Abstract
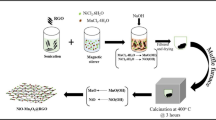
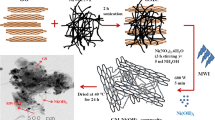
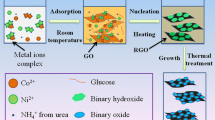
Nickel oxide-titanium dioxide/reduced graphene oxide (NiOTiO2/rGO) nanocomposites were synthesized for the first time using a simple solvothermal method for asymmetric supercapacitor applications. Crystalline NiO and TiO2 nanoparticles were dispersed uniformly on different ratios of rGO multilayers. The specific capacitance was enhanced significantly with an increase in the ratio of rGO. The electrochemical performance of the prepared composites was evaluated in 1.0 M KOH solution. The NTG10 composite showed the highest specific capacitance among all the samples, reaching 793.6 F g−1 at a current density of 5 A g−1 with capacity retention of 89.37% of its initial specific capacitance over 5000 cycles. The suggested electrode achieved a maximum energy density of 33.4 Wh Kg−1 at a power density of 1377 W Kg−1. This enhanced storage performance is attributed to the synergistic combination of NiO, TiO2, and rGO materials which offers superior electrical conductivity, and pseudocapacitive charge-storage mechanisms. The results indicate that NiOTiO2/rGO nanocomposite is a promising electrode material for supercapacitor applications.








Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data availability
All data are included in the paper.
References
Mohd Zaid NA, Idris NH (2016) Enhanced capacitance of hybrid layered graphene/nickel nanocomposite for supercapacitors. Sci Rep. https://doi.org/10.1038/srep32082
Niu L, Wang J, Hong W, Sun J, Fan Z, Ye X, Wang H, Yang S (2014) Solvothermal synthesis of Ni/reduced graphene oxide composites as electrode material for supercapacitors. Electrochim Acta 123:560–568. https://doi.org/10.1016/j.electacta.2014.01.005
Sarangapani S, Tilak BV, Chen C-P (1996) REVIEW materials for electrochemical capacitors theoretical and experimental consfraints. Xxx. https://doi.org/10.1149/1.1837291
Liu H, Wang Y, Gou X, Qi T, Yang J, Ding Y (2013) Three-dimensional graphene/polyaniline composite material for high-performance supercapacitor applications. Mater Sci Eng B Solid-State Mater Adv Technol 178:293–298. https://doi.org/10.1016/j.mseb.2012.12.002
Libich J, Máca J, Vondrák J, Čech O, Sedlaříková M (2018) Supercapacitors: properties and applications. J Energy Storage 17:224–227. https://doi.org/10.1016/j.est.2018.03.012
Arbizzani C, Mawragostino M, Menechello L (1995) Characterization by impedance spectroscopy of a polymer-based supercapacitor. Electrochim Acta 40:2223–2228. https://doi.org/10.1016/0013-4686(95)00167-D
Dai J, Fu K, Palanisamy R, Gong A, Pastel G, Kornfeld R, Zhu H, Sanghadasa M, Bekyarova E, Hu L (2017) A solid state energy storage device with supercapacitor-battery hybrid design. J Mater Chem A 5:15266–15272. https://doi.org/10.1039/c7ta02638b
Qu D, Feng X, Wei X, Guo L, Cai H, Tang H, Xie Z (2017) Synthesis of MnO nano-particle@Flourine doped carbon and its application in hybrid supercapacitor. Appl Surf Sci 413:344–350. https://doi.org/10.1016/j.apsusc.2017.03.305
Zhang SS (2017) Eliminating pre-lithiation step for making high energy density hybrid Li-ion capacitor. J Power Sources 343:322–328. https://doi.org/10.1016/j.jpowsour.2017.01.061
Hudak NS, Schlichting AD, Eisenbeiser K (2017) Structural supercapacitors with enhanced performance using carbon nanotubes and polyaniline. J Electrochem Soc 164:A691–A700. https://doi.org/10.1149/2.0721704jes
Sedlakova V, Sikula J, Majzner J, Sedlak P, Kuparowitz T, Buergler B, Vasina P (2015) Supercapacitor equivalent electrical circuit model based on charges redistribution by diffusion. J Power Sources 286:58–65. https://doi.org/10.1016/j.jpowsour.2015.03.122
AlShammari AS, Halim MM, Yam FK, Kaus NHM (2020) Synthesis of Titanium Dioxide (TiO2)/Reduced Graphene Oxide (rGO) thin film composite by spray pyrolysis technique and its physical properties. Mater Sci Semicond Process 116:105140. https://doi.org/10.1016/j.mssp.2020.105140
Stoller MD, Park S, Yanwu Z, An J, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 8:3498–3502. https://doi.org/10.1021/nl802558y
Vivekchand SRC, Sekhar C, Govindaraj A, Rao R (2008) Graphene-based electrochemical supercapacitors. J Chem Sci 120:9–13. https://doi.org/10.1007/s12039-008-0002-7
Wang HW, Hu ZA, Chang YQ, Chen YL, Wu HY, Zhang ZY, Yang YY (2011) Design and synthesis of NiCo2O4-reduced graphene oxide composites for high performance supercapacitors. J Mater Chem 21:10504–10511. https://doi.org/10.1039/c1jm10758e
Li J, Li Y, Guo Y, Lv J, Yi W, Ma P (2018) A facile method to enhance electrochemical performance of high-nickel cathode material Li(Ni0.8Co0.1Mn0.1)O2 via Ti doping. J Mater Sci Mater Electron 29:10702–10708. https://doi.org/10.1007/s10854-018-9093-1
Lu CH, Naresh N, Kumar PS, Som S (2019) Microwave-assisted solvothermal synthesis and electrochemical characterizations of ternary perovskite NiTiO3 anode materials for lithium-ion batteries. Ceram Int 45:19517–19521. https://doi.org/10.1016/j.ceramint.2019.06.057
Chen Y, Tang W, Ma J, Ge B, Wang X, Wang Y, Ren P, Liu R (2020) Nickel-decorated TiO2 nanotube arrays as a self-supporting cathode for lithium-sulfur batteries. Front Mater Sci 14:266–274. https://doi.org/10.1007/s11706-020-0509-5
Liu Y, Cai X, Jiang J, Yan M, Shi W (2017) Nitrogen and carbon co-doped Ni-TiO 2 spindles for high performance electrochemical capacitor electrodes. Appl Surf Sci 396:774–779. https://doi.org/10.1016/j.apsusc.2016.11.023
Loka C, Yu H, Lee KS, Cho J (2013) Nanocomposite Si/(NiTi) anode materials synthesized by high-energy mechanical milling for lithium-ion rechargeable batteries. J Power Sources 244:259–265. https://doi.org/10.1016/j.jpowsour.2013.01.107
Yang R-K, Wu Z-G, Li Y-C, Li R, Qiu L, Wang D, Yang L, Guo X-D (2020) Optimization of the electrochemical properties of LiNi 0.8 Co 0.1 Mn 0.1 O 2 cathode material by titanium doping. Ionics 26:3223–3230. https://doi.org/10.1007/s11581-019-03399-2/Published
Vangapally N, Jindal S, Gaffoor SA, Martha SK (2018) Titanium dioxide-reduced graphene oxide hybrid as negative electrode additive for high performance lead-acid batteries. J Energy Storage 20:204–212. https://doi.org/10.1016/j.est.2018.09.015
Liu Y, Gao C, Li Q, Pang H (2019) Nickel oxide/graphene composites: synthesis and applications. Chem Eur J. https://doi.org/10.1002/chem.201803982
Wang H, Yi H, Chen X, Wang X (2014) Asymmetric supercapacitors based on nano-architectured nickel oxide/graphene foam and hierarchical porous nitrogen-doped carbon nanotubes with ultrahigh-rate performance. J Mater Chem A 2:3223–3230. https://doi.org/10.1039/c3ta15046a
Zhu X, Zhu Y, Murali S, Stoller MD, Ruoff RS (2011) Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 5:3333–3338. https://doi.org/10.1021/nn200493r
Gu H, Ding Z, Yang Z, Yu W, Zhang W, Lu W, Zhang L, Wang K, Wang L, Fu Y (2019) Microstructure evolution and electrochemical properties of TiO 2 / Ti-35Nb-2Ta-3Zr micro / nano-composites fabricated by friction stir processing. Mater Des 169:107680
Kate RS, Khalate SA, Deokate RJ (2017) Electrochemical properties of spray deposited nickel oxide (NiO) thin films for energy storage systems. J Anal Appl Pyrolysis 125:289–295. https://doi.org/10.1016/j.jaap.2017.03.014
Bonomo M, Dini D, Decker F (2018) Electrochemical and photoelectrochemical properties of nickel oxide (NiO) with nanostructured morphology for photoconversion applications. Front Chem 6:1–16. https://doi.org/10.3389/fchem.2018.00601
Sajid F, Jabeen N, Khan LU, Sohail M, Rehman A, Akhter Z (2023) Local atomic structure order and electrochemical properties of NiO based nano-catalysts for ethanol sensing at room temperature. J Phys Chem Solids 175:111201. https://doi.org/10.1016/j.jpcs.2022.111201
Nguyen K, Hoa ND, Hung CM, Le Thanh DT, Van Duy N, Van Hieu N (2018) A comparative study on the electrochemical properties of nanoporous nickel oxide nanowires and nanosheets prepared by a hydrothermal method. RSC Adv 8:19449–19455. https://doi.org/10.1039/c8ra02862a
Gebretinsae H, Welegergs G, Matinise N, Maazab M, Nurua ZY (2020) Electrochemical study of Nickel Oxide (NiO) nanoparticles from cactus plant extract. MRS Adv 5:1095–1102. https://doi.org/10.1557/adv.2020.118
Hummers WS, Offeman E (1957) Preparation of graphitic oxide. J Am Chem Soc 208:1937. https://doi.org/10.1021/ja01539a017
Sun N, Ma J, Wang C, Xue J, Qiang L, Tang J (2018) A facile and efficient method to directly synthesize TiO2/rGO with enhanced photocatalytic performance. Superlattices Microstruct 121:1–8. https://doi.org/10.1016/j.spmi.2018.07.017
Sharma N, Tomar S, Shkir M, Kant Choubey R, Singh A (2019) Study of optical and electrical properties of graphene oxide. Mater Today Proc 36:730–735. https://doi.org/10.1016/j.matpr.2020.04.861
Kunnamareddy M, Rajendran R, Sivagnanam M, Rajendran R, Diravidamani B (2021) Nickel and sulfur codoped TiO2 nanoparticles for efficient visible light photocatalytic activity. J Inorg Organomet Polym Mater 31:2615–2626. https://doi.org/10.1007/s10904-021-01914-5
Abdel-Baset TA, Bashal AH (2020) Structure and AC conductivity of zinc and nickel-doped TiO2 nanocomposite synthesized by simple incipient wet impregnation method. J Mater Sci Mater Electron 31:18533–18540. https://doi.org/10.1007/s10854-020-04397-1
Vasu D, Karthi Keyan A, Sakthinathan S, Chiu TW (2022) Investigation of electrocatalytic and photocatalytic ability of Cu/Ni/TiO2/MWCNTs nanocomposites for detection and degradation of antibiotic drug Furaltadone. Sci Rep 12:1–16. https://doi.org/10.1038/s41598-022-04890-z
Yu C, Li M, Yang D, Pan K, Yang F, Xu Y, Yuan L, Qu Y, Zhou W (2021) NiO nanoparticles dotted TiO2 nanosheets assembled nanotubes P-N heterojunctions for efficient interface charge separation and photocatalytic hydrogen evolution. Appl Surf Sci 568:150981. https://doi.org/10.1016/j.apsusc.2021.150981
Faisal M, Harraz FA, Ismail AA, El-Toni AM, Al-Sayari SA, Al-Hajry A, Al-Assiri MS (2018) Novel mesoporous NiO/TiO2 nanocomposites with enhanced photocatalytic activity under visible light illumination. Ceram Int 44:7047–7056. https://doi.org/10.1016/j.ceramint.2018.01.140
Sharma A, Lee BK (2016) Integrated ternary nanocomposite of TiO2/NiO/reduced graphene oxide as a visible light photocatalyst for efficient degradation of o-chlorophenol. J Environ Manag 181:563–573. https://doi.org/10.1016/j.jenvman.2016.07.016
Kusiak-Nejman E, Wanag A, Kowalczyk Ł, Kapica-Kozar J, Colbeau-Justin C, Mendez Medrano MG, Morawski AW (2017) Graphene oxide-TiO2 and reduced graphene oxide-TiO2 nanocomposites: Insight in charge-carrier lifetime measurements. Catal Today 287:189–195. https://doi.org/10.1016/j.cattod.2016.11.008
Liu Y (2014) Hydrothermal synthesis of TiO2-RGO composites and their improved photocatalytic activity in visible light. RSC Adv 4:36040–36045. https://doi.org/10.1039/c4ra06342b
Johra FT, Lee JW, Jung WG (2014) Facile and safe graphene preparation on solution based platform. J Ind Eng Chem 20:2883–2887. https://doi.org/10.1016/j.jiec.2013.11.022
Schönfelder R, Rümmeli MH, Gruner W, Löffler M, Acker J, Hoffmann V, Gemming T, Büchner B, Pichler T (2007) Purification-induced sidewall functionalization of magnetically pure single-walled carbon nanotubes. Nanotechnology 18:375601. https://doi.org/10.1088/0957-4484/18/37/375601
Liu Y, Zhang Y, Ma G, Wang Z, Liu K, Liu H (2013) Ethylene glycol reduced graphene oxide/polypyrrole composite for supercapacitor. Electrochim Acta 88:519–525. https://doi.org/10.1016/j.electacta.2012.10.082
Hidayah NMS, Liu WW, Lai CW, Noriman NZ, Khe CS, Hashim U, Lee HC (2017) Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. In: AIP Conf Proc 1892. https://doi.org/10.1063/1.5005764.
Ansari AR, Ansari SA, Parveen N, Ansari MO, Osman Z (2022) Silver nanoparticles decorated on the surface of reduced graphene oxide coated titanium oxide nanocomposite for enhanced electrochemical supercapacitance performance. Ionics (Kiel) 28:4793–4804. https://doi.org/10.1007/s11581-022-04685-2
Article FL, Meti S, Rahman MR, Bhat KU (2018) Chemical free synthesis of graphene oxide in the preparation of reduced graphene oxide-zinc oxide nanocomposite with improved photocatalytic properties. Appl Surf Sci 451:67–75. https://doi.org/10.1016/j.apsusc.2018.04.138
Ferrari A, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B Condens Matter Mater Phys 61:14095–14107. https://doi.org/10.1103/PhysRevB.61.14095
Pimenta MA, Dresselhaus G, Dresselhaus MS, Cançado LG, Jorio A, Saito R (2007) Studying disorder in graphite-based systems by Raman spectroscopy. Phys Chem Chem Phys 9:1276–1291. https://doi.org/10.1039/b613962k
López-Díaz D, López Holgado M, García-Fierro JL, Velázquez MM (2017) Evolution of the Raman spectrum with the chemical composition of graphene oxide. J Phys Chem C 121:20489–20497. https://doi.org/10.1021/acs.jpcc.7b06236
Lin Z, Ye X, Han J, Chen Q, Fan P, Zhang H, Xie D, Zhu H, Zhong M (2015) Precise control of the number of layers of graphene by picosecond laser thinning. Sci Rep 5:1–7. https://doi.org/10.1038/srep11662
Hwangbo Y, Lee CK, Mag-Isa AE, Jang JW, Lee HJ, Lee SB, Kim SS, Kim JH (2014) Interlayer non-coupled optical properties for determining the number of layers in arbitrarily stacked multilayer graphenes. Carbon N Y 77:454–461. https://doi.org/10.1016/j.carbon.2014.05.050
Cui T et al (2014) Effect of lattice stacking orientation and local thickness variation on the mechanical behavior of few layer graphene oxide. Int J Refrig 43:36–49. https://doi.org/10.1016/j.carbon.2018.04.074.This
Singh M, Jha HS, Agarwal P (2014) Growth of large sp2 domain size single and multi-layer graphene films at low substrate temperature using hot filament chemical vapor deposition. Mater Lett 126:249–252. https://doi.org/10.1016/j.matlet.2014.04.066
Salimian M, Ivanov MS, Bdikin I, Pohl D, Oswald S, Khomchenko VA, Paixão JA, Rellinghaus B, Marques PAAP, Gonçalves G (2019) Nanoengineered nickel/reduced graphene oxide composites: Control of interfacial nanostructure for tunable electrophysical properties. Appl Surf Sci 498:143781. https://doi.org/10.1016/j.apsusc.2019.143781
Vijaya Kumar P, Jafar Ahamed A, Karthikeyan M (2019) Synthesis and characterization of NiO nanoparticles by chemical as well as green routes and their comparisons with respect to cytotoxic effect and toxicity studies in microbial and MCF-7 cancer cell models. SN Appl Sci 1:1–15. https://doi.org/10.1007/s42452-019-1113-0
Abbas Z, Holmberg JP, Hellström AK, Hagström M, Bergenholtz J, Hassellöv M, Ahlberg E (2011) Synthesis, characterization and particle size distribution of TiO2 colloidal nanoparticles. Colloids Surfaces A Physicochem Eng Asp 384:254–261. https://doi.org/10.1016/j.colsurfa.2011.03.064
Kalaivani T, Anilkumar P, Jasmin J, Deepak S, Ranjith Kumar E (2024) A study on microstructural, optical, vibrational, and morphological features of TiO2 nanoparticles for ethanol sensor application. Inorg Chem Commun 161:112061. https://doi.org/10.1016/j.inoche.2024.112061
Nie S, Tao L, Li J, Wang W, Poldorn P, He Y, Yin X, Wu M (2024) A single response to reducing gases by NiO-TiO2 heterojunction nanocrystals. Appl Surf Sci 644:158821. https://doi.org/10.1016/j.apsusc.2023.158821
Mannaa MA, Qasim KF, Alshorifi FT, El-Bahy SM, Salama RS (2021) Role of NiO nanoparticles in enhancing structure properties of TiO2 and its applications in photodegradation and hydrogen evolution. ACS Omega 6:30386–30400. https://doi.org/10.1021/acsomega.1c03693
Lee JH, Kim JY, Mirzaei A, Nam MS, Kim HW, Kim SS (2023) Room-temperature detection of acetone gas by PANI/NiO-loaded TiO2 nanoparticles under UV irradiation. Sensors Actuators B Chem 374:132850. https://doi.org/10.1016/j.snb.2022.132850
Liu R, Guo W, Sun B, Pang J, Pei M, Zhou G (2015) Composites of rutile TiO2 nanorods loaded on graphene oxide nanosheet with enhanced electrochemical performance. Electrochim Acta 156:274–282. https://doi.org/10.1016/j.electacta.2015.01.012
Peng S, Fan L, Rao W, Bai Z, Xu W, Xu J (2017) Bacterial cellulose membranes coated by polypyrrole/copper oxide as flexible supercapacitor electrodes. J Mater Sci 52:1930–1942. https://doi.org/10.1007/s10853-016-0482-7
Yan X, Li Y, Du F, Zhu K, Zhang Y, Su A, Chen G, Wei Y (2014) Synthesis and optimizable electrochemical performance of reduced graphene oxide wrapped mesoporous TiO2 microspheres. Nanoscale 6:4108–4116. https://doi.org/10.1039/c3nr06393c
Li Q, Zhu YQ, Eichhorn SJ (2018) Structural supercapacitors using a solid resin electrolyte with carbonized electrospun cellulose/carbon nanotube electrodes. J Mater Sci 53:14598–14607. https://doi.org/10.1007/s10853-018-2665-x
Kreth FA, Balducci A (2023) Solid-liquid interfaces/interphases in electrochemical capacitors: theoretical considerations, practical relevance, and state-of-the-art in-situ/in-operando characterization tools. Elsevier. https://doi.org/10.1016/B978-0-323-85669-0.00077-5.
Rambabu Y, Jaiswal M, Roy SC (2016) Enhanced photo-electrochemical performance of reduced graphene-oxide wrapped TiO 2 multi-leg nanotubes. J Electrochem Soc 163:H652–H656. https://doi.org/10.1149/2.0351608jes
Cai H, Yang Q, Hu Z, Duan Z, You Q, Sun J, Xu N, Wu J (2014) Enhanced photoelectrochemical activity of vertically aligned ZnO-coated TiO2 nanotubes. Appl Phys Lett. https://doi.org/10.1063/1.4863852
Li W, Bu Y, Jin H, Wang J, Zhang W, Wang S, Wang J (2013) The preparation of hierarchical flowerlike NiO/reduced graphene oxide composites for high performance supercapacitor applications. Energy Fuels 27:6304–6310. https://doi.org/10.1021/ef401190b
Kahimbi H, Hong SB, Yang MH, Choi BG (2017) Simultaneous synthesis of NiO/reduced graphene oxide composites by ball milling using bulk Ni and graphite oxide for supercapacitor applications. J Electroanal Chem 786:14–19. https://doi.org/10.1016/j.jelechem.2017.01.013
Wu S, Cui T, Hu Q, Yin F, Feng Q, Zhou S, Su Q (2020) Mixing solvothermal synthesis nickel selenide on the surface of graphene for high-efficiency asymmetric supercapacitors. Synth Met 268:116490. https://doi.org/10.1016/j.synthmet.2020.116490
Kim H, Cho M, Kim M, Park K, Gwon H, Lee Y, Roh KC, Kang K (2013) FULL PAPER A novel high-energy hybrid supercapacitor with an anatase TiO 2—reduced graphene oxide anode and an activated carbon cathode. Adv Energy Mater 3(11):1500–1506. https://doi.org/10.1002/aenm.201300467
Ramadoss A, Kim G, Kim SJ (2013) for supercapacitor applications. CrystEngComm 15(47):10222–10229. https://doi.org/10.1039/c3ce41517a
Agharezaei P, Abdizadeh H, Reza M (2018) Flexible supercapacitor electrodes based on TiO 2 / rGO / TiO 2 sandwich type hybrids. Ceram Int 44:4132–4141
Pham VH, Tong X (2017) Hydrogenated TiO2@reduced graphene oxide sandwich-like nanosheets for high voltage supercapacitor applications. Carbon 126:135–144. https://doi.org/10.1016/j.carbon.2017.10.026.This
Ramadoss A, Kim SJ (2013) Improved activity of a graphene—TiO 2 hybrid electrode in an electrochemical supercapacitor. Carbon 63:434–445. https://doi.org/10.1016/j.carbon.2013.07.006
Sundriyal S, Sharma M, Kaur A, Mishra S, Deep A (2018) Improved electrochemical performance of rGO/TiO2 nanosheet composite based electrode for supercapacitor applications. J Mater Sci Mater Electron 29:12754–12764. https://doi.org/10.1007/s10854-018-9393-5
Anandhi P, Jawahar V, Kumar S, Harikrishnan S (2020) Preparation and improved capacitive behavior of NiO/TiO2 nanocompo- sites as electrode material for supercapacitor. Curr Nanosci 16(1):79–85. https://doi.org/10.2174/1573413715666190219114524
Lazarte JPL, Dipasupil RC, Pasco GYS, Eusebio RCP, Orbecido AH, Doong R, Bautista-patacsil L (2018) Synthesis of reduced graphene oxide/titanium dioxide nanotubes (rGO/TNT) composites as an electrical double layer capacitor. Nanomaterials 8:934. https://doi.org/10.3390/nano8110934
Ramana AVFMV, Jadhav RST, Dae GSG, Kim Y (2018) TiO2/reduced graphene oxide composite based nano-petals for supercapacitor application: effect of substrate. J Mater Sci Mater Electron 29:10814–10824. https://doi.org/10.1007/s10854-018-9146-5
Acknowledgements
We are thankful to the Electronics and Nano Devices Lab, Faculty of Science, South Valley University, Egypt, for giving us access to the facilities and equipment we needed for our research. We also acknowledge the guidance and support of M. I. Elsheikh lab of nanoscience and nanotechnology, Faculty of Science, Sohag University, Egypt, who helped us throughout this project with their expertise and insights. They have been very generous and professional in sharing their knowledge and resources with us and we have learned a lot from them.
Author information
Authors and Affiliations
Contributions
Muhammad Abd El-Monem involved in conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, and visualization. Mohamed Khairy involved in conceptualization, formal analysis, validation, investigation, and writing—review and editing. Khaled G. Mahmoud involved in investigation. M. Abdel Ghany involved in review and editing. A. A. Ebnalwaled involved in supervision, resources, and methodology. E.M.M. Ibrahim involved in conceptualization, validation, formal analysis, investigation, writing—review and editing, project administration, and supervision.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts or competing interests to declare.
Ethical approval
Not applicable.
Additional information
Handling Editor: Kevin Jones.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Monem, M.A., Khairy, M., Mahmoud, K.G. et al. Fabrication of nickel oxide-titanium dioxide/reduced graphene oxide nanocomposites for developing asymmetric supercapacitor. J Mater Sci 59, 8987–9002 (2024). https://doi.org/10.1007/s10853-024-09750-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09750-x