788 (2013)
N. Massonnet, A. Carella, O. Jaudouin, P. Rannou, G. Laval, C. Celle, J.-P. Simonato, J. Mater. Chem. C 2, 1278 (2014)
L.A.A. Pettersson, S. Ghosh, O. Inganäs, Org. Electron. 3, 143 (2002)
S. Timpanaro, M. Kemerink, F.J. Touwslager, M.M. De Kok, S. Schrader, Chem. Phys. Lett. 394, 339 (2004)
A.M. Nardes, M. Kemerink, M.M. de Kok, E. Vinken, K. Maturova, R.A.J. Janssen, Org. Electron. 9, 727 (2008)
S. Park, S.J. Tark, D. Kim, Curr. Appl. Phys. 11, 1299 (2011)
A.M. Nardes, R.A.J. Janssen, M. Kemerink, Adv. Funct. Mater. 18, 865 (2008)
P.-M. Allemand, K.C. Khemani, A. Koch, F. Wudl, K. Holczer, S. Donovan, G. Grüner, J.D. Thompson, Science 253, 301 (1991)
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2055322).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, E., Kim, J., Jung, B.J. et al. Enhanced thermoelectric properties of sorbitol-mixed PEDOT:PSS thin films by chemical reduction. J Mater Sci: Mater Electron 26, 2838–2843 (2015). https://doi.org/10.1007/s10854-015-2766-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2766-0