Abstract
The power conversion efficiency (PCE) of single structured perovskite solar cells (PSCs) has achieved a remarkable value of 25.2% over the last ten years. But these PSCs are not eco-friendly and suffer from the serious issue of stability. So it is very important to investigate greener and more stable alternatives to these perovskite materials. In recent years, lead-free double perovskite (DP) materials have surfaced as an ecologically favorable photovoltaic material due to their inherent chemical stability, modest carrier effective masses, suitable bandgaps, and low exciton binding energies. Herein, we have synthesized lead-free, highly stable, good quality, cheaper and environment friendly halide-based hybrid organic–inorganic double perovskite (MA)2XBiCl6 (MA = CH3NH3, X = K, Na, Ag) by adopting one step hydrothermal route having the morphology of vertical flakes and micro-rods. Further, we have analyzed the morphological, photophysical, chemical, electronic, and structural behavior of synthesized samples by using various characterization techniques. We elucidated the formation of high-quality, stable, and highly crystalline luminescent DP nanoparticles (NPs). Moreover, we fabricated three solar cell devices using the three as-synthesized DPs and further demonstrated that the solar cell device using MA2NaBiCl6 NPs as absorber layer have 2.09% PCE performed better as compared to MA2KBiCl6 and MA2AgBiCl6 based devices. Our work reports a first lead-free double perovskite solar cell with different three-dimensional morphology shedding light on the shape manipulation and providing a feasible alternative to toxicity issues in single perovskite and further encouraging rigorous research on these materials for future photovoltaic technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The constant rise in global energy consumption has promoted the development of PV technology. In this community, perovskite-based solar cells have been considered a leading PV material because Lead halide-based single perovskites have recently earned a lot of interest due to their adjustable direct bandgaps, balanced electron and hole effective masses, high optical absorption coefficient, defect tolerance, small exciton binding energies, and super-long photo-generated carrier diffusion lengths and lifetimes [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Depending on the A-site cation, halide-based single celled perovskites with the generic formula ABX3 (A: CH3NH3+, Cs+; B: Sn2+, Pb2+; X: Cl−, I−, Br−) can be split into two categories: One is inorganic halide perovskite having A as an inorganic element and other is organic–inorganic hybrid halide perovskite having A as organic compound. The first PSCs in photovoltaics is reported by A. Kojima in 2009 based on organic–inorganic hybrid halide perovskites Methyl ammonium lead iodide (MAPbI3, MA is CH3NH3+) and Methyl ammonium lead bromide (MAPbBr3) to replace the sensitizer in the liquid dye-sensitized-based solar cells (DSSC) with the PCE of 3.8% [16]. These PSCs did not attract significant attention due to quite a low efficiency and poor stability. At the laboratory scale, the recorded PCE of solar cells based on these perovskite materials has improved significantly from 3.8 to 29.8% from 2009 to 2023 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. In addition to solar cells, these perovskite materials have been used in various optoelectronic applications, like lasers, light-emitting diodes (LEDs), X-ray detectors, and photodetectors [34,35,36,37,38,39]. Along with the various advantages of the lead-based perovskites, there are two major concerns associated with them, first is the toxicity nature of lead, and the second is the long-term stability issue in the presence of moisture, light and heat, which can further stymie the development of lead halide perovskite-based photovoltaic and optical devices [39]. In this regard, substantial research has been done to find non or low-toxic, air-stable perovskite-based materials [40,41,42,43,44,45]. Significant critical efforts have been done in recent decades to explain the underlying origins of Lead halide perovskite's exceptional and unique photovoltaic characteristics. The remarkable symmetry of the perovskite structure, as well as the specific electronic arrangement of Lead (6s2 6p0), have played a major role in influencing the optoelectronic capabilities of lead halide perovskites [43, 44]. In this respect, analogous divalent lone-pair metal cations like Sn (II) and Ge (II) could be a possible substitute for Pb(II). But at the other hand, Sn (II) and Ge (II)-based halide perovskites, have more significant instability problems against the oxidation state because Sn (II) and Ge (II) can be easily converted into Sn (IV) and Ge (IV) in ambient temperature due to the exceptionally high-energy of Sn (II) 5s2 and Ge (II) 4s2 states, and also due to their small ionic radii [46]. Moreover, trivalent cations like Bi (III) and Sb (III) have been used in place of divalent Pb(II) but the resultant structures cannot maintain the charge neutrality with the chemical formula A3B2X9 which further leads to unwanted photovoltaic features such as huge anisotropic carrier effective masses, high bandgaps and low defect tolerance behavior [47, 48].
The concept of maintaining high electronic dimensionality in the framework of structural dimensionality is vital to consider while looking for Lead-free halide perovskites as promising and a potential candidate for solar cell absorbers [40]. Therefore maintenance of the three-dimensional (3D) structure with the replacement of Lead could be the most favorable strategy that can be achieved by cation transfiguration in lieu of replacing the two divalent Pb2+ cations with two different cations, one is a monovalent cation and another is a trivalent cation which further leads to a 3D double perovskite structure with formula A2B(I)B(III)X6 which further allow the additional flexibility in selecting B-cation elements [49, 50]. In addition to the double perovskites materials, Cs-based lead-free halide quadruple perovskites materials (Cs4MnBi2Cl12,, Cs4CdBi2Cl12, Cs4MnSb2Cl12, and Cs4CdSb2Cl12) are also reported having 3D structure which have been synthesized and found to be highly crystalline, along with long carrier lifetime, and low trap-state densities [51]. To date, few double perovskites have been synthesized having monovalent cation B(I) as Ag+, Li+, Rb+, Na+, K+, and Ti+ and trivalent cation B(III) as Sb3+, Ga3+, Al3+, Tl3+, In3+, Bi3+, and some other lanthanides and a trivalent transition metal. These halide-based lead-free double perovskites have gained a lot of attention as attractive optoelectronic prospects because of their cubic structure (Fm3m) that allows them to extend in all three dimensions with corner-sharing metal halide octahedra having application in various domains including X-ray photodetectors, photocatalyst, electrocatalyst, solar cells, and LEDs. But, among these proposed double perovskite materials, very few of them have been experimentally realized in the laboratory for the development of solar cell having significant poer conversion efficiency [52,53,54,55].
The quest for lead-free and low-cost photovoltaics has encouraged researchers to investigate organic materials having conducting and semiconducting properties for their possible alternatives [56]. The advantages of the organic materials investigated by past researchers over the years is having low manufacturing cost, and high absorption coefficient which leads to significant impact on organic photovoltaic solar cell [57, 58]. Leveraging all these benefits and current considerable improvements in power conversion efficiency (PCE) has resulted in the creation of several initial commercial organic photovoltaic (OPV) devices. To further enhance the efficiency of photovoltaic (PV) devices, researcher has found Organic–inorganic hybrid solar cells combination is having advantages of both organic as well as inorganic material [59]. The advantages associated with the addition of inorganic material to organic material is to reduce the photo induced degradation of the organic photovoltaic devices in addition to increase in the charge carriers photo generation through the absorbed exciton in the inorganic material [60]. Moreover, inorganic materials have high light absorption in comparison with the organic materials [61, 62]. These interesting properties encourages us to build a hybrid organic–inorganic lead-free solar cell in replacement of the lead-based halide perovskites solar cell.
Here in this study, we report the first solar cell devices based on double perovskite nanomaterials MA2XBiCl6 (X = K, Na, Ag). Firstly we have synthesized MA-based non-toxic and non-degradable halide double perovskite MA2XBiCl6 (X = K, Na, Ag) using a simple one-step hydrothermal synthesis technique. Further, we have characterized these three synthesized materials to investigate the phase, optical behavior, chemical nature, surface morphology, absorption nature, and band gap. The obtained characterization results of all these three materials suggest them as promising and stable absorber candidates and solar cells based on these materials showed remarkable PCE having the highest value for MA2NaBiCl6 NPs. Our present study provides the access to the high-quality and large-scale fabrication of solar cell devices and throws light on further investigation of morphologically tuned non-toxic and cheaper DP materials for a wide range of applications with very high and stable performance in the photovoltaics domain.
Details of experiments
Chemicals used
The chemicals which were used in the synthesis process of material were purchased from Sigma Aldrich and have we utilized these materials in their original configuration without any additional treatment. Methyl ammonium (MA) solution, Potassium chloride (KCl, 99%), Silver chloride (AgCl, 99%), Sodium chloride (NaCl, 99%), Bismuth chloride (BiCl3, 99%), Acetone (≥ 99.9%), Hydrochloric acid (HCl), and Ethyl acetate (≥ 95%) were utilized into our synthesis method.
Synthesis process details
Preparation of methylammonium chloride (MACl) solution
Initially, we synthesized MACl solution by taking the equimolar amount of MA solution in HCl at room temperature and then heated the solution mixture at 50 °C till the solution is completely dry. Further, we cleaned the obtained product with acetone to remove the unwanted byproducts and then dried it for 24 h in an oven. The prepared MACl was used to synthesize the three double perovskites, i.e., MA2XBiCl6 (X is K, Na, Ag) by one-step hydrothermal process.
Preparation of MA2KBiX6 NPs, X is K, Na, Ag
The MA2KBiCl6 NPs were synthesized by adopting a one-step hydrothermal method after taking MACl, KCl, and BiCl3 precursors in 2, 1, and 1 molar ratios, respectively, in the 1 ml HCl solution kept in a stainless steel autoclave. Then, the complete solution was heated at 160 °C for 8 h to perform the high-pressure reaction. Further, the temperature was cooled down to room temperature and the obtained product was filtered and cleaned using the solvent precipitation technique to remove the byproducts. Finally, the cleaned product was heated at 60 °C in a vacuum oven to dryness.
Characterization details
The X-ray diffraction (XRD) experiment was performed by X-ray powder diffractometer with the Copper (Cu) Kα radiation having wavelength of 1.54 Å at the voltage and current of 40 kV and 30 mA value respectively, with variation of the angle (2θ) values (10° to 50°) with peak intensity (arbitrary unit) at an increment of 0.1° to investigate the quality, and to confirm the crystalline phases and purity of the synthesized DP materials. The sample for XRD measurement was prepared by drop casting a concentrated NP solution on the substrate and the diffraction peaks were observed using symmetric reflection mode and parallel beam geometry at room temperature. Further, the obtained results were analyzed using PANalytical Xpert HighScore 4.1 software. The optical property of the synthesized DP NPs was examined by doing room temperature Photoluminescence (PL) measurement which is a type of electromagnetic (EM) spectroscopy that evaluates the fluorescent compounds. The measurements were performed using PL Fluorolog-3 Spectroscopy with the help of a Light Source of 450 W Xenon Lamp having a scanning speed of 150 nm/sec, wavelength accuracy of 0.5 nm, and wavelength range of 200 nm to 700 nm. In the PL setup, a 300 nm continuous-wave laser source having an incidence intensity of 100 mW/cm2 with two monochromators (visible and near-infrared (NIR)) connected in series was used for the excitation purpose. The above setup can quickly and accurately determine the presence of molecular elements in the sample by utilizing the phenomena of electronic excitation caused by the interaction of high-energy particles (HEPs) such as excited electrons and photons with electrons. Using a Shimadzu ultraviolet–visible (UV–vis) absorption spectrometer having range of wavelength 200–800 nm, a resolution of 0.05 nm, slit width of 2 nm, and scan interval of 1 nm, UV–vis spectroscopy of the as-synthesized NPs was collected to examine the absorbance phenomena. Field emission gun-scanning electron microscopy (FEG-SEM) has been used to reveal the structural morphology using the JEOL JSM-7600F model with a secondary electron image (SEI) resolution of 1.0 nm at 15 kV with accelerating voltage varying from 0.1 to 30 kV at low & high magnification of 25 X and 1,000,000 X, respectively. A Schottky field emission gun and an electron column with semi-in-lens detectors were employed in the JEOL JSM-7600F to deliver ultrahigh-resolution with a variety of probe currents (1pA to more than 200 nA). In addition, the chemical composition and presence of functional groups in the synthesized DPs are analyzed by using Fourier Transform Infrared (FTIR) spectroscopy done using the Spectrum 100 FT-IR system of PerkinElmer.
Device fabrication detail
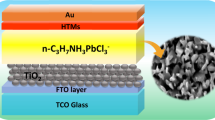
A simple solar cell has been fabricated with all the three as-synthesized DP as absorbing layer. Fluorine doped tin oxide (FTO) on transparent glass (7 Ω per square) substrate on which contact deposition of Gold (Au) has been used to fabricate the device. We have extensively cleaned substrate by deionized (DI) water, ethanol, and acetone by performing ultra-sonication for 45 min and further ultraviolet (UV)-ozone for 20 min treatment has been applied. SnO2 of FTO substrate is working as electron transport layer. SnO2 layer is came out by the fluorine doped tin oxide (FTO) coated transparent glass substrate which is modified by the oxygen plasma assisted reaction for 12 min at room temperature to form the pure SnO2 phase. Then obtained uniform solution of as-synthesized DPs materials MA2XBiCl6 (X is K, Na, Ag) was uniformly coated using spin-coating on the substrate at 900 rotation per minute (rpm) for 15 s and 2000 rpm for 20 s, respectively. The above process was repeated many times for obtaining a uniformity of absorber layer coated on substrate. Afterward, the DP-coated FTO substrate was annealed at 250 °C for 10 min and then allowed to ramp down to the temperature of 30 °C. Finally, 100 nm thick Au contacts were deposited with a deposition rate of 0.5 nm/sec and a chamber pressure of 2.5 × 10 −6 mbar using a mask in a 6-target Electron beam evaporation system. The I–V characteristics of fabricated solar cell devices were measured in dark and light by utilizing an in-house solar simulator. Also, the Bentham PVE300 system was used to calculate the external quantum efficiency (EQE) of the fabricated devices using a Xenon lamp source with Si and Ge detectors having a wavelength range from 300 to 1800 nm with a resolution of 2 nm.
Results and discussions
The schematic 3D crystal structure of double perovskite materials MA2XBiCl6 (X = K, Na, Ag) formed by alternatively positioned KCl6 (NaCl6 or AgCl6) and BiCl6 octahedra resulting in a doubly celled perovskite as compare to its single perovskite counterpart is shown in Fig. 1a. In this crystal structure, the B site is occupied by the Potassium (K)/Sodium (Na)/Silver (Ag), and B’ site is occupied by the Bi (Bismuth) element. The structural investigation of synthesized DPs MA2XBiCl6 (X is K, Na, Ag) NPs was done using the room temperature XRD technique. The XRD pattern was observed in order to examine the phases and purity of the synthesized materials. Figure 1b–d represent the XRD pattern of MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6 DP, respectively, revealing that newly synthesized MA-based halide DPs NPs form a rhombohedral \(R\overline{3 }m\) space group [63]. The peaks shown in the XRD pattern of all three materials have good accord with the standard reported data with well-indexing and also, demonstrates that the newly described synthesis process yields high-quality, and crystalline double perovskite materials [63,64,65,66]. The additional peak (marked *) in the XRD pattern of MA2KBiCl6 DPs around 34 \(^\circ\) is due to the precursors utilized in the synthesis process of the material. The diffraction peaks are indexed as (111), (022), (113), (222), (004), (024), (224), (044), and (244) planes of the crystal structure for materials MA2XBiCl6 (X = K, Na, Ag) showing the absence of any secondary phase diffraction peaks.
Furthermore, from the XRD data, we have calculated the full-width half maxima (FWHM), and crystallite size as given in Table 1 using Scherrer’s equation [64]:
where D is crystallite size, λ is the wavelength of X-rays used in XRD characterization of samples, K is Scherrer’s constant, β is the full width at half maximum, θ is the peak position, and \({2\theta }_{\mathrm{max}}\) and \({2\theta }_{\mathrm{min}}\) is the right and left side 2θ value where intensity is half of the maximum at that peak [64] calculated from XRD plots. For calculation of FWHM, we have taken K = 0.9, and λ = 0.154 nm as used in the XRD experiment.
In addition, we have calculated the lattice constants (a, c) using Brag’s diffraction law and interplanar distance (d) for all the three synthesized double perovskite nanomaterials using the below formula [65], and the calculated values for all the planes are summarized in Table 2.
where \(\theta\) is the angle, n is the order of diffraction, \(\lambda\) is the wavelength of X-rays, and (h k l) is the corresponding plane.
To know the structural stability of all the three synthesized DPs, we calculated the tolerance factor (t) and octahedral factor (µ) summarized in Table 3. The obtained values are in the standard range required to form a stable double perovskite structure. The tolerance and octahedral factor are calculated by utilizing the below equations [66]:
Here, RA & RX is the ionic radii of cation A (MA) and halide X (Cl, Br, I); respectively, \(R_{{{\text{avg}}}}\) is the average ionic radii of B’ and B” ion [67,68,69,70].
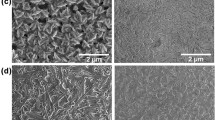
The FEG-SEM images were recorded to know the surface morphology and crystallinity of the synthesized double perovskite materials. Figure 2a–c shows FEG-SEM image of MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 DP NPs, respectively, at 1 μm scale of resolution. The morphology of MA2KBiCl6 was observed to be dense, and homogeneous with vertically aligned flakes structure with pure crystallinity throughout the area as shown in Fig. 2a. The micro-rod type surface morphology for MA2NaBiCl6 and MA2AgBiCl6 DP NPs was observed from the FEG-SEM images having length and diameter of 0.17 μm and 1.90 μm, respectively. The observed FEG-SEM images confirm the formation of excellent nanostructures as compared to the previously reported work on double perovskite nanostructures with a variation in the morphology from nanoflakes to nanorod [64]. Also, our group is reporting a rod-like microcrystal structure in MA2AgBiCl6 DP for the first time.
To examine the optical transitions, carrier dynamics, and applicability of synthesized materials for photovoltaic application, we performed the photoluminescence experiment at room temperature with a 300 nm fixed excitation wavelength and calculated their optical band gap. Figure 3a–c indicates the PL profile of MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6, respectively, at 300 K. The PL spectra of MA2KBiCl6 DP indicate a sharp and luminescent peak at the wavelength of 320 nm corresponding to an energy of 3.87 eV as shown in Fig. 3a. A broad PL peak at a wavelength of 480 nm (2.58 eV) and 300 nm (4.13 eV) has been observed for the MA2NaBiCl6 and MA2AgBiCl6 DP as shown in Fig. 3b, c, respectively. PL emission peaks of all three synthesized double perovskite material is comparable to the recent papers on related halide perovskites [71,72,73,74]. Also, the absorption profile of the as-synthesized double perovskite materials was determined by UV–VIS absorption spectrometer in 200–800 nm of the wavelength range. Figure 3d–f represent the absorption profile of MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6, respectively, at 300 K. From the absorption profile of all three material, a very good absorption behavior has been observed for the synthesized materials. The absorption peak rises at 465 nm (2.66 eV), 564 nm (2.19 eV), and 386 nm (3.21 eV with a sharp peak at 325 nm (3.81 eV), 482 nm (2.57 eV) and 325 nm (3.90 eV) for MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 as shown in Fig. 3d–f, respectively.
Further, from the optical absorption spectra, we have evaluated the band gap of synthesized double perovskite materials using the Tauc plot and these materials are showing an indirect band gap behavior which are in agreement with the previously reported results on similar type of materials [64, 75,76,77,78,79,80]. A Tauc plot of \({(\alpha h\vartheta )}^{0.5}\) verses wavelength indicate the material’s indirect band gap nature as shown in Fig. 4a–c for synthesized DP MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6, respectively. Here \(\alpha\) and \(h\vartheta\) represent the material’s absorption coefficient and energy of photons, respectively. The calculated indirect band gap are 2.51 eV, 2.06 eV, and 2.80 eV for MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6, respectively. In the reference [64], the reported band gap of MA2KBiCl6 is 3.37 eV and 3.04 eV but our evaluated indirect band gap is 2.51 eV. The reason behind the difference in results could be change of material’s phase to achieve stability leading to a change in band gap value [75, 81]. The materials is showing low band gap which clearly indicating that the materials are useful in the photovoltaic applications. Two distinct phenomena are responsible for the calculation of direct and indirect band gaps as Phonon-assisted recombination and absorption mechanisms create considerable coupling effects of carrier phonon in the indirect bandgap of all double perovskite materials which results in the dark self-trapped exciton state (STE) emission condition at room temperature whereas radiative STE has been formed by an insufficient exciton-phonon coupling power in the direct bandgap [82,83,84].
To check the stability of all the synthesized material in the exposure to light at room temperature, we have recorded the absorption profile, PL profile and XRD profile of all the materials after 14 and 28 days. Figure 5a–c demonstrate the absorption spectra of MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6, respectively, for freshly synthesized samples, after 14 days of synthesis and after 28 days of synthesis. It can be clearly seen from Fig. 5 that no remarkable change in absorption spectra has been observed for the synthesized material even after one month of exposure to light which further demonstrates the non-degradable and stable nature of synthesized double perovskites as compared to their single perovskite counterpart.
PL profile of the MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6 samples just after synthesis, after 14 days of synthesis and after 28 days of synthesis is demonstrated in Fig. 6a–c, respectively. The comparative PL plot clearly indicate that the material is showing similar luminescent behavior having negligible amount of change in the spectra even after one month also. In addition, we have performed the XRD of all the samples just after synthesis, after 14 days of synthesis and after 28 days of synthesis, which is demonstrated in Fig. 6d–f, respectively. The comparative XRD profile shows a negligible decrease in intensity of samples with increase in number of days which shows that the material is stable enough for photovoltaic application. Therefore, we can conclude that these synthesized DPs are showing an added advantage of having negligible degradation in the material after a month of synthesis.
FTIR spectroscopy has been done to know the presence of functional groups and other reaction by-products formed after the synthesis and cleaning of double perovskites. Figure 7a–c represent the FTIR spectra of MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 double perovskites materials respectively recorded at room temperature from 400 to 4000 cm−1. In Fig. 7a, the peaks observed at 585 cm−1, 766 cm−1, 1296 cm−1, and 2249 cm−1 correspond to halogen group, C–H, alkyl metal (K), and C = C stretching bond of alkynes molecules vibrational mode respectively [85] for the synthesized MA2KBiCl6 DP. In Fig. 7b, the peaks observed at 1463 cm−1, 1621 cm−1, and 3523 cm−1 correspond to the nitrosamine, diketones, and hydroxyl group vibrational mode respectively for the synthesized MA2NaBiCl6 DP. In Fig. 7c, the peak is observed at 486 cm−1, 764 cm−1, 1288 cm−1, and 2989 cm−1 corresponding to alkyl halide (AgCl), C-H group, alkyl ketones, and methyl group (MA, CH3NH3) for the synthesized DP MA2AgBiCl6. The other peaks in the FTIR spectra represent the presence of functional groups due to impurities and by-products formed during synthesis or in the cleaning of DP nanoparticles.
To test the dark current versus voltage characteristics of all the fabricated devices, we have utilized a solar simulator having −1 V to 1 V of voltage range. The dark I-V measurement utilizes electrical techniques to insert carriers into the device rather than light-generated carriers. Figure 8a–c represents the solar cell dark current verses voltage curve based on MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 material, respectively. Figure 8d shows the external quantum efficiency (EQE) to the wavelength curve of all three solar cell devices. EQE of all devices is almost at 20% which is quite remarkable as compared to other reported values [86]. The EQE is measured by utilizing the below equation
where \({N}_{\mathrm{electrons}}\) represent the flux of electrons taken from the solar cell when functioned in working conditions and \({N}_{\mathrm{photons}}\) represent the flux of photons falling on the solar cell.
Further, we have fabricated three photovoltaic devices based on synthesized materials in a planar device architecture to determine the photovoltaic properties. A schematic diagram of solar cell devices with glass/FTO/DP/Au structure which is shown in Fig. 9a. To calculate the power conversion efficiency of the solar cells, we tested the light current density verses voltage characteristics of all devices. Figure 8b–d show the light current density verses voltage characteristics of solar cell with absorber layer of MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 DP, respectively. Moreover, the photovoltaic characteristics were evaluated from the light current density verses voltage curve. The fill factor (FF) which is an important parameter to determine the PCE can be defined as the ratio of the maximum power to the multiplication of open circuit voltage and short circuit current density. FF is calculated by the formula below [86]
where \(V_{MP}\),\(I_{MP} , V_{OC} ,\) \(and\) \(I_{SC}\) represent the voltage where power is maximum, current where power is maximum, open circuit voltage, and short circuit current, respectively, in illuminated I–V. Further, the PCE of all devices is calculated by utilizing the equation below [84]
where \({P}_{IN}\) is the input power of the system having the value of 0.1 W/cm2. The \({V}_{OC}\) of solar cells based on MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6 DP were detected from illuminated I-V characteristics which is shown in Figs. 8b, 9c, and 9d and found to be 0.90 V, 0.98 V, and 0.95 V, respectively. Further, the short circuit current density calculated from Fig. 9b–d for the solar cell devices based on MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6 were found to be 0.3 mA/cm2, 4.0 mA/cm2, and 5.0 mA/cm2, respectively. From all the above-calculated parameters, the PCE of solar cell devices based on MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6 were evaluated and found to be 0.154%, 2.09%, and 1.639%, respectively, as indicated in Fig. 9b–d. The maximum PCE of solar cell is obtained for MA2NaBiCl6 DP as compared to the other two fabricated devices indicating this material is most promising for future photovoltaic applications. The obtained photovoltaic characteristics of all the three devices that is JSC, VOC, FF, and PCE are given in Table 4.
Researcher are nowadays exploring the solar cell device without using either electron transport layer (ETL) or hole transport layer (HTL) and getting better performance as compared to the device with ETL and HTL [87]. This motivates us to first fabricate the device without HTL or ETL layer. We have fabricated the device having electron transport layer only but no hole transport layer. In our work SnO2 is working as electron transport layer. SnO2 layer is present on FTO by the fluorine doped tin oxide (FTO) coated transparent glass substrate which is modified by the oxygen plasma assisted reaction for 12 min at room temperature to form the pure SnO2 phase [88]. In this paper, the fabricated device structure is glass/FTO/SnO2/DP/Au and the mechanism of transportation of the carriers is explained in details in our revised manuscript. Figure 11 shows the schematic of the energy band diagram of the fabricated device structure with SnO2 as ETL and MA2XBiCl6 as absorber layer. Our as-synthesized double perovskites materials are low band gap material having good carrier mobility Which results into a good match of the alignment of the energy band between ETL and DP to separate the charge carriers [89]. When light is exposed through sun, an electron–hole pairs is generated in the absorber layer and this would go through SnO2 to the FTO/Au electrode [90]. The conduction and valence band energy of FTO, ETL, DP, and Au is shown in Fig. 10. Due to the high electron mobility of SnO2 and low conduction band offset, photo-generated electrons will migrate and will reach to FTO and electron current will dominate in the device. Due to low offset of valence and offset, the depletion layer will form in DP layer which lead to the fast transportation of holes. This will further help to enhance the fill factor and hence the overall device performance.
The devices based on the MA2NaBiCl6 and MA2AgBiCl6 having rod-like microcrystal structure showing higher PCE values of 2.09% and 1.679% in comparison with the MA2KBiCl6-based device having PCE of 0.154%. The possible reason could be the large surface area to volume ratio due to rod like microcrystals structure. Another possible reason could be the high absorption of MA2NaBiCl6 and MA2AgBiCl6 in comparison with the MA2KBiCl6 which can be seen in the absorption profile of these materials. Due to high surface area to volume ratio and high absorption, the light will be absorbed more, which further leads to more electron hole pair generation which increases the reverse current. As the reverse current would increases, leads to high short circuit current density (JSC) and hence PCE will increase resulting a greater short circuit current density of MA2NaBiCl6 and MA2AgBiCl6 is greater (4 and 5mA cm−2) respectively in comparison with the JSC of MA2KBiCl6 is 0.3 mA cm−2. The device based on MA2KBiCl6 double perovskite which have vertical flakes morphology leading to low surface area to volume ratio further results into low absorption of light which could be the reason of low PCE as comparison to former two materials.
Further, we have checked the PCE of the all three solar cell devices to check the decay in the PCE as shown in Fig. 11a–c. For this, we have measure the PCE of all devices after one week, two week, three week and four week. We have found the PCE decay of 8.9%, 12.1% and 9.9% after one month in MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6-based devices, respectively, as shown in Table 5. This is in good agreement with the material stability observed from PL, XRD, and absorption profile of all the samples after a month. The rate of degradation in MA2NaBiCl6 double perovskite material is high as compare to other two double perovskite materials and this behavior can also be seen from PCE data.. In our future work, we will focus on improvement of our perovskite solar cell device functionality in lieu of reduction of PCE with time.
We have performed the experiment of current density–voltage characteristics in forward and reverse bias as well to check the hysteresis at room temperature. We have observed the hysteresis in all three devices. This current density–voltage curve hysteresis in the double perovskites solar cell can be due to the presence of mobile ions [91]. The investigation found that in MA2KBiCl6-based solar cell having high hysteresis while in MA2NaBiCl6 and MA2AgBiCl6-based device having very less current density–voltage hysteresis as shown in Fig. 12a–c. In literature there are evidence that device with high efficiency having low hysteresis while the device with low efficiency will have high hysteresis [88]. In our devices also, it is observed that MA2KBiCl6-based solar cell having low efficiency in comparison of MA2NaBiCl6 and MA2AgBiCl6-based device and therefore MA2KBiCl6-based solar cell showing high hysteresis. Mainly hysteresis is depends on the diffusion length of charge carriers and on the extent of surface recombination. Ion migration occurs in high-efficiency solar cells but does not result in a high hysteresis effect. Charge extraction in these devices is primarily driven by the diffusion of free electrons and holes [92]. Further, we have calculated the current density of all three devices from external quantum efficiency data also. And we find that the current density calculated from EQE data is almost same as calculated by I–V experiment. The calculated current density of MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 from EQE data has shown in Fig. 13a–c.
Conclusion
For the first time, we have chemically synthesized non-toxic, lead-free, non-degradable hybrid organic–inorganic halide double perovskites MA2KBiCl6, MA2NaBiCl6, and MA2AgBiCl6 material by adopting a novel cost-effective one-step hydrothermal synthesis route. The material characterization was done using various characterization techniques like XRD, PL, FEG-SEM, FTIR, and UV–VIS. The obtained characterization results of XRD have excellent agreement compare to the standard data and showing good crystallinity of the materials. FEG-SEM images of all the synthesized material is having a fine microstructure with vertical micro-flakes and micro-rod kind of structure indicating the possibility of morphology engineering. Our synthesized MA2NaBiCl6 and MA2AgBiCl6 DPs are showing rod like structure which is first time reported in the literature. Tolerance and octahedral factor of the synthesized DP NPs are in the required range for the formation of stable 3D structure. Moreover, these materials showed good stability even after one month of exposure of light confirming their non-degradable nature and thus can be used as promising absorber in solar cell applications. In addition, PL and UV–VIS characterization of the synthesized materials shows good photoluminescence and absorbance behavior confirming that the materials are suitable for optical devices. Calculated indirect gaps from Tauc plot of DPs MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 are 2.51 eV, 2.08 eV, and 2.8 eV, respectively, which is suitable band gap for photovoltaic device application. Further we have fabricated all together three solar cell devices and tested the illuminated and dark I-V and calculate PCE of all the three devices. The detected PCE of devices with MA2KBiCl6, MA2NaBiCl6 and MA2AgBiCl6 as absorbing layer was found to be 0.15%, 2.09% and 1.615%, respectively, which is quite remarkable as compare to other reports and its single perovskite counterpart. The solar cell device based on MA2NaBiCl6 DP showed the best performance as compare to other two devices. In conclusion, the overall material characterization and fabricated devices result shows that these double perovskites materials are promising replacements of the single perovskite in every aspects for photovoltaic utility. Our present work shows that other possible stable and high performing inorganic and organic–inorganic halide-based double perovskites should be explored for optoelectronic and photovoltaic utility. Our future efforts will involve in experimental realization of various double perovskites as a greener alternative to single perovskite for application in various domains.
Data and code availability
Due to the sensitive nature of the data, information created during and/or analyzed during the current study is available from the corresponding author on reasonable request to bona fide researchers.
References
Xing G, Mathews N, Sun S, Lim SS, Lam YM, Grätzel M, Sum TC (2013) Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 342:344–347
Giorgi G, Fujisawa JI, Segawa H, Yamashita K (2013) Small photocarrier effective masses featuring ambipolar transport in methylammonium lead iodide perovskite: a density functional analysis. J Phys Chem Lett 4:4213–4216
Frohna K, Deshpande T, Harter J, Peng W, Barker BA, Neaton JB, Bernardi M (2018) Inversion symmetry and bulk Rashba effect in methylammonium lead iodide perovskite single crystals. Nat Commun 9:1829
Stranks SD, Eperon GE, Grancini G, Menelaou C, Alcocer MJ, Leijtens T, Snaith HJ (2013) Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342:341–344
Wehrenfennig C, Eperon GE, Johnston MB, Snaith HJ, Herz LM (2014) High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv Mater 26:1584–1589
Edri E, Kirmayer S, Mukhopadhyay S, Gartsman K, Hodes G, Cahen D (2014) Elucidating the charge carrier separation and working mechanism of CH3NH3PbI3− x Cl x perovskite solar cells. Nat Commun 5:3461
Miyata A, Mitioglu A, Plochocka P, Portugall O, Wang JTW, Stranks SD, Nicholas RJ (2015) Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat Phys 11:582–587
Baumann A, Väth S, Rieder P, Heiber MC, Tvingstedt K, Dyakonov V (2015) Identification of trap states in perovskite solar cells. J Phys Chem Lett 6:2350–2354
Duan HS, Zhou H, Chen Q, Sun P, Luo S, Song TB, Yang Y (2015) The identification and characterization of defect states in hybrid organic–inorganic perovskite photovoltaics. Phys Chem Chem Phys 17:112–116
Wang JTW, Wang Z, Pathak S, Zhang W, deQuilettes DW, Wisnivesky-Rocca-Rivarola F, Snaith HJ (2016) Efficient perovskite solar cells by metal ion doping. Energy Environ Sci 9:2892–2901
Zhao D, Yu Y, Wang C, Liao W, Shrestha N, Grice CR, Yan Y (2017) Low-bandgap mixed tin–lead iodide perovskite absorbers with long carrier lifetimes for all-perovskite tandem solar cells. Nat Energy 2:1–7
Pandey N, Kumar A, Chakrabarti S (2019) Investigation of the structural, electronic, and optical properties of Mn-doped CsPbCl3: theory and experiment. RSC Adv 9:29556–29565
Pandey N, Chakrabarti S (2020) Understanding the effect of Mn doping in CsPbBr3 using ab-initio method with experimental validation. IEEE J Photovolt 10:1359–1364
Pandey N, Chakrabarti S (2021) Tuning the morphological, photophysical, and electronic properties of CsPb (Cl/Br)3 by impurity doping for optoelectronic applications: a theoretical and experimental study. IEEE J Photovolt 11:379–385
Pandey N, Chakrabarti S (2022) Ab initio computational details with facile high-temperature synthesis of pure and alloyed CsPbI3 with inherent stability analysis for optoelectronic applications. IEEE J Photovolt 12:625–633
Kojima A, Teshima K, Shirai Y, Miyasaka T (2009) Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc 131:6050–6051
Kim HS, Lee CR, Im JH, Lee KB, Moehl T, Marchioro A, Park NG (2012) Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci Rep 2:591
Saliba M, Matsui T, Domanski K, Seo JY, Ummadisingu A, Zakeeruddin SM, Grätzel M (2016) Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354:206–209
Saliba M, Matsui T, Seo JY, Domanski K, Correa-Baena JP, Nazeeruddin MK, Grätzel M (2016) Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ Sci 9:1989–1997
McMeekin DP, Sadoughi G, Rehman W, Eperon GE, Saliba M, Hörantner MT, Snaith HJ (2016) A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 351:151–155
Shao Y, Yuan Y, Huang J (2016) Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat Energy 1:1–6
Yang WS, Park BW, Jung EH, Jeon NJ, Kim YC, Lee DU, Seok SI (2017) Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 356:1376–1379
D’innocenzo V, Grancini G, Alcocer MJ, Kandada ARS, Stranks SD, Lee M, Petrozza A (2014) Excitons versus free charges in organo-lead tri-halide perovskites. Nat Commun 5:3586
Burschka J, Pellet N, Moon SJ, Humphry-Baker R, Gao P, Nazeeruddin MK, Grätzel M (2013) Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499:316–319
Liu M, Johnston MB, Snaith HJ (2013) Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501:395–398
Zhou H, Chen Q, Li G, Luo S, Song TB, Duan HS, Yang Y (2014) Interface engineering of highly efficient perovskite solar cells. Science 345:542–546
Jeon NJ, Noh JH, Kim YC, Yang WS, Ryu S, Seok SI (2014) Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat Mater 13:897–903
Jeon NJ, Noh JH, Yang WS, Kim YC, Ryu S, Seo J, Seok SI (2015) Compositional engineering of perovskite materials for high-performance solar cells. Nature 517:476–480
Yang WS, Noh JH, Jeon NJ, Kim YC, Ryu S, Seo J, Seok SI (2015) High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348:1234–1237
Chen W, Wu Y, Yue Y, Liu J, Zhang W, Yang X, Han L (2015) Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 350:944–948
National Renewable Energy Laboratory (NREL) (2018) https://www.nrel.gov/pv/assets/pdfs/pv-efciencies-chart.20181
Gao D, Bo L, Zhen L, Xin W, Shoufeng Z, Dan Z, Xiaofen J (2023) Highly efficient flexible perovskite solar cells through pentylammonium acetate modification with certified efficiency of 23.35%. Adv Mater 3: 2206387
Hui W, Xinxin K, Baohua W, Deli L, Zhenhuang S, Yaqi B, Lei G (2023) Stable electron-transport-layer-free perovskite solar cells with over 22% power conversion efficiency. Nano Lett 6:2195–2202
Chen Z, Li H, Tang Y, Huang X, Ho D, Lee CS (2014) Shape-controlled synthesis of organolead halide perovskite nanocrystals and their tunable optical absorption. Mater Res Express 1:015034
Stranks SD, Snaith HJ (2015) Metal-halide perovskites for photovoltaic and light-emitting devices. Nat Nanotechnol 10:391–402
Cho H, Jeong SH, Park MH, Kim YH, Wolf C, Lee CL, Lee TW (2015) Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 350:1222–1225
Chen Q, Wu J, Ou X, Huang B, Almutlaq J, Zhumekenov AA, Liu X (2018) All-inorganic perovskite nanocrystal scintillators. Nature 561:88–93
Zhang F, Yang B, Zheng K, Yang S, Li Y, Den W, He R (2018) Formamidinium lead bromide (FAPbBr3) perovskite microcrystals for sensitive and fast photodetectors. Nano-Micro Lett 10:1–8
Chu L, Hu R, Liu W, Ma Y, Zhang R, Yang J, Li XA (2018) Screen printing large-area organometal halide perovskite thin films for efficient photodetectors. Mater Res Bull 98:322–327
Qin X, Zhao Z, Wang Y, Wu J, Jiang Q, You J (2017) Recent progress in stability of perovskite solar cells. J Semicond 38:011002
Xiao Z, Meng W, Wang J, Mitzi DB, Yan Y (2017) Searching for promising new perovskite-based photovoltaic absorbers: the importance of electronic dimensionality. Mater Horiz 4:206–216
Zhang Q, Ting H, Wei S, Huang D, Wu C, Sun W, Xiao L (2018) Recent progress in lead-free perovskite (-like) solar cells. Mater Today Energy 8:157–165
Nasti G, Abate A (2020) Tin halide perovskite (ASnX3) solar cells: a comprehensive guide toward the highest power conversion efficiency. Adv Energy Mater 10:1902467
Liang L, Gao P (2018) Lead-free hybrid perovskite absorbers for viable application: can we eat the cake and have it too? Adv Sci 5:1700331
Abate A (2017) Perovskite solar cells go lead free. Joule 1:659–664
Yin WJ, Shi T, Yan Y (2014) Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv Mater 26:4653–4658
Zhao XG, Yang D, Ren JC, Sun Y, Xiao Z, Zhang L (2018) Rational design of halide double perovskites for optoelectronic applications. Joule 2:1662–1673
Todd S (2018) Pump probe studies of spin dynamics in the 2D perovskite butylammonium methylammonium lead iodide
Ghosh B, Chakraborty S, Wei H, Guet C, Li S, Mhaisalkar S, Mathews N (2017) Poor photovoltaic performance of Cs3Bi2I9: an insight through first-principles calculations. J Phys Chem C 121:17062–17067
Zhao XG, Yang JH, Fu Y, Yang D, Xu Q, Yu L, Zhang L (2017) Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J Am Chem Soc 139:2630–2638
Bai T, Yang B, Chen J, Zheng D, Tang Z, Wang X, Han K (2021) Efficient luminescent halide quadruple-perovskite nanocrystals via trap-engineering for highly sensitive photodetectors. Adv Mater 33:2007215
Hoseinpour V, Zahra S, Luis E (2023) Design, synthesis, optical studies, and application of all-inorganic layered double perovskites as stabilizers in ambient air processed perovskite solar cells. Mater Res Bull 159:112088
Pandey N, Neelu N, Chakrabarti S (2023) Room temperature synthesis of double perovskite Cs2AlBiCl6 for photovoltaic applications. Opt Mater 137:113570
Li B, Xin W, Shoufeng Z, Zhen L, Danpeng G, Xiankai C, Shuang X, Chu-Chen C, Alex KYJ, Zonglong Z (2022) Efficient and stable Cs2AgBiBr6 double perovskite solar cells through in-situ surface modulation. Chem Eng J 446:137144
Li, J, Xianghuan M, Zhiheng W, Yanyan D, Ruxin , Weidong X, Yongshang Z (2022) Pinning bromide ion with ionic liquid in lead‐free Cs2AgBiBr6 double perovskite solar cells. Adv Funct Mater 32:2112991
Tang, CW (1986) Two‐layer organic photovoltaic cell. Appl Phys Lett 48:183–185
Krebs Frederik C (2009) Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Solar Energy Mater Solar Cells 93:394–412
Zhokhavet U, Tobias E, Gerhard G, Maher A, Oliver A (2006) Relation between absorption and crystallinity of poly (3-hexylthiophene)/fullerene films for plastic solar cells. Chem Phys Lett 418:347–350
Zhou Y, Michael E, Michael K (2010) Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers. Energy Environ Sci 12:1851–1864
Zhou Y, Michael E, Clemens V, Birger Z, Frank R, Phenwisa N, Seyfullah Y (2011) Efficiency enhancement for bulk-heterojunction hybrid solar cells based on acid treated CdSe quantum dots and low bandgap polymer PCPDTBT. Sol Energy Mater Sol Cells 4:1232–1237
Dayal S, Kopidakis N, Olson DC, Ginley DS, Rumbles G (2010) Photovoltaic devices with a low band gap polymer and CdSe nanostructures exceeding 3% efficiency. Nano Lett 1:239–242
Nath A, Bhati N, Mahajan BK, Rakshit JK, Sarkar MB (2022) Silver nanoparticles textured oxide thin films for surface plasmon enhanced photovoltaic properties. Plasmonics 1:193–201
Zhao XG, Yang D, Sun Y, Li T, Zhang L, Yu L, Zunger A (2017) Cu–In halide perovskite solar absorbers. J Am Chem Soc 139:6718–6725
Wei F, Deng Z, Sun S, Xie F, Kieslich G, Evans DM, Cheetham AK (2016) The synthesis, structure and electronic properties of a lead-free hybrid inorganic–organic double perovskite (MA) 2 KBiCl6 (MA= methylammonium). Mater Horiz 3:328–332
Fatimah S, Ragadhita R, Al Husaeni DF, Nandiyanto ABD (2022) How to calculate crystallite size from x-ray diffraction (XRD) using Scherrer method. ASEAN J Sci Eng 2:65–76
Schmitz F, Horn J, Dengo N, Sedykh AE, Becker J, Maiworm E, Gatti T (2021) Large cation engineering in two-dimensional silver–bismuth bromide double perovskites. Chem Mater 33:4688–4700
Mir SA, Gupta DC (2021) Analysis of cage structured halide double perovskites Cs2NaMCl6 (M= Ti, V) by spin polarized calculations. J Alloy Compd 854:156000
Pering SR, Deng W, Troughton JR, Kubiak PS, Ghosh D, Niemann RG, Cameron PJ (2017) Azetidinium lead iodide for perovskite solar cells. J Mater Chem A 5:20658–20665
Boix PP, Agarwala S, Koh TM, Mathews N, Mhaisalkar SG (2015) Perovskite solar cells: beyond methylammonium lead iodide. J Phys Chem Lett 6:898–907
Dimesso L, Wussler M, Mayer T, Mankel E, Jaegermann W (2016) Inorganic alkali lead iodide semiconducting APbI3 (A= Li, Na, K, Cs) and NH4PbI3 films prepared from solution: structure, morphology, and electronic structure. AIMS Mater Sci 3:737–755
Li H, Sadler PJ, Sun H (1996) Unexpectedly strong binding of a large metal ion (Bi3+) to human serum transferrin (∗). J Biol Chem 271:9483–9489
Thind AS, Kavadiya S, Kouhnavard M, Wheelus R, Cho SB, Lin LY, Mishra R (2019) KBaTeBiO6: a lead-free, inorganic double-perovskite semiconductor for photovoltaic applications. Chem Mater 31:4769–4778
Yangui A, Garrot D, Lauret JS, Lusson A, Bouchez G, Deleporte E, Boukheddaden K (2015) Optical investigation of broadband white-light emission in self-assembled organic–inorganic perovskite (C6H11NH3) 2PbBr4. J Phys Chem C 119:23638–23647
McCall KM, Stoumpos CC, Kostina SS, Kanatzidis MG, Wessels BW (2017) Strong electron–phonon coupling and self-trapped excitons in the defect halide perovskites A3M2I9 (A= Cs, Rb; M= Bi, Sb). Chem Mater 29:4129–4145
Jeon IK, Abdullah A, Hussein AJ, Yong-Rak K, Ashrant A, Juan-Carlos B (2023) Effects of shape-stabilized phase change materials in cementitious composites on thermal-mechanical properties and economic benefits. Appl Therm Eng 219:119444
Ghasemi M, Mengmeng H, Mu X, Peng C, Dongxu H, Yurou Z, Weijian C (2020) Lead-free metal-halide double perovskites: from optoelectronic properties to applications. Nanophotonics 8:2181–2219
Wei F, Zeyu D, Shijing S, Fenghua Z, Donald M. E, Gregor K, Satoshi T (2017) Synthesis and properties of a lead-free hybrid double perovskite:(CH3NH3) 2AgBiBr6. Chem Mater 3:1089–1094
McClure ET, Molly RB, Wolfgang W, Patrick MW (2016) Cs2AgBiX6 (X= Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chem Mater 5:1348–1354
Volonakis G, Filip MR, Haghighirad AA, Sakai N, Wenger B, Snaith, HJ, Giustino F (2016) Lead-free halide double perovskites via heterovalent substitution of noble metals. J Phys Chem Lett
Slavney AH, Hu T, Lindenberg AM, Karunadasa HI (2016) A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J Am Chem Soc 7:2138–2141
Popple D, Mehmet D, Tony VH, Scott S, Peter E, Karen CB, Marvin C, Alex Z (2023) Charge-induced phase transition in encapsulated Hf Te2 nanoribbons. Phys Rev Mater 1:L013001
Li L, Shao H, Wu X, Chen W, Zhu J, Dong B, Bai X (2022) Aluminum-doped lead-free double perovskite Cs2AgBiCl6 nanocrystals with ultrahigh stability towards white light emitting diodes. Mater Res Bull 147:111645
Liu Z, Yang H, Wang J, Yuan Y, Hills-Kimball K, Cai T, Chen O (2021) Synthesis of lead-free Cs2AgBiX6 (X= Cl, Br, I) double perovskite nanoplatelets and their application in CO2 photocatalytic reduction. Nano Lett 21:1620–1627
Lu M, Zhang X, Bai X, Wu H, Shen X, Zhang Y, Rogach AL (2018) Spontaneous silver doping and surface passivation of CsPbI3 perovskite active layer enable light-emitting devices with an external quantum efficiency of 11.2%. ACS Energy Lett 3:1571–1577
Wang KQ, He Y, Zhang M, Shi JJ, Cai WW (2021) Promising lead-free double-perovskite photovoltaic materials Cs2MM′ Br 6 (M= Cu, Ag, and Au; M′= Ga, In, Sb, and Bi) with an ideal band gap and high power conversion efficiency. J Phys Chem C 125:21160–21168
Lingegowda DC, Kumar JK, Prasad AD, Zarei M, Gopal S (2012) FTIR spectroscopic studies on Cleome gynandra–comparative analysis of functional group before and after extraction. Rom J Biophys 22:137–143
Salem Marwa S, Mostafa MS, Mohamed MA, Ahmed S, Abdullah JA, Adwan A, Rabie R (2021) Efficient perovskite solar cell without electron transport layer. Int J Electr Eng Technol 34(12):1
Sun H, Yu Z, Yu X, Kaimo D, Linxing M, Jie X, Liang L (2019) Composition and energy band-modified commercial FTO substrate for in situ formed highly efficient electron transport layer in planar perovskite solar cells. Adv Funct Mater 11:1808667
Shalan AE, Tomoya O, Sudhakar N, Mahmoud ME, Kosei U, Hui-Ping W, Keisuke N, Xu S, Eric Wei-Guang D, Hiroaki M (2016) Cobalt oxide (CoOx) as an efficient hole-extracting layer for high-performance inverted planar perovskite solar cells. ACS Appl Mater Interfaces 49:33592–33600
Hussain I, Hoang Phong T, Jared J, Justin M, Nazmul I, Jasim M U (2018) Functional materials, device architecture, and flexibility of perovskite solar cell. Emerg Mater 1:133–154
Ren J, Kan Z (2020) Chapter 1: origin of hysteresis in perovskite solar cells. AIP Publishing LLC, Melville
Neukom MT, Züfle S, Knapp E, Makha M, Hany R, Ruhstaller B (2017) Why perovskite solar cells with high efficiency show small IV-curve hysteresis. Sol Energy Mater Sol Cells 169:159–166
Acknowledgements
The authors would like to thank the following funding sources that supported the research presented. 1. Sophisticated Analytical Instrument Facility (SAIF) at IIT Bombay for providing a characterization facility. 2. The Prime Minister Research Fellowship (PMRF) under Ministry of education (MOE) for research grant provide to Neelu. (PMRF-1301164 is PMRF ID under which research grant is provided). 3. The Indian Institute of Technology (IIT) Bombay, Mumbai, India for providing the Institute Post-Doctoral Fellow (IPDF) position to Nivedita Pandey.
Author information
Authors and Affiliations
Contributions
NN contributed to conceptualization, investigation, writing—original draft preparation, writing—review and editing. NP contributed to conceptualization, investigation, writing—original draft preparation, writing—review and editing. SC contributed to supervision, writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neelu, N., Pandey, N. & Chakrabarti, S. Morphology of highly stable lead-free hybrid organic–inorganic double perovskites (CH3NH3)2XBiCl6 (X = K, Na, Ag) for solar cell applications. J Mater Sci 58, 11139–11158 (2023). https://doi.org/10.1007/s10853-023-08704-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08704-z