Abstract
Lead-free piezoelectric ceramics of (Ba0.98Ca0.02)(Ti0.94Sn0.06)O3–x wt% ZnO (BCTS–xZnO) were synthesized by the conventional solid-state method. The microstructures and the electrical properties of BCTS–xZnO ceramics were systematically studied in the composition range of 0 ≤ x ≤ 0.20. It was found that the sintering temperature of BCTS ceramics was gradually decrease using ZnO as a sintering aid, and the addition of ZnO did not change the tetragonal phase structure of the ceramics. The BCTS–xZnO ceramics with x = 0.10 sintered at a lower temperature of ~1,350 °C for 3 h demonstrated an optimum electrical behavior: d 33 ~ 428 pC/N, k p ~ 53.3 %, 2P r ~ 24 μC/cm2, and 2E c ~ 3.0 kV/mm. As a result, the BCTS–xZnO ceramic is a promising candidate for lead-free piezoelectric ceramics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Piezoceramics mainly based on lead zirconate titanate (PZT) ceramics at present certainly show great applications in microelectronic and micro-electromechanical devices due to their superior piezoelectric properties especially when the composition of the ceramics is close to the morphotropic phase boundary (MPB) between rhombohedral and tetragonal phases. Nevertheless, the ceramics are not environmental friendly because of the lead oxide toxicity. With the increase of worldwide concern, it is vital to develop replacement lead-free ceramics with comparable piezoelectric properties to those of PZT [1–4]. In the search for lead-free piezoelectric ceramics which have relatively good piezoelectric properties and high curie temperatures, considerable attention has been drawn for such as (K0.50Na0.50)NbO3 (KNN)- and Bi0.50Na0.50TiO3 (BNT)-based ceramics [5–9]. However, the piezoelectric properties of the lead-free piezoelectric ceramics concerned are still lower than those of the Pb(Zr,Ti)O3 (PZT) family ceramics. Therefore, it is urgent to find a new lead-free material system with higher piezoelectric properties to replace the lead-based ones.
Recently, researchers further revealed that the BaTiO3 (BT) based ceramics, such as Ba(Sn0.12Ti0.88)O3–x(Ba0.7Ca0.3)O3 (BCTS), Ba(Zr0.2Ti0.8)O3–x(Ba0.7Ca0.3)TiO3 (BCTZ), have high piezoelectric coefficients of d 33 > 300–600 pC/N [10–14], i.e. the electrical properties of which ceramics are even higher than that of some lead-based ones. However, the ceramics with optimal composition and high d 33 value were always sintered at a higher temperature of ≥1,450 °C. Therefore, it was expected that these ceramics could be fabricated through the lower temperature sintering, which not only will be good at suppressing the compositional change during ceramics sintering and reducing the energy consumption, but also reduces the processing costs especially in production. The studies published recently indicate that some reduce of the sintering temperature of the ceramics can be realized if a liquid-phase is formed during the sintering process through the sintering aid of doping oxides, and the sintering aid, such as CuO, MnO2, CeO2, Pr2O3, and HfO2, is really an effective way to decrease the sintering temperature and improve the dense microstructure of the ceramics [14–22, 32]. However, there have been few systematical reports on the effect of ZnO used as a sintering aid on the microstructure and electrical properties of Ba(Ti,Sn)O3–x(Ba,Ca)TiO3 piezoelectric ceramics.
In this work, the BCTS–xZnO ceramics were prepared by the conventional solid-state method, and all ceramics were sintered at 1,350 °C for 3 h. The effects of ZnO as a sintering aid on the phase structure, microstructure, dielectric and piezoelectric properties of BCTS ceramics were studied systematically.
2 Experimental procedure
(Ba0.98Ca0.02)(Ti0.94Sn0.06)O3–x wt% ZnO piezoelectric ceramics were prepared by the conventional solid-state reaction process. Raw materials of BaCO3 (99.0 %), CaCO3 (99.0 %), SnO2 (99.0 %), TiO2 (99.5 %) and ZnO (99 %) were mixed with ZrO2 balls for 24 h by using the ethanol as a medium. After dried and calcined at 1,200 °C for 2 h, these powders were pressed into ~10 mm in diameter pellets and sintered at 1,350 °C for 3 h in air. Silver pastes were fired at ~700 °C for 10 min on both sides of these samples as electrodes for electrical measurements. All samples were poled at room temperature in a silicone oil bath under a dc field of ~4.0 kV/mm for 20 min.
The phase structure of these ceramics was measured by using X-ray diffraction (XRD) (Bruker D8 Advanced XRD, Bruker AXS Inc., Madison, WI, USA). Scanning electron microscopy (SEM, Philips, XL30) was employed to study the surface morphologies of these ceramics. The dielectric behavior as a function of the measurement temperature of these ceramics was measured by using an LCR meter (HP 4980, Agilent, USA), and their piezoelectric constant d 33 of the ceramics was measured by using a piezo-d 33 meter (ZJ-3A, China). The polarization versus electric field (P–E) hysteresis loops of the ceramics was measured by using a Radiant Precision Workstation (USA).
3 Results and discussion
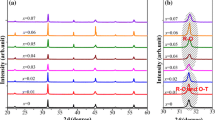
Figure 1a plots the room temperature XRD patterns of ZnO-doped BCTS ceramics with different x contents. It is evident that the ceramics show pure perovskite phase and no secondary phases can be certified in the investigated θ range of XRD. That is, the dopant has completely diffused into the BCTS lattice to form a homogeneous solid solution. As shown from Fig. 1b, the enlarged diffraction patterns at around 45° show that the diffraction peaks of all ceramics split into (002) and (200) double peaks, which well matches the PDF card of tetragonal BaTiO3 (PDF#05-0626). This result indicates that the ZnO-doped BCTS ceramics possess a tetragonal phase at room temperature. As a result, the addition of ZnO did not change the phase structure of BCTS ceramics. Moreover, compared with that of the pure BCTS ceramics, the XRD peaks of ZnO-doped BCTS ceramics shifted to a low angle with an increase in ZnO content when x ≤ 0.10, then it shifted to a high angle when x further increases to 0.20. Undoubtedly, the introduction of Zn is the reason for the shift of diffractions peaks. As we known, the ionic radius of Zn2+ (0.74 Å) is much smaller than those of A-site ions (Ba2+: 1.61 Å, Ca2+: 1.35 Å), while the radius of Zn2+ is close those of Ti4+ (0.61 Å) and Sn4+ (0.71 Å). Therefore, the Zn ions are more likely to substitute the B site of BCTS, which would lead to the increase of lattice parameter. And the above fact can qualitatively explain the shift of diffraction peaks towards lower angle when x ≤ 0.10. In addition, the underlying physical mechanisms of the abnormal phenomenon when x > 0.10 have been reasonably explained by two aspects. Firstly, owing to the limit of solid solubility of Zn2+ ions in the BCTS matrix, the excessive Zn2+ ions stockpile in the grain boundaries and suppress the grain growths. Secondly, it was reported that the oxygen vacancies can be induced by the substitutions for a higher valence B site using a lower valence [23]. This is, oxygen vacancies (ZnO = Zn(Ti,Sn)″ + VO.. + OO) are generated when the Zn substitutes for the (Ti, Sn) site. The generation of oxygen vacancies resulted in a distortion and contraction of unit cells from the viewpoint of crystal chemistry [24]. In this work, the shift of the diffraction peaks is caused by two distinct effects: the ion radius effect seems to be the main contributing factor at relatively low Zn content, while the oxygen vacancy effect appears to be the main one at relatively high Zn content.
To further confirm the phase structure of the ceramics, their Raman spectra was measured in the region of 100–1,000 cm−1, as shown in Fig. 2. The longitudinal (LO) and transverse (TO) components are split due to the long electrostatic force associated with the lattice iconicity induced by Ba2+ ions in BCTS [25]. The Raman modes of BaTiO3-based materials are assigned to be E(TO1), A1(TO1), A1(TO2), E(TO2), A1(TO3) and A1(LO3)/E(LO3) in the range of 150–1,000 cm−1. The tetragonal structure of BaTiO3-based materials could be confirmed by the E(TO2) phonon mode [26]. Therefore, the micro-Raman scattering spectra and the XRD pattern also conforms the tetragonal phase structure in BCTS ceramics.
To clearly study the ZnO effect on the surface morphologies of BCTS ceramics, the SEM patterns are shown in Fig. 3a–d. It is observed that the microstructure of ZnO-doped BCTS ceramics is strongly dependent on the introduction of ZnO. When the ZnO was doped (x ≤ 0.1 wt%), the grain size of the ceramics had a homogeneous microstructure. Moreover, small amounts of liquid phases were formed with increasing x (0.03–0.10), resulting in a denser microstructure of BCTS ceramics in this work. The fact that the lower valence states of Zn2+ substitute the higher valence states of Ti4+/Sn4+ generates oxygen vacancies, which enhances the transfer of mass and energy among reactions resulting the improvement of the sintering behavior [27–29]. With further addition of Zn content (x > 0.1 wt%), the grain growths are restrained, and some pores appear. The underlying physical mechanisms have been reasonably explained below: a low ZnO content promotes the grain growths of BCTS ceramics by entering the lattice, while the excess ZnO content may stockpile in the grain boundaries and suppress the grain growths.
Figures 4a, b plot the temperature dependence of the dielectric constant (ε r) and the dielectric loss (tan δ) of ZnO-doped BCTS ceramics respectively in the temperature range of room temperature to 200 °C at 10 kHz. It is observed from Fig. 3a that the tetragonal–cubic phase transition (T C) temperature of ZnO-doped BCTS ceramics decreases slightly with increasing ZnO content. The decrease in T C reflects the incorporation of Zn2+ into the BCTS lattice, and the amount of ZnO incorporated as solid solution also increases with increasing ZnO content, a similar result has been shown elsewhere [14, 33]. Moreover, a lower dielectric loss (tan δ < 1.6 %) is demonstrated in ZnO-doped BCTS ceramics, as shown in Fig. 4b.
Figure 5a plots the ε r and tan δ values of BCTS ceramics as a function of ZnO content, measured at room temperature of ~20 °C. It was observed that the ε r values of ZnO-doped BCTS ceramics obviously increase in the compositional range of x ≤ 0.15, getting a lager value (4,500–4,800) at 0.10 ≤ x ≤ 0.15, and then decrease with further rising ZnO content (x > 0.15) because of a lower density as well as the formation of some pores, as shown in Fig. 2d. Moreover, the ZnO-modified BCTS ceramics have a low tan δ value (0.012–0.016) regardless of ZnO contents. Figure 5b plots the piezoelectric behavior of ZnO-doped BCTS ceramics, measured at room temperature. The d 33 value increases with increasing ZnO content, reaching a maximum value of d 33 ~ 428 pC/N at the dopant of x = 0.10, and then sharply drops with further increasing the dopant. Similar to the change of the d 33 value, the k P value of the ZnO-doped BCTS ceramics also reaches a maximum value of ~53.3 % at x = 0.10. In this work, the improvement of the piezoelectric properties is fundamentally caused by two aspects. Firstly, the improved piezoelectric property of the ceramics is partly attributed to the dense and homogeneous microstructure of the optimized BCTS–0.1ZnO ceramics, which is benefited from the liquid sintering due to the introduction of Zn sintering aids, as shown in Fig. 3c. Secondly, the piezoelectric properties are also related to the dielectric and ferroelectric properties of ferroelectric materials, that is, d 33 ~ αε r P r [34, 35]. In this work, the ZnO-doped BCTS ceramic with x = 0.10 has a higher ε r value and a larger P r value, as shown in Figs. 5a and 6, so the d 33 and ε r P r simultaneously reach maximum for the ceramic with x = 0.1, confirming that enhanced dielectric and ferroelectric properties should be partly responsible for its enhanced piezoelectricity. As a result, the improvement in d 33 value could be attributed to the enhancement of the dielectric properties, ferroelectric properties and dense microstructure of ZnO-doped BCTS ceramic.
Table 1 gives the piezoelectric properties and the sintering temperature of BCTS-based ceramics published in the literature recently. From Table 1 one can clearly see that till now, there were few reports on relatively high piezoelectric properties (d 33 ~ 428 pC/N and k p ~ 53.3 %) of BCTS ceramics when sintered at such a low temperature of ~1,350 °C as in present work.
Figure 6 shows the P–E loops of ZnO-doped BCTS ceramics with different ZnO contents measured at 10 Hz and room temperature of ~20 °C. It is observed from Fig. 6 that all the loops are saturated and have a typical ferroelectric behavior. The insert of Fig. 6 shows the 2P r and 2E c values of ZnO-doped BCTS ceramics, the remnant polarization (P r) of ZnO-doped BCTS ceramics slightly increases in the compositional range of x ≤ 0.10, reaches a maximum value of ~24 μC/cm2 at the doping content of x = 0.10, and then decreases with further rising ZnO content. In addition, the change of E c value is similar as compared with the P r, the 2E c is in the range of 3.0–4.2 kV/cm.
4 Conclusions
(Ba0.98Ca0.02)(Ti0.94Sn0.06)O3–x wt% ZnO (BCTS–xZnO) lead-free piezoelectric ceramics were prepared by the conventional solid-state method with a lower sintering temperature of ~1,350 °C. The ceramics with x = 0.10 shows an excellent high piezoelectric performance of d 33 ~ 428 pC/N, k p ~ 53.3 %, 2P r ~ 24 μC/cm2, and 2E c ~ 3.0 kV/mm. Experimental results indicate that the addition of ZnO as a sintering aid plays an important role in the microstructure, dielectric and piezoelectric properties of BCTS ceramics with a relatively lower sintered temperature, which shows that the ceramics possess a promising future for lead-free piezoelectric devices.
References
Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, Nature 432, 84 (2004)
J. Rödel, W. Jo, K.T.P. Seifert, M. Anton, T. Granzow, D. Damjanovic, J. Am. Ceram. Soc. 92, 1153 (2009)
D.Q. Xiao, J.G. Wu, L. Wu, J.G. Zhu, P. Yu, D.M. Lin, Y.W. Liao, Y. Sun, J. Mater. Sci. 44, 5408 (2009)
L.E. Cross, Nature 432, 24 (2004)
J.F. Li, K. Wang, F.Y. Zhu, L.Q. Cheng, F.Z. Yao, J. Am. Ceram. Soc. 96, 3677 (2013)
D.M. Lin, D.Q. Xiao, J.G. Zhu, P. Yu, Appl. Phys. Lett. 88, 062901 (2006)
H. Nagata, M. Yoshida, Y. Makiuchi, T. Takenaka, Jpn. J. Appl. Phys. 42, 7401 (2003)
J.G. Wu, D.Q. Xiao, Y.Y. Wang, J.G. Zhu, L. Wu, Y.H. Jiang, Appl. Phys. Lett. 91, 252907 (2007)
D. Lin, D.Q. Xiao, J.G. Zhu, P. Yu, H.J. Yan, L.Z. Li, Mater. Lett. 58, 615–618 (2004)
W.F. Liu, X.B. Ren, Phys. Rev. Lett 103, 257602 (2009)
D.Z. Xue, Y.M. Zhou, H.X. Bao, J.H. Gao, C. Zhou, X.B. Ren, Appl. Phys. Lett. 99, 122901 (2011)
S.W. Zhang, H.L. Zhang, B.P. Zhang, S. Yang, J. Alloy. Compd. 506, 131 (2010)
J.G. Wu, D.Q. Xiao, W.J. Wu, J.G. Zhu, J. Wang, J. Alloys, Compd. 509, L359 (2011)
J.G. Wu, D.Q. Xiao, W.J. Wu, Q. Chen, J.G. Zhu, Z. Yang, J. Wang, Script. Mater. 65, 771 (2011)
I. Burn, US patent 4283753, 1981
C.F. Yang, L. Wu, T.S. Wu, J. Mat. Sci. 27, 6573 (1992)
P. Zheng, J.L. Zhang, S.F. Shao, Y.Q. Tan, C.L. Wang, Appl. Phys. Lett. 94, 032902 (2009)
C.C. Tsai, S.Y. Chu, C.H. Lu, IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 660 (2009)
Y.C. Lee, Y.L. Huang, J. Am. Ceram. Soc. 92, 2661 (2009)
S. Derling, Th Müller, H.-P. Abicht, K.-H. Felgner, H.T. Langhammer, J. Mater. Sci. 36, 1425 (2001)
A. Ramesh Babu, A.V. Prasadarao, J. Mater. Sci. Lett. 16, 313 (1997)
J.G. Wu, T. Wang, X.J. Cheng, X.P. Wang, B.Y. Zhang, J.G. Zhu, D.Q. Xiao, J. Alloys Compd. 576, 299 (2013)
H.C. Hu, M.K. Zhu, F.Y. Xie, N. Lei, J. Chen, Y.D. Hou, H. Yan, J. Am. Ceram. Soc. 92, 2039 (2009)
Q. Xu, M. Chen, W. Chen, H.X. Liu, B.H. Kim, B.K. Ahn, Acta Mater. 56, 642 (2008)
P.S. Dobal, R.S. Katiyar, J. Raman Spectrosc. 33, 405 (2002)
B.D. Begg, K.S. Finnie, E.R. Vance, J. Am. Ceram. Soc. 79, 2666 (1996)
T. Chen, T. Zhang, G.C. Wang, J.F. Zhou, J.W. Zhang, Y.H. Liu, J. Mater. Sci. 47, 4612 (2012)
M.K. Zhu, L.Y. Liu, Y.D. Hou, H. Wang, H. Yan, J. Am. Ceram. Soc. 90, 120 (2007)
A. Watcharapasorn, S. Jiansirisomboon, Ceram. Int. 34, 769 (2008)
M.L. Chen, Z.J. Xu, R.Q. Chu, H. Qiu, M. Li, Y. Liu, L. Shao, S. Ma, W.B. Ji, W. Li, S.W. Gong, G.R. Li, Phys. B 433, 43 (2014)
J.G. Wu, A. Habibul, X.J. Cheng, X.P. Wang, B.Y. Zhang, Mater. Res. Bull. 48, 4411 (2013)
B. Wu, D.Q. Xiao, J.G. Wu, T. Huang, Z. Wang, C. Liu, F.X. Li, J.G. Zhu, J. Electroceram. (2014). doi:10.1007/s10832-014-9949-6
A.C. Caballero, J.F. Fernandez, C. Moure, P. Duran, J. Eur. Ceram. Soc. 17, 513 (1997)
X.P. Wang, J.G. Wu, D.Q. Xiao, X.J. Cheng, T. Zheng, B.Y. Zhang, X.J. Lou, J.G. Zhu, J. Mater. Chem. A 2, 4122 (2014)
X.P. Wang, J.G. Wu, X.J. Cheng, B.Y. Zhang, D.Q. Xiao, J.G. Zhu, X.J. Wang, X.J. Lou, J. Phys. D Appl. Phys. 46, 495305 (2013)
Acknowledgments
Authors gratefully acknowledge the supports of the National Science Foundation of China (NSFC Nos. 50772068, 50972095, 51102173, 51272164, and 51332003), the Fundamental Research Funds for the Central Universities, and the introduction of talent start funds of Sichuan University (2082204144033). And the authors also thank Ms. Wang Hui for her help in the SEM measurement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, B., Xiao, D., Wu, J. et al. Microstructure and electrical properties of (Ba0.98Ca0.02)(Ti0.94Sn0.06)O3–x wt% ZnO lead-free piezoelectric ceramics sintered at lower temperature. J Mater Sci: Mater Electron 26, 2323–2328 (2015). https://doi.org/10.1007/s10854-015-2687-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2687-y