Abstract
The microstructures and microwave dielectric properties of (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 (x = 0.05–0.08) composite ceramics, prepared via a conventional solid-state ceramic route, were investigated. As expected, an increase in sintering temperature effectively promoted densification and enhanced the dielectric properties of the (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 ceramics used in this study. As the amount of Ca0.9Sr0.1TiO3 increased, temperature coefficient of resonant frequency (τ f ) increased; with a near-zero τ f obtained for samples where x = 0.06. The optimal microwave dielectric properties, a dielectric constant (ε r ) of 8.01, high quality factor (Q × f) of 58,389 GHz (at 14.6 GHz) and a τ f of −3.62 ppm/°C, were obtained for 0.94Mg2SiO4–0.06Ca0.9Sr0.1TiO3 sintered at 1,440 °C for 3 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, much attention has been drawn to the development of millimeter-wave telecommunications, which have the potential to transport a large amount of information with high data transfer rates. In order to satisfy this application, the microwave dielectric materials require a low dielectric constant (ε r ), a high quality factor Q × f for achieving frequency selectivity and stability, and a near-zero temperature coefficient of resonant frequency (τ f ) for temperature stability [1–8].
Silicates with low ε r are good candidate dielectrics for millimeter-wave applications. Forsterite (Mg2SiO4) is a silicate ceramic and has attracted a great deal of attention as a result of its low ε r (≈6.8) and high Q × f (240,000 GHz). However, Forsterite has a large negative τ f value (~−70 ppm/°C) [9], which limits its applicability. Some investigations have been made to adjust the τ f value of Forsterite to closer to 0 ppm/°C by combining it with TiO2 [10], CaTiO3 [11], or Ba3(VO4)2 [12], which have positive temperature coefficients of resonance frequency. In the present work, 0.91Mg2SiO4–0.09CaTiO3 composite ceramics with Bi2O3–Li2CO3–H3BO3 additions show good microwave dielectric properties (ε r ~ 7.7, Q × f ~ 11,300 GHz (f = 6.1 GHz) and τ f ~ −5.0 ppm/°C) [11]. A εr of 9.03, a Q × f of 52,500 GHz and a τ f of 0.6 ppm/°C were obtained for 0.55Mg2SiO4–0.45Ba3(VO4)2 ceramics [12]. However, V2O5 is used as an important raw chemical in the synthesis of Ba3(VO4)2 and it is harmful to our health and to the environment. In this paper, Ca0.9Sr0.1TiO3 (ε r ~ 170, Q × f ~ 8,319 GHz and τ f ~ 931 ppm/°C) [13], exhibiting a higher Q × f than CaTiO3, was added to the Mg2SiO4 as a τ f compensator. As reported, by adjusting the stoichiometry (Mg/Si = 2.05) in Mg2SiO4 ceramics, the formation of a MgSiO3 secondary phase could be suppressed effectively [14]. Therefore, the pure Mg2SiO4 was prepared by adjusting stoichiometry (Mg/Si = 2.05). The (1 − x)Mg2SiO4(Mg/Si = 2.05)–xCa0.9Sr0.1TiO3 system is likely to yield an environmentally-friendly material with excellent dielectric properties. In this work, microstructure and microwave dielectric properties of (1 − x)Mg2SiO4(Mg/Si = 2.05)–xCa0.9Sr0.1TiO3 (x = 0.05–0.08) ceramics were investigated.
2 Experimental procedures
(1 − x)Mg2SiO4(Mg/Si = 2.05)–xCa0.9Sr0.1TiO3 (x = 0.05, 0.06, 0.07 and 0.08) composite ceramics were prepared using a conventional solid-state reaction method. Both Mg2SiO4 (Mg/Si = 2.05) and Ca0.9Sr0.1TiO3 compounds were individually synthesized using reagent-grade powders: MgO (98.0 %), SiO2 (99.5 %), CaCO3 (99.0 %) and TiO2 (99.0 %). Mg2SiO4 (Mg/Si = 2.05) and Ca0.9Sr0.1TiO3 were weighed according to the above formula and then ball milled for 6 h whilst submersed in distilled water using zirconia balls, calcined for 4 h at 1,240 and 1,100 °C respectively. After this, the (1 − x)Mg2SiO4(Mg/Si = 2.05)–xCa0.9Sr0.1TiO3 mixtures were prepared by mixing Ca0.9Sr0.1TiO3 and Mg2SiO4 powders with several weight ratios. The mixtures were then ball milled for 6 h using zirconia balls in distilled water. After drying, the fine powders were mixed with appropriate PVA binders and pressed into pellets (11 mm in diameter and 5 mm in thickness) at 300 MPa. The pellets were then sintered at 1,380–1,500 °C for 3 h in air.
The crystalline phase compositions of the sintered samples were investigated by X-ray diffraction (XRD), using Cu Kα radiation [RIGAKU SmartLab(3)]. The surface microstructure of the thermally etched specimens was observed using a JEOL (JSM-5900) scanning electron microscopy (SEM) equipped with a NORAN VANTAGE DSI energy dispersive spectroscope (EDS). The bulk density of the sintered samples was measured using the Archimedean method. The dielectric properties of the samples at microwave frequency were measured using a network analyzer (AGILENT 8722ET) and analyzed using the modified Hakki and Coleman and Courtney’s methods [15, 16]. τ f was evaluated in the temperature range of 25–80 °C. It was determined by noting the change in resonant frequency and using the following equation:
where f 25 and f 80 represent resonant frequencies at temperatures 25 and 80 °C, respectively.
3 Results and discussion
Figure 1 illustrates the apparent porosity and bulk densities of the (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 ceramics sintered at 1,380–1,500 °C. The bulk densities increase as the x values increase from 0.05 to 0.08, largely due to an increase in the Ca0.9Sr0.1TiO3 content. The theoretical density of a Mg2SiO4 ceramic is 3.223 g/cm3, whereas the density of Ca0.8Sr0.2TiO3 is higher at 4.082 g/cm3. This suggests that the higher Ca0.9Sr0.1TiO3 content would show a relatively higher density in the ceramics. It also can be observed that the apparent porosity decreases and then increases minimally with an increase in sintering temperature, In contrast, the densities increase and then decrease with an increase in sintering temperature. The minimum apparent porosity and subsequent maximum densities of samples are obtained at 1,440 °C; likely to be due to the well-developed grain growth, as illustrated in Fig. 7.
Figures 2 and 3 show that ε r and Q × f of (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 ceramics separately. The trend seen in the increase in dielectric constant with an increase in the proportion of the Ca0.9Sr0.1TiO3 phase is in agreement with predictions, owing to the higher ε r value of Ca0.9Sr0.1TiO3. Where sintering temperatures were increased from 1,380 to 1,500 °C, ε r values increased to a maximum and subsequently decreased. The maximum εr values for the (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 ceramics were obtained at 1,440 °C. This is likely to be a result of the densification of the ceramics having a greater influence on their εr as εr increases when the bulk densities of samples increase.
Q × f values showed a similar trend with a variation in bulk density of the samples as a function of sintering temperature (Fig. 3). Q × f values increase to a maximum then subsequently decrease. At lower temperatures, the reduction of Q × f is due to a change in porosity and overall bulk density. However, at higher temperatures, the reduction of Q × f is largely due to porosity as well as abnormal grain growth, which can be seen in Figs. 1 and 8e.
Figure 4 illustrates the variation in τ f for (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 ceramics sintered at 1,440 °C as a function of x value. τ f increases from −19.29 to 33.7 ppm/°C with an increase in the Ca0.9Sr0.1TiO3 content. Moreover, this trend in the variation of τ f is consistent with the Lichtenecker empirical rule. As reported, τ f for Mg2SiO4 is −70 ppm/°C and τ f for Ca0.9Sr0.1TiO3 is 931 ppm/°C. In addition, a near-zero τ f (−3.62 ppm/°C) is obtained for 0.94Mg2SiO4–0.06Ca0.9Sr0.1TiO3 ceramics sintered at 1,440 °C for 3 h.
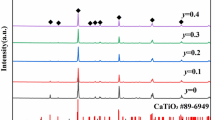
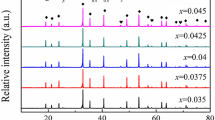
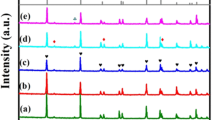
Figure 5 shows XRD patterns for (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 composite ceramics, Mg2SiO4 powder and Ca0.9Sr0.1TiO3 powder sintered at 1,440 °C for 3 h. Figure 6 shows XRD patterns for 0.94Mg2SiO4–0.06Ca0.9Sr0.1TiO3 ceramics sintered at different temperatures for just 3 h. All the main peaks can be indexed using the perovskite structure (orthorhombic: ICDD-PDF no. 82-0231) and Mg2SiO4 (orthorhombic: ICDD-PDF no. 04-0768). XRD phase analysis reveals that Ca0.9Sr0.1TiO3 and Mg2SiO4 coexist in a sintered composite ceramic and no other crystalline phases are detected. XRD phase analysis also shows that the MgSiO3 secondary phase could be suppressed by adjusting the ratio of Mg/Si in the Mg2SiO4 ceramics [14]. The intensities of the diffraction peaks of the Ca0.9Sr0.1TiO3 phases do not vary dramatically with an increase in the proportion of Ca0.9Sr0.1TiO3 in the compound. This is likely to be because the content of Ca0.9Sr0.1TiO3 is only small and varies only within a small range. Perovskite and Mg2SiO4 phases are observed for all samples, indicating that the sintering temperature has little influence on the composition of the 0.94Mg2SiO4–0.06Ca0.9Sr0.1TiO3 ceramics.
Figure 7a–d illustrates the representative SEM micrographs of (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 (x = 0.05, 0.06, 0.07 and 0.08) composite ceramics sintered at 1,440 °C for 3 h. All micrographs exhibit ceramics with dense microstructures with only small pores in Fig. 7a. However, the grain size is not uniformly distributed in all of the samples. The SEM micrographs of the 0.94Mg2SiO4–0.06Ca0.9Sr0.1TiO3 ceramics, sintered at different temperatures for 3 h, are shown in Fig. 8a–e. The number of pores decrease with an increase in sintering temperature from 1,380 to 1,470 °C. However, when the sintering temperature increases to 1,500 °C, the number of pores increases oppositely. In addition, the grain size increases abnormally (Fig. 8e), resulting in a decrease in Q × f. The samples with well-developed grain growth and dense microstructures are obtained at 1,440 °C for 3 h. In order to further verify the proportion of (1 − x)Mg2SiO4–xCa0.9Sr0.1TiO3 composite ceramics, the grains were analyzed using EDS. The EDS data is shown in Table 1 and is marked in Fig. 8c. Based on the EDS analyses, the grain marked A is Mg2SiO4 and that marked B is Mg2SiO4 and Ca0.9Sr0.1TiO3, which could be certified by the ratios of Mg:Si:O and Ca:Sr:Ti:O that are approximately 2:1:4 and 9:1:10:30 respectively. The observations of SEM and EDS are consistent with the XRD results shown in Fig. 5.
4 Conclusions
In this study, for the purpose of adjusting τ f values, Ca0.9Sr0.1TiO3 was added to Mg2SiO4. The microstructure and microwave dielectric properties of the (1 − x)Mg2SiO4 –xCa0.9Sr0.1TiO3 (x = 0.05–0.08) ceramics were investigated as a function of composition (x) and sintering temperature. All the samples exhibited two crystalline phases: Perovskite and Forsterite. The microwave dielectric prosperities and bulk densities were found to be associated with the varying contents of Ca0.9Sr0.1TiO3 and sintering temperatures. At the composition of x = 0.06, the 0.94Mg2SiO4–0.06Ca0.9Sr0.1TiO3 ceramics sintered at 1,440 °C for 3 h show excellent microwave dielectric properties (ε r = 8.01, Q × f = 58,389 GHz and τ f = −3.62 ppm/°C), indicating that they could be used as candidate materials for many applications.
References
R.J. Cava, J. Mater. Chem. 11, 54–62 (2001)
T. Fujii, A. Ando, Y. Sakabe, J. Eur. Ceram. Soc. 26, 1857–1860 (2006)
T. Fujii, A. Ando, Y. Sakabe, Jpn. J. Appl. Phys. 43, 6765–6768 (2004)
H. Ohsato, T. Tsunooka, A. Kan, Y. Ohishi, Y. Miyauchi, Y. Tohdo, T. Okawa, K. Kakimoto, H. Ogawa, Key Eng. Mater. 269, 195–198 (2004)
K.X. Song, X.M. Chen, C.W. Zheng, Ceram. Int. 34, 917–920 (2008)
M.E. Song, J.S. Kim, M.R. Joung, S. Nahm, J. Am. Ceram. Soc. 91, 2747–2750 (2008)
L. Cheng, P. Liu, X.M. Chen, J. Alloys Compd. 513, 373–377 (2012)
J. Sugihara, K. Kakimoto, I. Kagomiya, H. Ohsato, J. Eur. Ceram. Soc. 27, 3105–3108 (2007)
T. Tsunooka, T. Sugiyama, H. Ohsato, K. Kakimoto, M. Andou, Y. Higashida, H. Sugiura, Key Eng. Mater. 269, 199–202 (2004)
I. Kagomiya, J. Sugihara, K. Kakimoto, H. Ohsato, J. Electroceramics 22, 327–333 (2009)
G. Dou, D. Zhou, M. Guo, S. Gong, Y. Hu, J. Mater. Sci. Mater. Electron. 24, 1431–1438 (2013)
S.Q. Meng, Z.X. Yue, H. Zhuang, F. Zhao, L.T. Li, J. Am. Ceram. Soc. 93, 359–361 (2010)
P.L. Wise, I.M. Reaney, W.E. Lee, T.J. Price, D.M. Iddles, D.S. Cannell, J. Eur. Ceram. Soc. 21, 1723–1726 (2001)
K.X. Song, X.M. Chen, X.C. Fan, J. Am. Ceram. Soc. 90, 1808–1811 (2007)
B.W. Hakki, P.D. Coleman, IEEE Trans. Microw. Theory Tech. 8, 402–410 (1960)
W.E. Courtney, IEEE Trans. Microw. Theory Tech. 18, 476–485 (1970)
Acknowledgments
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT), IRT1146.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Feng, Y., Qiu, T. et al. Microstructures and microwave dielectric properties of Mg2SiO4–Ca0.9Sr0.1TiO3 ceramics. J Mater Sci: Mater Electron 26, 1316–1321 (2015). https://doi.org/10.1007/s10854-014-2540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2540-8