Abstract
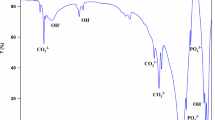
Bovine bone-derived hydroxyapatite (BBHAp) has been reported to be a biocompatible and efficient bone graft substitute. In this study, hydroxyapatite (HAp) was produced by combining the cleaning process with alkali and thermal treatment of bovine bone. The calcination temperature was 700°C. The functionality of the BBHAp scaffold as a bone graft substitute was determined by its physicochemical properties and in vitro biocompatibility. Physicochemical properties such as mechanical properties, degradation, ion composition, crystallinity, crystallite size, surface characteristics, and porosity were characterized using several analytical tools, including X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray analysis (EDX), X-ray fluorescence (XRF), Vickers hardness, contact angle (CA), and biodegradation. XRD, SEM, and TEM analyses confirmed the hexagonal and spherical crystalline structure of BBHAp with a size of approximately 100 nm. Elemental analysis using EDX and XRF confirmed the presence of Sr, Zn, Mg, and Na in the formulation, while SEM showed the hierarchical porous structures of BBHAp. The microhardness values were approximately 229.44 ± 82.52 HV or 2.2501 ± 1.18 GPa.The porosity of BBHAp ranged from 25% to 47.18%, with a bulk density of 0,0324 g/cc. The biodegradation rate was approximately 0.7 mg/month. These results indicated that the BBHAp scaffold did not collapse under the conditions of in vitro cell cultivation. However, the results of this study, in which the contact angles of the materials were 0–11°, showed that the materials were hydrophilic. Cell activities were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) and Hoechst fluorescent staining assays. MTT assay results showed no cytotoxicity. Hoechst fluorescent staining showed cell adhesion and proliferation on the material surfaces after 72 h and 120 h. In conclusion, BBHAp can be considered a potential bone substitute material.








Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Anita Lett J, Sagadevan S, Fatimah I, Hoque ME, Lokanathan Y, Léonard E et al (2021) Recent advances in natural polymer-based hydroxyapatite scaffolds: properties and applications. Eur Polym J 148:110360
Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, Koval KJ (2019) Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma 33(4):203–213
Sathiyavimal S, Vasantharaj S, LewisOscar F, Selvaraj R, Brindhadevi K, Pugazhendhi A (2020) Natural organic and inorganic–hydroxyapatite biopolymer composite for biomedical applications. Progress Org Coat 147:105858
Wong SK, Yee MMF, Chin KY, Ima-Nirwana S (2023) A review of the application of natural and synthetic scaffolds in bone regeneration. J Funct Biomater 14(5):286
Li B, Moriarty TF, Webster T, Xing M (2020) Racing for the surface: antimicrobial and interface tissue engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-34471-9
Ye K, Cao L, Li S, Yu L, Ding J (2016) Interplay of matrix stiffness and cell-cell contact in regulating differentiation of stem cells. ACS Appl Mater Interfaces 8(34):21903–21913
Wan W, Cheng B, Zhang C, Ma Y, Li A, Xu F et al (2019) Synergistic effect of matrix stiffness and inflammatory factors on osteogenic differentiation of MSC. Biophys J 117(1):129–142
Narayan R (2009) Biomedical materials. Springer, Boston. https://doi.org/10.1007/978-0-387-84872-3
Hoornaert A, Layrolle P (2020) Chapter 8—bone regenerative issues related to bone grafting biomaterials. In: Alghamdi H, Jansen J (eds) Dental implants and bone grafts. Woodhead Publishing, Sawston, pp 207–215
Hayakawa S (2015) In vitro degradation behavior of hydroxyapatite. In: Mucalo M (ed) Hydroxyapatite (Hap) for biomedical applications. Woodhead Publishing, Sawston, pp 85–105
Odusote JK, Danyuo Y, Baruwa AD, Azeez AA (2019) Synthesis and characterization of hydroxyapatite from bovine bone for production of dental implants. J Appl Biomater Funct Mater 17(2):228080001983682. https://doi.org/10.1177/2280800019836829
Martinez-Marquez D, Delmar Y, Sun S, Stewart RA (2020) Exploring macroporosity of additively manufactured titanium metamaterials for bone regeneration with quality by design: a systematic literature review. Materials 13(21):4794
Abifarin JK, Obada DO, Dauda ET, Dodoo-Arhin D (2019) Experimental data on the characterization of hydroxyapatite synthesized from biowastes. Data Brief 26:104485
Ha LV, Mai DNT, Cao HT (2021) Fabrication of nano- or micro-powder for hydroxyapatite particles derived from bovine bone. VNUHCM J Sci Technol Dev 24(1):875–883
Hà L, Tien C, Tran D (2020) The The variable size consideration of hydroxyapatite extracted from bovine bone at different temperature furnaces. Sci Technol Dev J—Nat Sci 5(1):1005–1014
Manalu J, Soegijono B, Indrani D.(2015) Characterization of hydroxyapatite derived from bovine bone. Asian J Appl Sci. 3(4)
Pramanik S, Hanif A, Pingguan-Murphy B, Abu ON (2012) Morphological change of heat treated bovine bone: a comparative study. Materials 6(1):65–75
Forero-Sossa PA, Olvera-Alvarez IU, Salazar-Martinez JD, Espinosa-Arbelaez DG, Segura-Giraldo B, Giraldo-Betancur AL (2021) Biogenic hydroxyapatite powders: effects of source and processing methodologies on physicochemical properties and bioactive response. Mater Charact 173:110950
Nagy G, Lorand T, Patonai Z, Montsko G, Bajnoczky I, Marcsik A et al (2008) Analysis of pathological and non-pathological human skeletal remains by FT-IR spectroscopy. Forensic Sci Int 175(1):55–60
Thompson TJU, Gauthier M, Islam M (2009) The application of a new method of fourier transform infrared spectroscopy to the analysis of burned bone. J Archaeol Sci 36(3):910–914
Airini R, Iordache F, Alexandru D, Savu L, Epureanu FB, Mihailescu D et al (2019) Senescence-induced immunophenotype, gene expression and electrophysiology changes in human amniocytes. J Cell Mol Medi 23(11):7233–7245. https://doi.org/10.1111/jcmm.14495
de Oliveira PGFP, de Melo Soares MS, Silveira E Souza AMM, TabaPalioto MDB, Messora MR et al (2021) Influence of nano-hydroxyapatite coating implants on gene expression of osteogenic markers and micro-CT parameters. An in vivo study in diabetic rats. J Biomed Mater Res A 109(5):682–694
Dumitrescu CR, Neacsu IA, Surdu VA, Nicoara AI, Iordache F, Trusca R et al (2021) Nano-hydroxyapatite vs. xenografts: synthesis, characterization, and in vitro behavior. Nanomaterials 11(9):2289
Rincón-López JA, Hermann-Muñoz JA, Giraldo-Betancur AL, De Vizcaya-Ruiz A, Alvarado-Orozco JM, Muñoz-Saldaña J (2018) Synthesis, characterization and in vitro study of synthetic and bovine-derived hydroxyapatite ceramics: a comparison. Materials 11(3):333
Mucalo M (2015) Hydroxyapatite (Hap) for biomedical applications. Woodhead Publishing, Sawston
Pires LA, de Meira CR, Tokuhara CK, de Oliveira FA, Dainezi VB, Zardin Graeff MS et al (2020) Wettability and pre-osteoblastic behavior evaluations of a dense bovine hydroxyapatite ceramics. J Oral Sci 62(3):259–264
Canillas M, Pena P, de Aza AH, Rodríguez MA (2017) Calcium phosphates for biomedical applications. Boletín de la Sociedad Española de Cerámica y Vidrio 56(3):91–112
Chang H, Chen Y, Tuan W, Wu C, Lai P (2021) Attachment and migration of cells on porous bone graft. J Am Ceram Soc 104(4):1649–1654. https://doi.org/10.1111/jace.17591
Petrova OV, Bakina KA, Ehrlich H (2021) NEXAFS study of carbonate substituted bioapatite. J Phys: Conf Ser 2103(1):012153
Dorozhkin SV (2018) Current state of bioceramics. J Ceram Sci Technol. https://doi.org/10.4416/JCST2018-00026
Rahavi SS, Ghaderi O, Monshi A, Fathi MH (2017) A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ J Non-ferrous Metals. 58(3):276–286
Xiao D, Zhang J, Zhang C, Barbieri D, Yuan H, Moroni L et al (2020) The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater 106:22–33
Han KS, Sathiyaseelan A, Saravanakumar K, Wang MH (2022) Wound healing efficacy of biocompatible hydroxyapatite from bovine bone waste for bone tissue engineering application. J Environ Chem Eng 10(1):106888
Prathap S, Rajesh KS, Thomas NG et al (2022) Synthesis and extraction of hydroxyapatite grafts from animal sources. J Dental Sci Res Rep 139:1–6
Burduel AC, Gherasim O, Andronescu E, Grumezescu AM, Ficai A (2022) Inorganic nanoparticles in bone healing applications. Pharmaceutics 14(4):770
Rabiei A (2009) Hip prosthesis. In: Narayan R (ed) Biomedical materials. Springer, Boston. https://doi.org/10.1007/978-0-387-84872-3_13
Londoño-Restrepo SM, Ramirez-Gutierrez CF, Del Real A, Rubio-Rosas E, Rodriguez-García ME (2016) Study of bovine hydroxyapatite obtained by calcination at low heating rates and cooled in furnace air. J Mater Sci 51(9):4431–4441
Marques A, Miranda G, Silva F, Pinto P, Carvalho Ó (2021) Review on current limits and potentialities of technologies for biomedical ceramic scaffolds production. J Biomed Mater Res 109(3):377–393. https://doi.org/10.1002/jbm.b.34706
Chen Z, Zhang X, Yang Y, Zhou K, Wragg N, Liu Y et al (2017) Fabrication and characterization of 3D complex hydroxyapatite scaffolds with hierarchical porosity of different features for optimal bioactive performance. Ceramics Int 43(1):336–344
Kazimierczak P, Przekora A (2020) Osteoconductive and osteoinductive surface modifications of biomaterials for bone regeneration: a concise review. Coatings 10(10):971
LeGeros RZ (2008) Calcium phosphate-based osteoinductive materials. Chem Rev 108(11):4742–4753s
Cell responses to surface and architecture of tissue engineering scaffolds | IntechOpen [Internet]. [cited 2024 Feb 18]. Available from: https://www.intechopen.com/chapters/19013
Webster BE Thomas.(2014) Cell response to nanoscale features and its implications in tissue regeneration: an orthopedic perspective. In: Nanotechnology and regenerative engineering. 2nd ed. CRC Press
Wang X, Chen X, Hodgson P, Wen C (2006) Elastic modulus and hardness of cortical and trabecular bovine bone measured by nanoindentation. Trans Nonferrous Metals Soc China. 16:e744–e748
Heidari F, Razavi M, Zamani MAA, Tahriri M, Tayebi L (2018) A comparison between the properties of natural hydroxyapatite produced by cold isostatic pressing and spark plasma sintering techniques. J Aust Ceram Soc 54(2):337–344. https://doi.org/10.1007/s41779-017-0158-z
Asgharzadeh Shirazi H, Ayatollahi MR, Beigzadeh B (2016) Preparation and characterisation of hydroxyapatite derived from natural bovine bone and PMMA/BHA composite for biomedical applications. Mater Technol 31(8):448–453. https://doi.org/10.1080/10667857.2015.1105577
Perić Kačarević Z, Kavehei F, Houshmand A, Franke J, Smeets R, Rimashevskiy D et al (2018) Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int J Artif Organs 41(11):789–800. https://doi.org/10.1177/0391398818771530
Valencia-Llano CH, López-Tenorio D, Grande-Tovar CD (2022) Biocompatibility assessment of two commercial bone xenografts by in vitro and in vivo methods. Polymers 14(13):2672
Liu Q, Douglas T, Zamponi C, Becker ST, Sherry E, Sivananthan S et al (2023) Comparison of in vitro biocompatibility of NanoBone® and BioOss® for human osteoblasts comparison of in vitro biocompatibility. Clin Oral Implant Res 22(11):1259–1264. https://doi.org/10.1111/j.1600-0501.2010.02100.x
Acknowledgements
Not Applicable
Author information
Authors and Affiliations
Contributions
Huu Tien Cao provided conceptualization, project administration, and funding acquisition. Van Linh Ha performed methodology, visualization, writing–review and editing, and resource. Diem Ngoc Thi Mai presented investigation and writing–original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical approval
Not applicable.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, H.T., Ha, V.L. & Mai, D.N.T. In vitro assessment of bovine-derived hydroxyapatite for bone xenografts. J Mater Sci 59, 10406–10418 (2024). https://doi.org/10.1007/s10853-024-09794-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09794-z