Abstract
Free-standing TiO2 nanotube (NT) array films were prepared by anodization approach as support materials for quantum dots sensitized solar cells (QDSSCs). The ZnxCd1−xSe quantum dots (QDs) layers with tunable compositions have been deposited on the TiO2 NT array film via a simple ion-exchange approach. The optical absorptions of the ZnxCd1−xSe/TiO2 NT can be controllably tuned to cover almost the entire visible region by changing the atomic ratio of Zn to Cd. In the fabricated QDSSCs based on these ZnxCd1−xSe/TiO2 NT array photoanodes, the photovoltaic performance gradually improved as the composition changes from Zn-rich to Cd-rich of ZnxCd1−xSe QDs layers on TiO2 NT arrays. In contrast with other ZnxCd1−xSe/TiO2 NT solar cells, the Zn0.13Cd0.87Se/TiO2 NT solar cell yielded a highest power conversion efficiency of 2.15 %, indicating the most effective band alignment is obtained with Cd-rich ZnxCd1−xSe QDs layers. It can be anticipated that tunable energy band of QDs controlled by the ratio of atoms can contribute to higher efficiency QDSSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of one-dimensional nanostructure materials including nanorods, and nanotubes that can be used as substrate in photovoltaic system is quite a challenging subject [1, 2]. The vertically aligned one-dimensional architectures provide a direct pathway for rapid transport of photogenerated carriers to the collection electrodes [3]. In particular, TiO2 nanotube (NT) arrays sheathed with semiconductor nanocrystals are emerging as novel photoelectrode for photoelectrochemical cells [4, 5]. Band gap engineering is an important strategy for custom tuning physical properties of semiconductors in photoelectronic devices. On comparison with bulk semiconductors, nanocrystals which are also known as quantum dots (QDs) provide a novel way for band gap modulation through their tunable sizes and shapes due to the quantum confinement effects [6].
Recently, much attention has been paid on QD sensitized solar cells (QDSSCs) based on one-dimensional TiO2 NT arrays, sensitized by various QDs such as CdS [7], CdSe [8], and CdTe [9]. However, power conversion efficiencies of these QDSSCs are usually in a low level. The co-sensitization of different component QDs is a promising technique to improve the efficiency of QDSSCs. For instance, CdS/CdSe system showed higher solar cell efficiency than either of the single QDs [10–13]. Ternary alloyed QDs have also been employed as efficient sensitizers due to their advantage of tunable band gap by varying their relative composition [14–17]. Furthermore, gradient heterostructures can be formed in alloyed QDs to engineering band gap. For example, ZnO–ZnxCd1−xSe core/shell nanowire arrays have band-gaps that from a stepwise energy alignment at the heterojunctions, where both the conduction and valence bands of the shell are either higher or lower in energy than those of core [18, 19]. The photogenerated electrons and holes would preferably transfer across the interface in opposite directions to form a charge separation state.
Herein, we design and report an ion-exchange route to prepare ZnxCd1−xSe layers on a pre-anodic TiO2 NT arrays with tunable composition (0 ≤ x ≤ 1) and band-gaps from 1.80 to 2.67 eV. The strategy for the synthesis of ZnxCd1−xSe/TiO2 NT arrays is based on the difference between solubility product constant (K sp) of ZnSe and CdSe [20]. The K sp of ZnSe (3.6 × 10−26) is larger than the K sp of CdSe (6.31 × 10−36), which implies that the pre-prepared ZnSe/TiO2 NT arrays can be used as sacrificial templates to convert into ZnxCd1−xSe/TiO2 NT arrays by cation exchange between Zn2+ and Cd2+. Although alloyed QDs sensitized solar cells have been reported in previous research work [16, 18, 21], surprisingly, the QDSSCs based on ZnxCd1−xSe/TiO2 NT photoanode synthesized by ion-exchange route has rarely referred. On the basis of the ZnxCd1−xSe/TiO2 NT arrays, a photovoltaic device with a maximum power conversion efficiency of 2.15 % has been demonstrated. Based on the experimental results, a possible formation mechanism involving the band alignment has been proposed in this study.
2 Experiments
2.1 Chemicals
Titanium (Ti) foils (99.6 % purity), ammonium fluoride (NH4F), ethylene glycol (HOCH2CH2OH), hydrogen peroxide (30 wt% H2O2), deionized water (H2O, 18.2 ΩM cm resistivity), ethyl cellulose, terpinol (C10H18O), tetrabutyl titanate (Ti(OC4H9)4), zinc acetate dihydrate (Zn(AC)2·H2O), cadmium nitrate tetrahydrate (Cd(NO)3·4H2O), and selenium powder (Se) were employed as starting materials. Unless otherwise indicated, all these reagents used were of analytical purity and were used directly, without further purification.
2.2 Experimental details
2.2.1 Fabrication of crystallized and free-standing TiO2 NT films
Titanium foils were cut into the required size and shape, following by being cleaned in acetone, ethanol and deionized water, respectively, then dried in air and processed for anodization. The free-standing TiO2 NT films were prepared by a two-step potentiostatic anodization in a two-electrode electrochemical cell similar to previous report [22]. Ti foil is used as the anode and graphite sheet as the cathode. The Ti foil was first anodized in a electrolyte solution consisted of 150 mL ethylene glycol, 0.3 wt% NH4F, and 3 vol% deionized water at 50 V using a laboratory DC power supply for 8 h. The first anodized products were rinsed with ethanol and slightly ultrasonicated in ethanol to remove debris adsorbed on the surface, and dried in ambient temperature. The as-prepared TiO2 NT film on Ti foil was annealed at 450 °C for 1 h to ensure the complete crystallization. Following this annealing treatment, the annealed TiO2 NT film on Ti foil was anodized again with applied voltage of 10 V for 6 h in the electrolyte which was used in the first anodization process. After being rinsed in ethanol, the re-anodized products were immersed into 10 wt% H2O2 aqueous solution for another 6 h to resolve the new formed amorphous TiO2 layer beneath the crystallized as-prepared TiO2 NT film. This post-treatment will lead to gradual detachment of the crystalline TiO2 NT membrane from the Ti substrate.
2.2.2 Fixation of TiO2 NT film on FTO glass photoanode
A paste used as adhesive was prepared by mixing 0.5 mL tetrabutyl titanate, 0.5 g ethyl cellulose, 15 mL terpinol, and 5 mL ethanol, followed by magnetic stirring at 60 °C until transparent. Fluorine-doped tin oxide (FTO) conductive glass (OPV-FTO22-7, Liaoning, China) with 2 cm × 1.5 cm in size was employed as substrate and cleaned by rinsing in an ultrasonic bath of acetone and isopropanol for 20 min in turn, then rinsing with a large amount of ethanol and finally blowing to dry with a drier. The edges of the FTO glass were covered with scotch tape as the frame. The paste which functioned as adhesive was flattened with a glass rod on the surface of FTO glass. The free-standing TiO2 NT film was then transferred onto the paste followed by drying the film at ambient temperature. In order to completely eliminate the organic component in the paste, the TiO2 NT film on FTO glass was sintered again at 450 °C for 30 min.
2.2.3 Fabrication of ZnxCd1−xSe/TiO2 NT photoanode by ion-exchange method
A Se source solution (0.1 M NaSeSO3) was prepared by refluxing Se powder and NaSO3 at 96 °C for 12 h in advance. The successive ionic layer adsorption and reaction (SILAR) cycles process similar to previous report [23] was used to prepare ZnSe/TiO2 NT arrays template which involved dipping the TiO2 NT film in a 0.1 M Zn(AC)2 aqueous solution for 5 min, rinsing it with ethanol, and then dipping it for another 5 min in a 0.1 M NaSeSO3 aqueous solution, and rinsing it again with ethanol. The two-step dipping procedure is termed as one SILAR cycle. In this experiment, 15 SILAR cycles were repeated to ensure the enough amount of ZnSe can be incorporated onto the TiO2 NT film.
The ZnxCd1−xSe/TiO2 NT photoanodes were obtained by the immersion of the ZnSe/TiO2 NT template into a Cd2+ source solution (0.1 M Cd(NO)3 aqueous solution) at 90 °C for different periods (4, 8 and 12 h). In this process, the cation of Cd2+ will gradually substitutes the Zn2+ in the ZnSe, forming ZnxCd1−xSe with different atomic ratios of Cd to Zn. The variation of Zn and Cd in ZnxCd1−xSe can be tuned by controlling the reaction time of the ZnSe/TiO2 NT template with the Cd2+ ions.
For photovoltaic applications, the prepared ZnxCd1−xSe/TiO2 NT photoanodes were assembled with Pt/FTO counter electrode which prepared by pyrolysis of hydrochloroplatinic acid in a fashion similar to sandwich. The space between the two electrodes was filled with polysulfide electrolyte as holes scavenger which was consisted of 0.5 M sodium sulfide, and 0.1 M sulfur aqueous solution.
2.3 Characterization
Field-emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were carried out with a Hitachi S-4200 field emission SEM and a Tecnai G2 F20 TEM, respectively. The compositional analysis and elemental mapping of the as-prepared sample were performed with an energy-dispersive X-ray spectroscopy (EDX), an accessory of the SEM. The crystalline nature and structure of the TiO2 NT films were characterized by X-ray diffraction (XRD, D/MAX-2400, Rigaku) using a Cu Kα source operated at 40 kV and 30 mA with scanning rate of 4° min. The optical absorption of the samples was recorded using a UV2501PC (SHIMADZU) spectrometer with an ISR-2200 integrating sphere attachment for diffuse reflection measurement.
The photocurrent–voltage (J–V) curves were measured under an illumination of a solar simulator (Pecell-L15, Japan) at one sun (AM1.5, 100 mW cm−2), which calibrated by standard silicon solar cell (BS-520, Japan). The incident photon-to-current conversion efficiency (IPCE) was measured as a function of wavelength from 300 to 800 nm by employing a 150-W Xe lamp coupled with a computer-controlled monochromator. A mask with exposure area of 0.16 cm2 was used to cover on the photoanodes when conducting photovoltaic measurement.
3 Results and discussion
Figure 1a shows the SEM image of the as-prepared TiO2 NT arrays. It can be seen that the TiO2 NT arrays are highly ordered distributed over the entire surface. The tubular structure with a nearly uniform wall thickness possesses an outer diameter approximate to 100 nm. The inset of Fig. 1a is the part cross-section view of the TiO2 NT arrays, showing that the nanotubes regularly aligned together. The growth direction of the TiO2 NT is also pointed as the arrow indicates, showing that the TiO2 NTs were vertically grown. The evolution of the TiO2 NT is an electrochemical etching process accompanying with two main reactions [24]:
Initially, TiO2 layer is formed on the surface of Ti foil under anodization as reaction (1) indicated, meantime, reaction (2) occurs, the F− begin to etching the TiO2 layer to form pits on the its surface, which is called ‘pitting attack’ process. As the anodization time prolongs, the pits would be gradually developed into tubular structure.
Figure 1b presents the back-side morphology of the free-standing TiO2 NT film, displaying that the bulges of TiO2 NT arrays are packed in approximately circle shape and possess sealed ends, and the integrity of the arrays preserves well. The whole free-standing TiO2 NT film detached from Ti foil is presented in the inset of Fig. 1b, exhibiting a light brown color and translucence, which indicates its potential application as photoanode materials in sensitized solar cells. The TEM image of the free-standing TiO2 NT arrays is shown in Fig. 1c. Obviously, the TiO2 NTs aligned with each other and have hollow tubular structure, providing the space for adsorption of ZnSe QDs and channels for excited electrons to transport in sensitized solar cells. Figure 1d shows the TEM of a single TiO2 NT in large magnification, from which the hollow tubular structure can be clearly identified. The inner and outer diameter of the nanotube can be estimated to be around 80 and 100 nm, respectively, which is almost consistent with the SEM result, and the wall thickness of the TiO2 NT is around 20 nm. The selected area electron diffraction (SAED) pattern from the TEM of the free-standing TiO2 NT is displayed in the inset of Fig. 1d. The appearance of diffraction rings indicates that the free-standing TiO2 film obtained from two-step anodization process is in polycrystalline phase (Fig. 2).
The XRD data reveal that the TiO2 NT film experienced a phase transformation between the first and second step anodization process. Figure 2 a is the XRD patterns of the first anodized TiO2 NT film (without annealing treatment) which scraped from the Ti substrate. None of diffraction peaks can be observed in this XRD pattern, indicating that the TiO2 NT film formed in the first anodization process is in amorphous phase. However, strong diffraction peaks appeared for the free-standing TiO2 NT film obtained from the second step anodization. This phase transformation can be ascribed to the annealing treatment of the first anodized Ti foil, which changes the amorphous TiO2 NT to the crystalline TiO2 NT. The crystallized TiO2 is critical for the successful separation of TiO2 NT film due to the chemical stability of the crystallized TiO2 to H2O2 aqueous solution [22]. A new amorphous TiO2 layer would be formed between crystallized TiO2 NT and Ti foil during secondary anodization process, and this amorphous layer TiO2 can be resolved by 10 wt% H2O2 aqueous solution, leading to the separation of crystallized TiO2 NT film from Ti substrate. For comparison, the standard XRD pattern of anatase TiO2 (JCPDS # 21-1272) is supplied. The diffraction peaks corresponding to (101), (004), (200), (105), (211) and (213) planes confirm that the free-standing TiO2 NT film belong to the anatase TiO2.
Figure 3a shows the SEM image of the ZnxCd1−xSe/TiO2 NT arrays which is obtained by ion-exchange of ZnSe/TiO2 NT photoanode in Cd2+ solution for 8 h. Obviously, the ZnxCd1−xSe/TiO2 NT arrays have a reduced inner diameter and rougher surface than that of the bare TiO2 NT arrays, indicating the successful adsorption of sensitizers into the TiO2 NT arrays. The structures of the ZnxCd1−xSe/TiO2 NT arrays are further demonstrated by their TEM images. Figure 3b exhibits a typical TEM image of the ZnxCd1−xSe/TiO2 NT arrays. Evidently, a large amount of nanoparticles are distributed across the nanotubes. Figure 3c provides the TEM image of single ZnxCd1−xSe/TiO2 NT in higher magnification, clearly showing the tubular structure as the scaffold to support nanoparticles. As illustrated in Fig. 3d, the EDX elemental mapping confirms that the presence and distribution of the Zn, Cd and Se elements which are based on TiO2 NT. Figure 3d is the HRTEM image of the ZnxCd1−xSe/TiO2 NT prepared by ion-exchange of ZnSe/TiO2 NT photoanode in Cd2+ solution for 8 h. The observed lattice spacing of 0.35 nm corresponds to the (101) plane of anatase TiO2, confirming that the supporter of the sensitizers is TiO2. Along the TiO2 crystalline edge, fine crystallites could be discerned. The crystallites close to TiO2 have lattice fringe of 0.34, 0.21, and 0.19 nm, which can be ascribed to the (111), (220), and (311) planes of ZnxCd1−xSe. These results of SEM, TEM, and EDX elemental mapping characterization have confirmed that the successful formation of ZnxCd1−xSe/TiO2 NT arrays can be obtained from the ion-exchange strategy of ZnSe/TiO2 NT in Cd2+ solution.
In order to estimate the composition of ZnxCd1−xSe, EDX spectra and data were used to approximately calculate the x value of ZnxCd1−xSe. The EDX spectra of the ZnxCd1−xSe/TiO2 NT and their corresponding quantitative data are shown in the supplementary material (Fig. S1). The ZnxCd1−xSe/TiO2 NT obtained from ion-exchange process in various periods, with different compositions, are listed in Table 1. With regard to the EDX spectra in Fig. S1, the atomic ratio of Zn to Se is calculated to be around 1:1.19 for the ZnSe/TiO2 NT (Fig. S1a). The deviation from a stoichiometric ratio of ZnSe is probably caused by the oxidation of Se2− on the surface of the TiO2 NT into amorphous Se, in the air [19]. After ion-exchange process of ZnSe/TiO2 NT in the Cd cations solution for different periods, the EDX spectra (supplementary materials, Fig. S1b-d) indicates that the Cd2+ ions have replaced the Zn2+ to form ZnxCd1−xSe on TiO2 NT. The estimated results (Table 1) of the ZnxCd1−xSe prepared in different time are presented as ZnSe, Zn0.64Cd0.36Se, Zn0.27Cd0.73Se, and Zn0.13Cd0.87Se, corresponding to ion-exchange time for 0, 4, 8, and 12 h, respectively.
The ZnxCd1−xSe/TiO2 NT was prepared by ion-exchange process by immersing ZnSe/TiO2 NT in Cd2+ solution. This ion-exchange reaction occurred on the surface between ZnSe layers and Cd2+ solution as the following reaction (3) indicated:
Therefore, it is reasonable to speculate that a composition gradient ZnxCd1−xSe with three different phases would probably be formed due to the diffusion of Cd2+ from surface of ZnSe layers to the deep layer of ZnSe, which is illustrated in Fig. 4. Initially, once the ZnSe/TiO2 NT is immersed into the Cd2+ solution, the Cd2+ would substitute some of Zn2+ for form ZnxCd1−xSe fast. However, the quickly formed ZnxCd1−xSe layer upper the ZnSe layers could prevent the Cd2+ from further infusing into the deep layers of ZnSe to replace Zn2+, and in contrast the upper layer would be rich of Cd, leading to form a gradient composition with CdSe, ZnxCd1−xSe, and ZnSe as upper, middle, and deep layers (indicated in A and B selected zone in Fig. 4), respectively. The similar gradient composition of ZnxCd1−xSe has also been observed in previous report by Yang et al. [19].
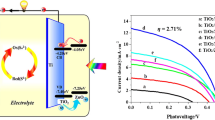
The current density–voltage (J–V) performance curves of the solar cells, assembled with ZnxCd1−xSe/TiO2 NT photoanodes, Pt/FTO counter electrodes and electrolyte based on mixture of 0.5 M sodium sulfide and 0.1 M sulfur aqueous solution, are presented in Fig. 5. The photovoltaic parameters of the ZnxCd1−xTiO2 NT solar cells including short circuit current density (J sc), open circuit voltage (V oc), fill factor (FF), and power conversion efficiency (PCE) are also summarized in Table 2. The solar cell based on ZnSe/TiO2 NT photoanode exhibits a J sc of 4.78 mA cm−2, a V oc of 0.34 V, and a FF of 0.36, producing a PCE of 0.59 %. With increasing Cd content in the ZnxCd1−xSe QDs, the photovoltaic parameters are also gradually enhanced. The solar cell based on Zn0.64Cd0.36Se/TiO2 NT photoanode gives a J sc of 5.61 mA cm−2, a V oc of 0.37 V, and a FF of 0.38, yielding a PCE of 0.79 %. The Zn0.27Cd0.73Se/TiO2 NT solar cell shows a drastically improved J sc of 8.79 mA cm−2, a V oc of 0.4 V, and a FF of 0.49, leading to a PCE of 1.72 %. Compared with the other sample solar cells, the solar cells based on Zn0.13Cd0.87Se/TiO2 NT photoanode produces a further enhanced J sc of 10.49 mA cm−2, V oc of 0.41 V, and FF of 0.50, yielding the PCE of 2.15 %. The significant improvement of photovoltaic performance for the solar cells based on various of ZnxCd1−x/TiO2 NT photoanode is mainly attributed to a broader light absorption, a more effective generation of photoexcited electrons, and a more efficient electron injection efficiency. The specific reasons will be further discussed later.
Compared with the ZnSe/TiO2 NT solar cell, the solar cells based on ZnxCd1−xSe/TiO2 NT photoanodes with different composition could effectively improve the photoelectric conversion efficiency due to a broader absorption of visible light as the increase of Cd content in ZnxCd1−xSe. The UV–Vis absorption spectra of the bare TiO2 NT, ZnSe/TiO2 NT, Zn0.64Cd0.36Se/TiO2 NT, Zn0.27Cd0.73Se/TiO2 NT, and Zn0.13Cd0.87Se/TiO2 NT photoanodes were characterized and the results are shown in Fig. 6. It could be observed that the bare TiO2 NT exhibits an absorption onset at around 380 nm, which corresponds to the band gap of anatase TiO2 (3.26 eV) [25]. The absorption edge of ZnSe/TiO2 NT at around 450 nm, corresponding to the band gap of 2.75 eV, which is higher than the band gap of ZnSe in bulk (2.67 eV) [15], suggesting that the ZnSe particles deposited on TiO2 NT are within the scale of QDs. Further expanded absorption edge in the visible light region is demonstrated upon the formation of ZnxCd1−xSe alloyed QDs. As the increase of Cd relative atomic ratio in the ZnxCd1−xSe alloyed QDs, the absorption edge of the photoanodes is gradually red-shifted to around 530 (Zn0.64Cd0.36Se/TiO2 NT), 600 (Zn0.27Cd0.73Se/TiO2 NT), and 690 nm (Zn0.13Cd0.87Se/TiO2 NT), corresponding the band gap of 2.34, 2.07, and 1.80 eV, respectively. This expanded absorption range in visible light region shows that the decrease of the optical band gap stems from the stoichiometry variation in the composition of the ZnxCd1−xSe alloy semiconductors. Thus, it can be concluded that the Cd-rich composition of ZnxCd1−xSe semiconductors amplify the photon absorption at the longer wavelength, which means more photons would be involved in the generation of photoexcited electrons, leading to an improved light harvesting efficiency.
The maximum J sc of 10.49 mA cm−2 obtained from Zn0.13Cd0.87Se/TiO2 NT solar cell can be attributed to the expanded absorption range in visible light region. Here, J sc is determined by the following equation:
where q is the elementary charge, I o is the incident photon flux of light (6.6 × 1016 cm−2S−1), η lh is the light harvesting efficiency, η ing is the charge injection efficiency, and η cc is the charge collection efficiency [26]. As discussed earlier, the increase of Cd content in ZnxCd1−xSe alloy semiconductors significantly enlarge the visible light absorption range. Moreover, the light absorption was expanded to the nearly entire visible light region with Zn0.13Cd0.87Se, which drastically improve the η lh of Zn0.13Cd0.87Se/TiO2 NT photoanode, thus resulting the increment of J sc.
The IPCE spectra was monitored to compare the photoresponse of ZnxCd1−xSe/TiO2 NT photoanodes, which is presented in Fig. 7. Comparison of these IPCE spectra yields two interesting observations. The first observation is the ability to tune the photoresponse of the photoanodes by controlling the Cd content in ZnxCd1−xSe. Similarities between the IPCE spectra and absorption spectra confirm the excitation of the ZnxCd1−xSe as the primary event responsible for photocurrent generation. The onset in photocurrent gradually shift to the red region with increasing Cd content in ZnxCd1−xSe is in agreement with the expanded absorption at longer wavelengths, which further confirmed that the light harvesting efficiency could be enhanced by forming Cd-rich ZnxCd1−xSe alloy QDs on TiO2 NT. The second observation is that the maximum IPCE value for various ZnxCd1−xSe/TiO2 NT photoanodes is on the rise with increasing Cd content. This incremental trend of IPCE may be attributed to the gradient composition of ZnxCd1−xSe, forming a stepwise structure of conduction band edge alignment in order of ZnSe < ZnxCd1−xSe < CdSe, which favours the collection of photoexcited electrons to the conduction band of TiO2 NT, leading to improvement of the charge collection efficiency. As a result, the maximum IPCE of 52 % with photocurrent onset around 690 nm, which obtained by the Zn0.13Cd0.87Se/TiO2 NT photoanode, highlights the contribution to the significant increase of J sc by forming Cd-rich ZnxCd1−xSe alloy semiconductors.
It is well-known that the band alignment of semiconductors in the photosensitizing architecture has an important effect on the electron injection from photoexcited QDs to the conduction band of TiO2, which influence the open circuit voltage (V oc) of QDSSCs. The V oc can be determined by the following equation [27]:
where E fn is the quasi-Fermi level of the electrons in semiconductor photoanode under illumination; E redox is the potential of redox electrolyte; e is the positive elementary charge; k BT is the thermal energy; n is the electron concentration in conduction band of the semiconductor photoanode under illumination; n 0 is the electron concentration in the dark condition. When the collection and injection efficiency of electrons are increased, more electrons would be accumulated in the conduction band of TiO2, which results in an increment of n, while the E redox remains unchangeable. This accumulation of electrons in conduction band of TiO2 is related with the band positions of semiconductor sensitizers. Figure 8a exhibits the band positions of TiO2, ZnSe, and CdSe in bulk, showing that ZnSe has a higher conduction band edge in comparison with that of TiO2 NT, which is beneficial to the injection of photoexcited electron from ZnSe. On the contrary, the CdSe has a lower conduction band edge than that of TiO2 NT, leading to a less efficient electron injection from CdSe. However, when TiO2, ZnSe, and CdSe are brought in contact as cascade architecture, the energy levels difference between ZnSe and CdSe would result in the electrons flow from ZnSe to CdSe, which is known as Fermi level alignment [11]. This redistribution of the electrons between ZnSe and CdSe can trigger a downward and upward shift of the band edges for ZnSe and CdSe respectively, leading to a large driving force for the injection of photoexcited electrons to the conduction band of TiO2 NT. Therefore, a band realignment model, as illustrated in Fig. 8b, is proposed for the Zn0.13Cd0.87Se/TiO2 NT photoanode. It is simply supposed that the Zn0.13Cd0.87Se/TiO2 NT photoanode possesses a gradient structure with ZnSe as inner layer (Zn-rich layer), Zn0.13Cd0.87Se as intermediate layer, and CdSe as out layer (Cd-rich layer). Based on this gradient structure, a realignment of the band positions among Zn-rich inner layer, Zn0.13Cd0.87Se intermediate layer, and Cd-rich outer layer occurs to form a step-wise band position structure, which is favorable to the collection and injection of photoexcited electrons to the conduction band of TiO2 NT. It can be concluded that such a gradient structure induces a synergistic effect, which increases the electron collection efficiency and driving force for accumulation of electrons in conduction band of TiO2, resulting in an enhancement V oc of solar cells.
4 Conclusion
In summary, the ZnxCd1−x/TiO2 NT photoanode have been constructed through a facile ion-exchange process after the formation of ZnSe QDs on translucent TiO2 NT array film for QDSSCs. The light absorption property of ternary ZnxCd1−x QDs layers can be tuned by changing the atomic ratio of Zn to Cd in alloyed ZnxCd1−x QDs. The power conversion efficiency of ZnxCd1−x/TiO2 NT solar cells gradually enhanced as the increase of Cd content in ZnxCd1−x alloyed QDs. The gradient structure of ZnxCd1−xSe layers composed of ZnSe (Zn-rich layer), ZnxCd1−x intermediate layer, and CdSe (Cd-rich layer) is proposed due to the diffusion of Cd cations. The band positions alignment of ternary sensitizers caused by the gradient structure contribute to the enhancement of photovoltaic performance for QDSSC based on Zn0.13Cd0.87Se/TiO2 NT photoanode, yielding a maximum power conversion efficiency up to 2.15 %. Further investigation into the tunable energy band alloyed QDs with a range of atoms and ratios could be expected to improve the performance of QDSSCs. The flexibility of tailoring various materials via the simple ion-exchange approach makes it a very promising synthetic method for preparation of TiO2 nanotube-based heterostructures with custom-designed properties for different applications.
References
K. Shankar, J.I. Basham, N.K. Allam, O.K. Varghese, G.K. Mor, X. Feng, M. Paulose, J.A. Seabold, K.-S. Choi, C.A. Grimes, J. Phys. Chem. C 113, 6327 (2009)
K.S. Leschkies, R. Divakar, J. Basu, E. Enache-Pommer, J.E. Boercker, C.B. Carter, U.R. Kortshagen, D.J. Norris, E.S. Aydil, Nano Lett. 7, 1793 (2007)
D.R. Baker, P.V. Kamat, Adv. Funct. Mater. 19, 805 (2009)
W.-T. Sun, Y. Yu, H.-Y. Pan, X.-F. Gao, Q. Chen, L.-M. Peng, J. Am. Chem. Soc. 130, 1124 (2008)
T. Toyoda, Q. Shen, J. Phys. Chem. Lett. 3, 1885 (2012)
S. Rühle, M. Shalom, A. Zaban, ChemPhysChem 11, 2290 (2010)
X. Ma, Y. Shen, Q. Wu, T. Shen, M. Cao, F. Gu, L. Wang, J. Inorg. Organomet. Polym. 23, 798 (2013)
B. Mukherjee, Y.R. Smith, V. Subramanian, J. Phys. Chem. C 116, 15175 (2012)
X.F. Gao, H.B. Li, W.T. Sun, Q. Chen, F.Q. Tang, L.M. Peng, J. Phys. Chem. C 113, 7531 (2009)
J. Luo, L. Ma, T. He, C.F. Ng, S. Wang, H. Sun, H.J. Fan, J. Phys. Chem. C 116, 11956 (2012)
Y.-L. Lee, Y.-S. Lo, Adv. Funct. Mater. 19, 604 (2009)
B.-M. Kim, M.-K. Son, S.-K. Kim, N.-Y. Hong, S. Park, M.-S. Jeong, H. Seo, K. Prabakar, H.-J. Kim, Electrochim. Acta 117, 92 (2014)
Z. Li, L. Yu, Y. Liu, S. Sun, J. Mater. Sci. 49, 6392 (2014)
X. Song, M. Wang, J. Deng, Z. Yang, C. Ran, X. Zhang, X. Yao, ACS Appl. Mater. Interfaces 5, 5139 (2013)
Y. Myung, J.H. Kang, J.W. Choi, D.M. Jang, J. Park, J. Mater. Chem. 22, 2157 (2012)
T. Shu, Z. Zhou, H. Wang, G. Liu, P. Xiang, Y. Rong, H. Han, Y. Zhao, J. Mater. Chem. 22, 10525 (2012)
A. Ranjitha, N. Muthukumarasamy, M. Thambidurai, D. Velauthapillai, R. Balasundaraprabhu, S. Agilan, J. Mater. Sci. Mater. Electron. 24, 3014 (2013)
J. Xu, X. Yang, H. Wang, X. Chen, C. Luan, Z. Xu, Z. Lu, V.A. Roy, W. Zhang, C.-S. Lee, Nano Lett. 11, 4138 (2011)
L. Yang, R. Zhou, J. Lan, Q. Zhang, G. Cao, J. Zhu, J. Mater. Chem. A 2, 3669 (2014)
X. Wang, Q. Peng, Y. Li, Acc. Chem. Res. 40, 635 (2007)
Q. Kang, Q. Cai, S.Z. Yao, C.A. Grimes, J. Ye, J. Phys. Chem. C 116, 16885 (2012)
Q. Chen, D. Xu, J. Phys. Chem. C 113, 6310 (2009)
H. Lee, M. Wang, P. Chen, D.R. Gamelin, S.M. Zakeeruddin, M. Grätzel, M.K. Nazeeruddin, Nano Lett. 9, 4221 (2009)
S. Huang, W. Peng, C. Ning, Q. Hu, H. Dong, J. Phys. Chem. C 116, 22359 (2012)
X. Chen, S.S. Mao, Chem. Rev. 107, 2891 (2007)
J.-Y. Hwang, S.-A. Lee, Y.H. Lee, S.-I. Seok, ACS Appl. Mater. Interfaces 2, 1343 (2010)
L. Yu, Z. Li, Y. Liu, F. Cheng, S. Sun, J. Power Sources 270, 42 (2014)
Acknowledgments
The authors gratefully acknowledge the support for this work from the Key Project of Tianjin Sci-Tech Support Program (No. 08ZCKFH01400).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Yu, L., Liu, Y. et al. Arrays of ZnxCd1−xSe/TiO2 nanotubes: fabrication by ion-exchange and photovoltaic applications. J Mater Sci: Mater Electron 26, 1625–1633 (2015). https://doi.org/10.1007/s10854-014-2586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2586-7