Abstract
A series of Fe2O3/BiVO4 composite photo-Fenton catalysts were prepared by a simple ultrasonic-assisted calcination method. By changing the calcination temperature, FB-2 with tetragonal structure BiVO4 has excellent photo-Fenton performance. The crystal structure and chemical composition of the composite catalyst were studied by a series of characterizations. Under 5 W LED illumination, 97% rhodamine B dye (Rh B) was degraded within 40 min in FB-2 sample, which was attributed to the high efficiency of photogenic electron–hole pair separation. The subsequent capture experiment and Tafel test proved that h+, e−, ·OH were the main active substances in the degradation process and the flow direction of electrons in the reaction process. The composite catalyst has good cyclic stability and efficient photo-Fenton catalytic degradation performance, and has good reference value for the degradation of water dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid progress of industrialization in society, many water pollution problems have come along [1, 2], among which, dye pollution accounts for most of them. Generally speaking, dyes have complex structures, high molecular weights, are readily soluble in water, resist degradation, have potential carcinogenicity and mutagenicity. Therefore, dye-containing wastewater will directly destroy the ecological balance of water bodies and harm on human health [1].In order to solve the problem of dye pollution in water, the photo-Fenton process has been widely concerned. Under the condition of additional introduction of light, Fe2+ can not only accelerate the reduction of Fe3+, but also directly photolysis H2O2 to produce ·OH. Therefore, the synergistic action of Fe2+ and light can produce more ·OH, thus improving the degradation efficiency of photo-Fenton.
BiVO4 has three crystal forms: tetragonal zircon structure (t-z BiVO4), tetragonal scheelite (t-s BiVO4) and monoclinic scheelite (m-s BiVO4) (Fig. 1a–c). The most studied BiVO4 photocatalyst is the scheelite BiVO4, with a band gap of 2.4 eV [2]. The crystal structure of m-s BiVO4 is similar to that of t-s BiVO4, and it has a scheelite structure. The Bi–O polyhedron in m-s BiVO4 is more severely deformed by the 6S2 lone pair of Bi3+ than the Bi–O polyhedron in t-s BiVO4 [3], so m-s BiVO4 exhibits higher photocatalytic performance [4].
In the field of photo-Fenton, Fe2O3 and BiVO4 are both hot research materials in recent years. In these years, a series of two-dimensional photo-Fenton catalytic materials such as Fe2O3/BiVO4 [5], Fe2O3/Bi2WO6 [6], and Fe2O3/Bi2MoO6 [7] have been prepared by simple method, which is effective for methylene blue (MB) and tetracycline (TC) have good degradation properties. The Fe2O3/BiOI [8] synthesized by the two-step hydrothermal emulsion polymerization method and the immersion precipitation method showed good degradation performance for methyl orange (MO), phenol and tetracycline hydrochloride (TCH). Therefore, it is a practical measure to use Fe2O3 as a Fenton catalyst to couple with a semiconductor photocatalyst matching the band gap to prepare a composite catalyst with good photo-Fenton performance.
Therefore, in this paper, Fe2O3/BiVO4 two-dimensional composite photo-Fenton catalyst was prepared by ultrasound-assisted calcination for the first time in the field of photo-Fenton. A series of photo-Fenton catalysts with different crystal structures of BiVO4 were prepared by adjusting the calcination temperature. Using rhodamine B (Rh B), MB and MO as target pollutants, the performance of Fe2O3/BiVO4 composite photo-Fenton catalyst was tested, and the catalytic mechanism of the catalyst was further analyzed. The composite catalyst has high efficiency photo-Fenton catalytic performance and has good reference value for the degradation of dyes in water.
Materials and methods
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), ammonium metavanadate (NH4VO3), ferric chloride hexahydrate (FeCl3·6H2O), sodium lauryl sulfate (C12H25SO4Na) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Rhodamine B (Rh B) was purchased from Shanghai Aladdin Industrial Company. All reagents are of analytical grade, and no further purification is required when used. All experiments use deionized water.
Synthesis of BiVO4
The tetragonal BiVO4 was synthesized following the reported literature [9]. 4 mmol (1.94 g) Bi(NO3)3·5H2O and 4 mmol (0.468 g) NH4VO3 were dissolved in 50 mL of deionized water and stirred vigorously for 1 h at room temperature. The aqueous solution was then sonicated for an additional 1 h in a high-intensity ultrasonic bath. In an instant, the color of the precipitate turned yellow. The yellow precipitate was then washed with deionized water, centrifuged, and excess water molecules were removed with absolute ethanol, and then placed in an oven at 60 °C for 12 h. The powder material was calcined at 300 °C for 4 h, and the resulting bright yellow material was denoted as BiVO4.
Synthesis of Fe2O3/BiVO4 photo-Fenton catalysts
The Fe2O3/BiVO4 was synthesized following the reported literature [10] (Fig. 2). 1 mmol (0.324 g) BiVO4 was uniformly dispersed in 50 mL deionized water, then 3 mmol (0.81 g) FeCl3·6H2O and 1.5 mmol (0.45 g) SDS were added, and ultrasonically treated for 2 h. Then remove excess water with ethanol. Put it in a 60 °C oven for 12 h, and heat the dried powder at 100 °C, 300 °C, 500 °C, and 700 °C for 4 h to remove the organic content. Substances obtained after calcination were designated as FB-1, FB-2, FB-3, and FB-4, respectively. For comparison, Fe2O3 could also be synthesized following a similar procedure.
Characterization
XRD patterns were obtained on a D/Max-RB X-ray diffractometer (Rigaku) with Cu Kα as scan rate (2θ) and irradiation at 5°/min in the range of 10°–80°. Powder morphologies were characterized using SEM (Zeiss Supra 55), HR-TEM (FEI Tecnai F30) and STEM techniques equipped with energy dispersive X-ray (EDX) compositional analysis. XPS measurements were performed on a PHI-5000 C ESCA system operating at 14.0 kV and 25 mA using a Mg Kα source. Fourier transform infrared spectra were measured between 400 and 4000 cm−1 using a Nicolet Aexus 470 infrared spectrometer with KBr as a reference background. Diffuse reflectance spectroscopy (DRS) was measured by UV–visible spectrophotometry (PERSEE T9), with BaSO4 as the reference sample. The RF-5301PC fluorescence spectrometer was used for testing, and the specific parameters were: 150 W xenon lamp, wavelength measurement range of 340–750 nm, and wavelength moving speed of 20000 nm/min. Transient photocurrent, electrochemical impedance spectroscopy (EIS) and Mott–Schottky test (M–S), all in a three-electrode system (one ITO glass electrode (working electrode), platinum electrode (counter electrode) and saturated calomel electrode (SCE, reference electrode)) on a PARSTAT 2273 electrochemical workstation.
Photo-Fenton experiments
The degradation experiments of different dyes proved the catalytic activity of Fe2O3/BiVO4 composite photo-Fenton catalysts. The photo-Fenton reaction was carried out in a PCX50A discover multi-channel photocatalytic reaction instrument produced by Beijing Perfect-light Technology Company. The photocatalytic reactor uses 9 LED lamps with a power of 5 W as the cold light source. During the reaction, the disc is set to rotate one grid per minute, thereby ensuring that each reaction vial is evenly irradiated with light. Usually, 0.1 g of the prepared photo-Fenton catalyst was added to 100 mL of dye solution (Rh B = 20 mg/L, MB = 35 mg/L, MO = 40 mg/L), and after 30 min adsorption under dark conditions, the dye was the adsorption–desorption equilibrium was reached with the catalyst sample. Then add a 5 W LED light source, and at the same time add an appropriate amount of H2O2 (30%). 4 mL of the suspension was taken at intervals and centrifuged to remove solid particles. The concentration of the dye was determined by UV–vis spectrophotometry (PERSEE T9) at the corresponding maximum absorbance. Where C0 is the initial concentration of the dye, C is the dye concentration sampled at different times during the reaction process, the degradation efficiency of the composite photo-Fenton catalyst to organic pollutants was calculated by C/C0, and the first-order kinetic curve ln (the relationship between C0/C) and time t yields the photoreaction rate constant.
For the Fenton-like reaction process, except that the 5 W LED light source was not introduced, the rest of the experimental process was the same as the above operation. For the photocatalytic experiments, the rest of the experimental procedures were the same as those described above, except that no H2O2 was added.
Results
Morphologies and structures
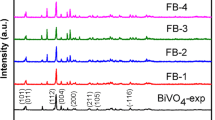
First, the crystal structures of the as-prepared BiVO4 and a series of Fe2O3/BiVO4 (FB-1 ~ FB-4) were characterized (Fig. 3a). The XRD pattern of pure BiVO4 prepared by calcination at 300 °C was matched with that of tetragonal BiVO4 (PDF# 14-0133) [9]. In 18.41°, 24.4°, 30.76°, 32.68°, 34.83°, 39.64°, 43.92°, 47.19°, 48.51° and 49.99° in the diffraction peak of respectively with (101), (200), (211), (112), (220), (301), (103), (321), (312), (400) crystal plane matching. Tetragonal BiVO4 (PDF# 14-0133) corresponds to FB-1 and FB-2 prepared by calcination at 100 °C and 300 °C. Because monoclinic BiVO4 and tetragonal BiVO4 are calcined at 528 K, a reversible crystal phase transition [4] can occur, that is, from tetragonal BiVO4 at low temperature to monoclinic BiVO4. FB-3 and FB-4 were obtained by increasing the calcination temperature at 500 °C and 700 °C. The diffraction peaks of the two at 18.8°, 28.92°, 30.64° and 53.34° are consistent with the monoclinal BiVO4 (PDF# 14-0688) (011), (121), (040) and (161) planes, which is consistent with the previously reported view that calcination can change the crystal phase of BiVO4 [6]. In the XRD patterns of FB-1 ~ FB-4, no pronounced Fe2O3 diffraction peaks were observed, which may be related to its low content or poor crystallinity. However, the existence of Fe2O3 can be further confirmed by the surface element scanning of TEM. The characterization results of XRD can prove that by changing the calcination temperature, BiVO4 with two different crystal phases are obtained, and Fe2O3 is compounded with BiVO4 without changing the crystal structure of the original BiVO4. Furthermore, no other diffraction peaks were observed, confirming the phase purity of all composites [11].
In order to study the chemical composition and chemical structure of the heterogeneous catalysts, the XPS technique was used for further analysis. Only the peaks of four elements, Bi, V, O, and Fe, were detected in the full spectrum in Fig. 3b, which proves that the prepared catalyst is of high purity. The characteristic peaks at 516.5 eV and 524.2 eV in Fig. 3c correspond to the V 2p3/2 and V 2p1/2 spin orbits of V5+, respectively [12]. In the high-resolution XPS image of Bi 4f in Fig. 3d, Bi 4f5/2 and Bi 4f7/2 were observed to have two symmetrical characteristic peaks at 159.1 eV and 164.5 eV [13], which indicates that the primary element valence of Bi is Bi3+ [14]. The two distinct peaks at 711.3 eV and 724.7 eV in Fig. 3e belong to the Fe 2p3/2 and Fe 2p1/2 spin–orbit states [15] of Fe2O3 nanoparticles, respectively. In addition, the XPS spectrum of O 1s of Fe2O3/BiVO4 is divided into two peaks around 529.8 eV and 531.3 eV (Fig. 3f), and an apparent binding energy peak is formed at 529.8 eV, which belongs to Bi–O in BiVO4 and is centred at 531.3 eV. The rise belongs to the Fe2O3 species Fe–O [16]. The above results confirmed that Fe2O3/BiVO4 nanocomposites were successfully prepared.
On this basis, the chemical composition and chemical structure of the composite catalyst was further confirmed by FT-IR (Fig. 4a). In the FT-IR of Fe2O3, 598 cm−1 corresponds to the stretching vibration of Fe–O [17], 1134 cm−1 corresponds to the characteristic absorption peak of Fe2O3 [18], and 1595 cm−1 corresponds to the N–H vibration [18] caused by the residual SDS in the preparation process, while 3404 cm−1 corresponds to the stretching vibration [19] of the hydroxyl group adsorbed on the surface of Fe2O3. The FT-IR of BiVO4 at 736 cm−1 corresponds to the stretching vibration of VO43− [20]. From the enlarged FT-IR image (Fig. 4b), it can be observed that the characteristic peak of VO43− in the FB-1 ~ FB-4 composite catalyst has shifted and the intensity has decreased, which may be caused by the successful contact between Fe2O3 and BiVO4 [18]. The outflow of electrons from the conduction band of BiVO4 will lead to changes in the local electronic structure [21], which corresponds to the mechanism explanation in the following part. Possibly due to the low loading content of Fe2O3, there is no prominent FT-IR diffraction peak of Fe2O3 in the FB-1 ~ FB-4 composites. The FT-IR characterization results are consistent with the XRD and XPS analysis results.
The morphologies and microstructures of Fe2O3, BiVO4 and Fe2O3/BiVO4 nanocomposites were observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Figure 5a, b are the SEM images of the Fe2O3 sample and the BiVO4 sample, respectively. It can be seen that Fe2O3 is a varying block in a stacked state, while the BiVO4 sample is a sphere with varying size and rough surface. Figure 5c–f shows the FB sample prepared under different temperature calcination, with an average size of about 1–2 μm. With the increase of the calcination temperature, the morphology of the FB sample gradually changed from regular spherical to irregular because more irregular Fe2O3 [10] would crystallize on the surface of the BiVO4 sample during this process, covering the active sites on the surface of the BiVO4 sample. Compared with the other three FB samples, FB-2 is smaller in size and closer in shape to regular spherical particles, which is similar to the BiVO4 sample [9]. The SEM–EDS (Supplementary Fig. S1) analysis results show that Bi, V, Fe, and O are well distributed in their respective regions [22], which agrees with the XRD analysis results. As depicted by the TEM image in Fig. 5g, h, the interplanar distances are 0.16 and 0.27 nm, matching Fe2O3 (121) and BiVO4 (400), respectively.
Photoelectric properties
The absorbance of the initial samples and composite samples were characterized by UV–vis diffuse reflectance spectroscopy (UV–vis DRS), and the results are shown in Fig. 6a. The absorption sideband of BiVO4 is 530 nm, which is in line with a previous report [9]. Fe2O3 exhibits broad absorption up to 700 nm due to transitions in the d orbital [23], indicating high activity in the visible region. Furthermore, due to the lower Fe2O3 content in the FB samples, the FB composites exhibited similar absorption properties to pure BiVO4. The recombined FB-2 ~ FB-4 all undergo a red shift, attributed to the charge transition between Fe2O3 and BiVO4 [24]. This indicates that the photo-response range has been expanded, and more photoinduced electrons and holes can be excited to form [14], that is, the utilization of visible light by the composite sample is enhanced. Calculate the band gap value of the semiconductor according to the Kubelka–Munk formula (Eq. 1) [25], where A is the absorption coefficient, h is Planck’s constant, and v is optical frequency, Eg is the band gap, and the value of n depends on the semiconductor type.
a UV–Vis diffuse reflectance spectra of Fe2O3, BiVO4 and FB series samples. b Estimate the band gap values of Fe2O3 and BiVO4 based on the plotted (αhν)1/2 versus hν. c PL emission spectra (the excitation wavelength is 340 nm). d Transient photocurrent curves of prepared samples. e, f Mott-Schottky and EIS curves of the prepared samples (in a three-electrode system with ITO glass electrode as working electrode, platinum electrode as counter electrode and saturated calomel electrode as reference electrode. 0.5 M Na2SO4 solution was used as electrolyte solution)
BiVO4 and Fe2O3 are direct-gap semiconductors and indirect-gap semiconductors, respectively, with n values of 2 and 1/2 [14]. The material's band gap [21] was estimated from the inflexion point between the tangent line on the inflexion point and the horizontal line, and the band gaps (Eg) of BiVO4 and Fe2O3 were determined to be 2.2 eV and 1.9 eV, respectively (Fig. 6b). The band gap of composite samples FB-1 ~ FB-4 is between 2.04 and 2.14 eV (Fig. S2a, b), which is smaller than that of pure BiVO4 samples.
Photoluminescence spectroscopy can study the efficiency of photogenerated carrier recombination and separation. The results of photoluminescence spectroscopy can reveal electron–hole pair transfer, migration and recombination processes [26]. When the semiconductor is illuminated by light, the catalyst generates electrons and holes, and the fluorescence emission spectrum represents the released energy of some of the electrons and holes recombination. Higher emissivity indicates better charge recombination efficiency. The emission spectra of Fe2O3 and BiVO4 show higher intensities (Fig. 6c), indicating that the electron–hole pairs of Fe2O3 and BiVO4 are rapidly recombined. As expected, the PL intensity of the FB-2 composite is lower than that of Fe2O3 and BiVO4. Therefore, the FB-2 composite structure is illustrated to facilitate the efficient separation of electron–hole pairs, which is consistent with the optical properties of DRS [27].
Figure 6d compares the transient photocurrent densities of the obtained BiVO4, Fe2O3 and a series of FB electrodes under visible light irradiation. All electrodes produced a fast photocurrent response when the light was present. Then, when the light is cut off, it instantly drops to zero, these materials are photosensitive. Under visible light irradiation, the photocurrent of FB-2 was significantly higher than pure BiVO4. These results show that BiVO4 has a higher photogenerated electron and hole combination rate than FB-2. That is, FB-2 has efficient charge separation, consistent with the results of PL spectroscopy [28].
It is well known that the photocatalytic activity of a catalyst is closely related to its band structure and charge transfer kinetics. The band structures of the samples were prepared, and their charge transfer and recombination properties were analyzed. First, the flat-band potential of the sample was measured using M–S. Figure 6e is the M–S curve of the measured sample. The slopes of Fe2O3 and BiVO4 are positive, indicating that the semiconductor is n-type, consistent with the results reported in the previous literature [18]. Meanwhile, the flat-band potentials (approximately equal to the conduction band in n-type semiconductors) of Fe2O3 and BiVO4 materials are − 0.27 V and − 0.7 V, respectively (relative to a common hydrogen electrode (RHE)). In general, the bottom of the conduction band of an n-type semiconductor is 0.1 V [29] minus the flat band potential. Therefore, the conduction band potentials of Fe2O3 and BiVO4 materials are − 0.37 V and − 0.8 V, respectively (relative to standard hydrogen electrode (RHE)). After that, the conduction band values of Fe2O3 and BiVO4 materials close to normal hydrogen electrode (NHE) were calculated according to Eq. (2) [30], which were − 0.78 V and − 1.21 V, respectively.
Combined with the above DRS test, the band gap values of Fe2O3 and BiVO4 materials are 1.9 eV and 2.2 eV, respectively. Therefore, according to Eq. (3), the valence band positions of Fe2O3 and BiVO4 materials are further calculated to be 1.12 eV and 0.99 eV, respectively. In summary, the specific energy band structure values of Fe2O3 and BiVO4 materials are shown in Table 1.
The changes in charge separation and transfer in the FB composite were further determined by electrochemical impedance spectroscopy (EIS). The smaller the diameter of the semicircle in the EIS diagram, the faster the electron mobility and the more efficient the separation of carriers [17]. The semicircle diameter of the FB-2 composite sample in Fig. 6f is much smaller than that of the other composite samples, indicating that the FB-2 interfacial layer resistance is reduced, which is beneficial to charge separation and transfer.
The above series of photoelectric performance characterizations further confirmed the successful preparation of Fe2O3/BiVO4 composite photo-Fenton materials. The prepared FB-2 sample has excellent photo-response performance and efficient electron transfer efficiency. This is inseparable from its excellent photo-Fenton degradation efficiency below.
Photo-Fenton catalytic performance
Taking the degradation of Rh B as an example, the performance of other photo-Fenton catalysts was evaluated. Under the condition of adding an appropriate amount of Fe2O3, different calcination temperatures have different affect the photo-Fenton performance of the prepared catalysts. With Rh B as the target pollutant, FB-2 calcined at 300 °C had the highest photo-Fenton degradation efficiency (Fig. 7a). As the calcination temperature increased, more Fe2O3 nanoparticles were crystallized, covering the active sites on the FB surface. Moreover, the accumulation of excess Fe2O3 nanoparticles due to unbalanced forces hinders the efficient formation of heterojunctions [31], which results in the ineffective separation of photogenerated carriers and reduces the catalytic activity. In addition, the photo-Fenton degradation kinetics curves of FB-1 ~ FB-4 can be approximated as a quasi-first-order process: ln(C0/C) = kt. By fitting the obtained ln(C0/C) curve, the photoreaction rate constant k was obtained (Fig. 7c), in which the photodegradation rate of FB-2 was the highest, reaching 0.086 min−1.
a, b FB-1 ~ FB-4 photo-Fenton degradation performance curve and degradation rate histogram c First-order kinetic curves of FB-1 ~ FB-4 after fitting d UV absorbance at different times during photo-Fenton degradation of FB-2 sample e photo-Fenton properties of BiVO4, Fe2O3 and FB-2 f photo-Fenton cycle test of the FB-2 sample
In Fig. 7d, the concentration changes of Rh B under different degradation time during the photo-Fenton degradation of Rh B by FB-2 were monitored. With the increase of illumination times, the characteristic peak intensity of Rh B decreased continuously. After 60 min, the absorption peak almost disappeared. The final degradation rate of the FB-1 ~ FB-4 samples can be observed in the bar graph of Fig. 7b, and the broken line in the figure intuitively reflects the changing trend of the degradation rate. It is further confirmed that FB-2 has excellent photo-Fenton degradation performance. Supplementary Fig. S3a–c show the photo-Fenton, Fenton-like and photocatalytic degradation performance curves of BiVO4, Fe2O3 and composite FB-2 for Rh B. The photo-Fenton performance of the composite FB-2 was significantly improved (Fig. 7e), and the degradation rate of Rh B reached 97% within 40 min. It is proved that the FB-2 composite sample obtained by calcination of BiVO4 loaded with an appropriate amount of Fe2O3 at 300 °C can promote the separation and migration of photogenerated carriers. At the same time, the exposure of more active sites enables the photocatalytic and Fenton oxidation has the best synergistic effect. It is also proved that the photocatalytic response of BiVO4 is dominant in the Fenton-photocatalytic synergistic reaction of FB-2.
It is generally believed that the cyclic stability of catalysts is one of the critical signs of good catalytic performance. The catalytic stability of FB-2 catalyst for five consecutive degradations of Rh B (20 mg L−1) under visible light was investigated. After five photo-Fenton catalytic cycles, the FB-2 composite catalyst still has good degradation performance (Fig. 7f), confirming the good long-term reusability of the FB-2 catalyst, which proposed a new idea for the practical industrial application of composite catalyst.
It is generally believed that the photo-Fenton catalysis reaction is carried out on the surface of the catalyst, so the positive and negative charges on the surface of the catalyst will directly affect the adsorption process of the catalyst to the dye molecules, thereby affecting the catalytic effect of the photo-Fenton [32]. In this experiment, FB-2 has good degradation performance on Rh B and MB cationic dyes. However, the anionic dye MO did not achieve the expected effect. It is speculated that the surface of the FB-2 catalyst has many negative charges. At the moment of adding the cationic dyes (Rh B and MB), the negative charges on the catalyst’s surface quickly attract the cationic dyes. When the light source is introduced, and H2O2 is added, the cationic dyes adsorbed on the catalyst surface rapidly undergo a series of redox reactions with H2O2. They are degraded (Fig. 8a, d).
a, d Photo-Fenton degradation performance curves and bar graphs of FB-2 for different dyes (Rh B, MB, MO) b, c the effects of different H2O2 dosage and catalyst content on the photo-Fenton performance of FB-2 samples e, f corresponding fitting first-order kinetic linear diagram of photo-Fenton degradation performance of FB-2 samples with different H2O2 dosage and catalyst content
To clarify the photo-Fenton reaction mechanism of the catalyst, the effects of H2O2 dosage (Fig. 8b) and catalyst dosage (Fig. 8c) on the degradation performance of Rh B were discussed. Figure 8b shows the degradation efficiency of Rh B (20 mg L−1) by FB-2 by adding different amounts of H2O2. It can be seen that the decomposition rate of Rh B increases significantly with the increase in the quantity of H2O2 added. From the fitted first-order kinetic line graph (Fig. 8e) and the corresponding kinetic constant bar graph (Supplementary Fig. S4a), it can be seen that as the H2O2 dosage increases from 0 to 1 mL, the fitted rate constant rapidly increased to 0.07731 min−1. This is because with the addition of H2O2, the generation rate of ·OH is accelerated, promoting the degradation of the target pollutants. However, when more than 1 mL of H2O2 was added, the fitted rate constant decreased from 0.07731 to 0.06162 min−1. This may be because excess H2O2 captures ·OH [Eq. (4)], resulting in a continuous increase in the degradation rate of ·OH, and many HO2· is also generated. Compared with ·OH, the oxidation potential of HO2· is lower, which inhibits the entire photo-Fenton reaction process [Eq. (5)], in turn, reduces the rate of pollutant degradation [33].
Usually, the catalyst degradation rate increases with the increase of catalyst dosage. According to the fitted first-order kinetic line (Fig. 8f) and the corresponding kinetic constant histogram (Supplementary Fig. S4b), as the catalyst dosage increased from 0.04 to 0.08 g, its fitted rate constant Increased from 0.07336 to 0.11745 min−1. However, when the dosage continued to increase, the fitted rate consistent decreased to 0.06021 min−1, which may be due to the decrease of the transmittance of the solution due to the excess catalyst, which reduced the photocatalytic efficiency of BiVO4 and hindered the electron from BiVO4 flows to Fe2O3, which in turn reduces the photo-Fenton catalytic degradation efficiency of the composite catalyst.
Mechanism of catalyst performance improvement
To study the experimental mechanism of the Fe2O3/BiVO4 photo-Fenton reaction, a capture experiment was carried out to determine the active species that played a significant role in the response process. In the photo-Fenton reaction, silver nitrate (AgNO3), isopropanol (IPA), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) and p-benzoquinone (BQ) were used as trapping agents for e−, ·OH, h+ and O2·−, respectively [34] (Fig. 9a). Among them, IPA, EDTA-2Na, and BQ inhibited the degradation rate of the catalyst to the dye by 60%, 60%, and 24%, respectively (Fig. 9b). The results of capture experiments showed that e− and ·OH were involved in the photo-Fenton reaction, and h+ was the main active species in the photo-Fenton reaction, which played a crucial role in the degradation of Rh B. When BQ was added to the photo-Fenton reaction, there was a slight inhibitory effect during the reaction. It is speculated that the addition of BQ may reduce solution’s transparency, thereby inhibiting the degradation efficiency of the catalyst. However, the degradation efficiency of Rh B did not change in the end, indicating that O2·− was not the active species in the reaction process.
To judge the electron flow direction between Fe2O3 and BiVO4, the Tafel curves of Fe2O3 and BiVO4 under light and without light were tested (Fig. 9c). Under visible light irradiation, the redox equilibrium potential of BiVO4 shifted to a more negative direction [13] and the redox potential of Fe2O3 moves in a more positive direction. Under visible light irradiation, BiVO4 has a more negative redox equilibrium potential than Fe2O3, indicating that BiVO4 has a higher surface electron energy level in Na2SO4 solution under visible light irradiation [13]. At the same time, it can be seen that the redox properties of Fe2O3 are more affected by visible light irradiation, indicating that the energy level structure of Fe2O3 is more affected by minority carrier injection. The open-circuit voltages of Fe2O3/BiVO4 under dark and light conditions were measured to be − 0.88 V and − 0.81 V, respectively. These open circuit voltages lie between the redox potentials of Fe2O3 and BiVO4, representing the flow of electrons from BiVO4 to Fe2O3 during photo-Fenton catalysis.
Based on the above series of photoelectric characterization, performance testing experiments, trapping experiments and Tafel curves, the electron flow diagram during the reaction of the FB-2 composite photo-Fenton catalyst (Fig. 9d, f) and the reaction mechanism of Rh B degradation (Fig. 10) are proposed. Under illumination, BiVO4 and Fe2O3 are excited to generate photogenerated electrons (e−) and holes (h+) simultaneously [Eq. (6)]. Since the conduction band and valence band of BiVO4 are more negative than Fe2O3, the e− on the conduction band can be easily transferred to the conduction band of Fe2O3 which is closer to it (Fig. 9e), further reducing Fe3+ to Fe2+ [Eq. (7)], the Fe2+ generated by the reduction reaction reacts with H2O2 to generate ·OH active species that participate in the photo-Fenton reaction to degrade Rh B [Eq. (9), (11)]. The added H2O2 can not only oxidize the generated Fe2+ to Fe3+ (Eq. (9)), but also react with e− to generate ·OH active species to promote the degradation of the dye [Eq. (8)]. At the same time, the holes in the valence band of Fe2O3 can also react with the OH− generated by the decomposition of H2O2 to generate ·OH to promote the degradation of pollutants [Eq. (10)] (Fig. 9f). The Fe2O3/BiVO4 photo-Fenton catalyst system accelerates the transfer of electrons and promotes the cycle between Fe3+ and Fe2+, thereby improving the catalytic degradation efficiency of the FB-2 composite photo-Fenton catalyst.
Discussion
In this paper, the photo-Fenton catalyst with high catalytic degradation performance was prepared by ultrasound-assisted calcination. FB-2 with tetragonal BiVO4 structure was obtained by calcination at 300 °C with the best photo-Fenton degradation performance. The capture experiment and Tafel test proved that h+, e− and ·OH were the main active species in the reaction process and the specific direction of electrons in the reaction process.
Compared with other Fe2O3/BiVO4 photocatalytic degradation experiments (Table 2), under the irradiation of a weak light source (5 W LED) in this experiment, Rh B (20 mg/L) and MB (35 mg/L) dyes still have good degradation efficiency within 40 min. That is, under low-intensity light, the FB-2 composite photo-Fenton catalyst prepared in this experiment still has good catalytic degradation activity.
Conclusions
Fe2O3/BiVO4 composite photo-Fenton catalyst was prepared by a simple ultrasonic-assisted calcination method. The change of calcination temperature affects the photo-Fenton performance of FB series composite catalysts. FB-2 with tetragonal BiVO4 structure, which was calcined at 300 °C, degraded 97% of Rh B (20 mg L−1) within 40 min, and maintained high activity after five cycles of testing. The capture experiment and Tafel test further demonstrated the main active species and electron flow between catalyst interfaces during the reaction. The close interfacial contact and efficient charge transfer between Fe2O3 and BiVO4 greatly enhance the synergistic reaction between them. At the same time, it provides a new idea and solution for the practical application of Fe2O3 based heterogeneous photo-Fenton catalyst.
Data and code availability
The date that support the findings of this study are available from the corresponding author upon reasonable request.
References
Hitam CNC, Jalil AA (2020) A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J Environ Manag 258:110050. https://doi.org/10.1016/j.jenvman.2019.110050
Xu M, Yang J, Sun C, Liu L, Cui Y, Liang B (2020) Performance enhancement strategies of bi-based photocatalysts: a review on recent progress. Chem Eng J. https://doi.org/10.1016/j.cej.2020.124402
Li J, You J, Wang Z et al (2022) Application of α-Fe2O3-based heterogeneous photo-Fenton catalyst in wastewater treatment: a review of recent advances. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2022.108329
Tokunaga S, Kato H, Kudo A (2001) Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem Mater 13:4624–4628. https://doi.org/10.1021/cm0103390
Wen Y, Zhao Y, Guo M, Xu Y (2019) Synergetic effect of Fe2O3 and BiVO4 as photocatalyst nanocomposites for improved photo-Fenton catalytic activity. J Mater Sci 54:8236–8246. https://doi.org/10.1007/s10853-019-03511-x
Wang W, Zhao W, Zhang H, Dou X, Shi H (2021) 2D/2D step-scheme α-Fe2O3/Bi2WO6 photocatalyst with efficient charge transfer for enhanced photo-Fenton catalytic activity. Chin J Catal 42:97–106. https://doi.org/10.1016/s1872-2067(20)63602-6
Wang W, Zhao W, Huang H, Chen R, Shi H (2021) A 2D/2D S-scheme photo-Fenton catalyst based on ultrathin Bi2MoO6 and Fe2O3 hexagonal nanosheets for efficient tetracycline degradation. Catal Sci Technol 11:2948–2956. https://doi.org/10.1039/d1cy00051a
Jiang J, Gao J, Li T et al (2019) Visible-light-driven photo-Fenton reaction with α-Fe2O3/BiOI at near neutral pH: boosted photogenerated charge separation, optimum operating parameters and mechanism insight. J Colloid Interface Sci 554:531–543. https://doi.org/10.1016/j.jcis.2019.07.038
Zhou L, Wang W, Liu S, Zhang L, Xu H, Zhu W (2006) A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. J Mol Catal A Chem 252:120–124. https://doi.org/10.1016/j.molcata.2006.01.052
Hao C, Feng F, Wang X et al (2015) The preparation of Fe2O3 nanoparticles by liquid phase-based ultrasonic-assisted method and its application as enzyme-free sensor for the detection of H2O2. RSC Adv 5:21161–21169. https://doi.org/10.1039/c4ra17226d
Liu S, Tang H, Zhou H, Dai G, Wang W (2017) Photocatalytic perfermance of sandwich-like BiVO4 sheets by microwave assisted synthesis. Appl Surf Sci 391:542–547. https://doi.org/10.1016/j.apsusc.2016.06.184
Ji K, Dai H, Deng J et al (2015) 3DOM BiVO4 supported silver bromide and noble metals: high-performance photocatalysts for the visible-light-driven degradation of 4-chlorophenol. Appl Catal B 168–169:274–282. https://doi.org/10.1016/j.apcatb.2014.12.045
Guo R, Yan A, Xu J et al (2020) Effects of morphology on the visible-light-driven photocatalytic and bactericidal properties of BiVO4/CdS heterojunctions: a discussion on photocatalysis mechanism. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2019.153246
Ma C, Lee J, Kim Y, Cheol Seo W, Jung H, Yang W (2021) Rational design of α-Fe2O3 nanocubes supported BiVO4 Z-scheme photocatalyst for photocatalytic degradation of antibiotic under visible light. J Colloid Interface Sci 581:514–522. https://doi.org/10.1016/j.jcis.2020.07.127
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449. https://doi.org/10.1016/j.apsusc.2007.09.063
Gao X, Ma C, Liu Y, Xing L, Yan Y (2019) Self-induced Fenton reaction constructed by Fe (III) grafted BiVO4 nanosheets with improved photocatalytic performance and mechanism insight. Appl Surf Sci 467–468:673–683. https://doi.org/10.1016/j.apsusc.2018.10.172
Xie T, Zhang Y, Yao W, Liu Y, Wang H, Wu Z (2019) Synthesis of Bi-deficient monolayered Bi2WO6 nanosheets with enhanced photocatalytic activity under visible light irradiation. Catal Sci Technol 9:1178–1188. https://doi.org/10.1039/c8cy02344a
Liu N, Ouyang D, Cai Y, Li Y (2020) Heterostructured Fe2O3/BiVO4 nano-photocatalyst for the reduction of nitroarenes into amines. Ceram Int 46:24534–24543. https://doi.org/10.1016/j.ceramint.2020.06.240
Liu Y, Ge HY, Zhang XZ, Huang SF, Cheng X (2013) Surface chemical modification and characterization of nano Fe2O3. Appl Mech Mater 345:180–183. https://doi.org/10.4028/www.scientific.net/AMM.345.180
Gotić M, Musić S, Ivanda M, Šoufek M, Popović S (2005) Synthesis and characterisation of bismuth (III) vanadate. J Mol Struct 744–747:535–570. https://doi.org/10.1016/j.molstruc.2004.10.075
Xi J, Xia H, Ning X et al (2019) Carbon-intercalated 0D/2D hybrid of hematite quantum dots/graphitic carbon nitride nanosheets as superior catalyst for advanced oxidation. Small 15:e1902744. https://doi.org/10.1002/smll.201902744
Xu T, Zhu R, Zhu G et al (2017) Mechanisms for the enhanced photo-Fenton activity of ferrihydrite modified with BiVO4 at neutral pH. Appl Catal B 212:50–58. https://doi.org/10.1016/j.apcatb.2017.04.064
Zhou L, Wang L, Zhang J, Lei J, Liu Y (2016) Well‐dispersed Fe2O3 nanoparticles on g‐C3N4for efficient and stable photo‐fenton photocatalysis under visible‐light irradiation. Eur J Inorg Chem 2016:5387–5392. https://doi.org/10.1002/ejic.201600959
Sahar S, Zeb A, Liu Y, Ullah N, Xu A (2017) Enhanced Fenton, photo-Fenton and peroxidase-like activity and stability over Fe3O4/g-C3N4 nanocomposites. Chin J Catal 38:2110–2119. https://doi.org/10.1016/s1872-2067(17)62957-7
Zhao Y, Tao C, Xiao G et al (2016) Controlled synthesis and photocatalysis of sea urchin-like Fe3O4@TiO2@Ag nanocomposites. Nanoscale 8:5313–5326. https://doi.org/10.1039/c5nr08624h
Wei B, Wang C, He Y, Ran G, Song Q (2021) A novel FeS2@g-C3N4 composite with enhanced photo-Fenton catalytic activity for pollutant degradation. Compos Commun. https://doi.org/10.1016/j.coco.2021.100652
Wang T, Liu X, Men Q et al (2019) Photocatalytic removal using g-C3N4 quantum dots/Bi2Ti2O7 composites. Spectrochim Acta A Mol Biomol Spectrosc 213:19–27. https://doi.org/10.1016/j.saa.2019.01.051
Zhang M, Zhang Y, Tang L et al (2019) Ultrathin Bi2WO6 nanosheets loaded g-C3N4 quantum dots: a direct Z-scheme photocatalyst with enhanced photocatalytic activity towards degradation of organic pollutants under wide spectrum light irradiation. J Colloid Interface Sci 539:654–664. https://doi.org/10.1016/j.jcis.2018.12.112
Liu L, Hu P, Cui W, Li X, Zhang Z (2017) Increased photocatalytic hydrogen evolution and stability over nano-sheet g-C3N4 hybridized CdS core@shell structure. Int J Hydrog Energy 42:17435–17445. https://doi.org/10.1016/j.ijhydene.2017.02.171
Bornoz P, Prevot MS, Yu X, Guijarro N, Sivula K (2015) Direct light-driven water oxidation by a ladder-type conjugated polymer photoanode. J Am Chem Soc 137:15338–15341. https://doi.org/10.1021/jacs.5b05724
Chakraborty A, Sarangapany S, Mishra U, Mohanty K (2021) Green synthesized magnetically separable iron oxide nanoparticles for efficient heterogeneous photo-Fenton degradation of dye pollutants. J Clust Sci 33:675. https://doi.org/10.1007/s10876-021-02010-x
Xu H, Wu L, Jin L, Wu K (2017) Synthesis of Fe2O3/TiO2 monoliths for the enhanced degradation of industrial dye and pesticide via photo-Fenton catalysis. J Mater Sci Technol 33:30–38. https://doi.org/10.1016/j.jmst.2016.04.008
Singh J, Sharma S, Aanchal, Basu S (2019) Synthesis of Fe2O3/TiO2 monoliths for the enhanced degradation of industrial dye and pesticide via photo-Fenton catalysis. J Photochem Photobiol A Chem 376:32–42. https://doi.org/10.1016/j.jphotochem.2019.03.004
Liu X, Xu J, Ni Z, Wang R, You J, Guo R (2019) Adsorption and visible-light-driven photocatalytic properties of Ag3PO4/WO3 composites: a discussion of the mechanism. Chem Eng J 356:22–33. https://doi.org/10.1016/j.cej.2018.09.001
Zhang K, Liu Y, Deng J et al (2017) Fe2O3/3DOM BiVO4: high-performance photocatalysts for the visible light-driven degradation of 4-nitrophenol. Appl Catal B 202:569–579. https://doi.org/10.1016/j.apcatb.2016.09.069
Acknowledgements
This work was financially supported by the Liaoning Applied Basic Research Program (No. 2023JH2/101300011, No. 2023JH2/101300018), and the Basic scientific research project of Liaoning Province Department of Education (No. LJKZZ20220024).
Author information
Authors and Affiliations
Contributions
JL: Conceptualization, methodology, data curation, writing—original draft. JY: Funding acquisition. ZW: Validation. YZ: Formal analysis. JX: Resources. MD: Supervision. HZ: Project administration.
Corresponding authors
Ethics declarations
Conflicts of interest
All authors disclosed no relevant relationships.
Ethical approval
Not Applicable.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., You, J., Wang, Z. et al. Fe2O3/BiVO4 heterogeneous photo-Fenton catalyst with excellent dye degradation performance prepared by ultrasonic-assisted calcination. J Mater Sci 58, 10274–10287 (2023). https://doi.org/10.1007/s10853-023-08683-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08683-1