Abstract
The investigation of the Al–Ag–Zr system is crucial for the development of heat-resistant Al alloys, which are essential for applications in high-temperature environments. In this work, we first determined the isothermal sections of the Al–Ag–Zr system at 500 and 600 °C using equilibrium alloys. Simultaneously, the τ3 (Al6AgZr6) phase was identified by transmission electron microscopy (TEM). In addition, the three-phase equilibrium of τ3 + AgZr + (Ag) instead of Al3Zr4 + AgZr + (Ag) was confirmed at 500 and 600 °C. The formation enthalpies of the end-members and ternary compounds in the Al–Ag–Zr system were calculated via first-principles calculations. Based on the experimental and computational results, a thermodynamic database of the Al–Ag–Zr system was established using the CALPHAD (calculation of phase diagrams) method. The hardness of τ1 (Al2.68Ag0.32Zr) and τ2 (Al1.84Ag0.16Zr) were determined to be 3.82 and 10.20 GPa, respectively, using nanoindentation. The introduction of the τ2 phase in the design of Al alloys can considerably increase the mechanical properties of the alloys.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum alloys are commonly strengthened using methods such as solid solution strengthening [1, 2], grain refinement strengthening [3], work hardening [4, 5], second phase strengthening [6, 7], and heterostructure strengthening [8,9,10]. In practical applications, multiple strengthening methods are often combined to optimize alloy performance. Adding alloying elements to aluminum alloys can form heterostructures or introduce second phases to improve their heat resistance and strength. For example, in Al–Sc–Zr alloys, core–shell nanoparticles with a Sc-rich core and a Zr-rich shell about 1–2 nm thickness can precipitate. These nanoparticles can provide the alloys with high strength and excellent thermal stability [11]. The addition of equal amounts of Fe and Ni to Al–Cu–Mg alloys introduces a stable dispersed strengthening Al9FeNi phase [6]. This phase exhibits hardness values of 7.71 GPa and 5.83 GPa at room temperature and 350 °C, respectively, which are higher than that of Al2Cu at room temperature (5.77 GPa) [12]. Therefore, the introduction of Al9FeNi can considerably improve the mechanical properties of the alloy. Currently, the most used Al–Cu–Mg–Fe–Ni alloy is 2618, which retains good mechanical properties above 200 °C. Adding Mn to Al–Cu alloys can form a dispersed Al20Cu2Mn3 phase [13]. The high-temperature creep resistance of an Al–Cu–Mn alloy reinforced by coarse Al20Cu2Mn3 phases is better than that of Al–Cu–Mg alloys consisting of small θ' phases [7].
Using phase diagrams to guide the design of heat-resistant aluminum alloys can reduce time and cost [14]. Zirconium, a transition element, is one of the microalloying elements commonly used in Al–Cu–Mg–Ag alloys [15, 16]. Kotur et al. [17] experimentally verified the existence of τ1 and τ2 ternary phases in the Al–Ag–Zr system. Currently, reports on the thermodynamic data and phase properties of the ternary phases in the Al–Ag–Zr system are limited [17, 18]. Understanding the phase equilibrium and thermodynamic properties of the Al–Ag–Zr system is crucial for designing alloy compositions and developing heat treatment regimes. In this work, the isothermal sections of the Al–Ag–Zr system at 500 and 600 °C were determined experimentally. The enthalpy of formation of phases such as τ1 and τ2 was calculated via first-principles calculations, and a thermodynamic database for the Al-Ag–Zr system was established. Additionally, the hardness of the τ1 and τ2 ternary phases was measured to provide theoretical references for studying the effect of the introduction of the second phases on the properties of aluminum alloys.
Literature review
The Al–Ag system
An evaluation of the Al–Ag system can be found in our recent report [14], and the thermodynamic parameters reported by Wit et al. [19] were used in this work. The corresponding Al–Ag binary phase diagram is shown in Fig. 1.
The Al–Zr system
The Al–Zr binary phase diagram [20] is shown in Fig. 1. In this system, 10 stable intermetallic compounds are listed: AlZr3, AlZr2, Al3Zr5, Al2Zr3, Al3Zr4, Al4Zr5, AlZr, Al3Zr2, Al2Zr and Al3Zr. In 1954, McPherson et al. [21] conducted the first systematic investigation of the Al–Zr phase diagram using metallographic methods, X-ray diffraction (XRD) and thermal analysis. Later, Peruzzi [22] re-determined the phase diagram of the Zr-rich region at 500–1300 °C via metallography, XRD, electron probe analysis, and resistance measurement. The samples used by Peruzzi are of higher purity than those used in previous experiments, and the phase diagrams are very reliable. Saunders [23, 24] evaluated the Al–Zr system twice, but neither evaluation considered the Al3Zr4 phase. In 2001, Wang et al. [25] used a new thermodynamic model to evaluate the Al–Zr binary system, and the calculated enthalpy of formation of the compound was in good agreement with the experimental data. However, Wang et al. [25] did not apply any thermodynamic constraint to the Zr-rich intermetallic compounds. Therefore, Fischer and Colinet [20] reassessed the Al–Zr binary system, attributing high reliability to the ab-initio results.
The Ag–Zr system
Figure 1 also shows the binary phase diagram of the Ag–Zr system [26]. Two intermetallic compounds AgZr and AgZr2 exist. Raub et al. [27] measured the liquidus and solid solubility curve in the 4–51 at.% Zr phase region and found that the eutectic reaction L ↔ Ag + AgZr occurred at 955 °C. Betterton et al. [28] studied the phase diagram of the Zr-rich region and proved the existence of intermetallic compounds AgZr and AgZr2, and measured the eutectoid reaction (βZr) ↔ (αZr) + AgZr2. Zhang [29] et al. surveyed the phase diagram of the Zr content in the 20–80 at.% range via thermal analysis. Karakaya et al. [30] optimized the Ag–Zr system but ignored the experimental data of Zhang et al. [29] in the optimization process. In 2010, Kang et al. [31] evaluated Ag–Zr system using a modified quasi-chemical solution model of FactSage [32], but this model only works with FactSage and is not compatible with Pandat [33] and Thermo-Calc [34]. In 2016, Hsiao et al. [26] re-evaluated the Ag–Zr system, and most of the calculated data are consistent with the experimental data.
The Al–Ag–Zr ternary system
The isothermal section of the Al–Ag–Zr ternary system at 500 °C was first described by Kotur et al. [17] in 2003. Three ternary intermetallic compounds exist in this system: τ1 (AuCu3 structure type), τ2 (MgCu2), and τ3 (W6Fe7). Recently, Hsiao et al. [18] also determined the isothermal section of Al–Ag–Zr ternary system at 500 °C using the equilibrium alloy method, and confirmed the existence of τ2 and τ3. A comparison of the experimental results of Kotur et al. and Hsiao et al. indicates that Hsiao et al. suggest that AgZr2 and AgZr are stoichiometric compounds, whereas Kotur et al. suggest that they comprise a certain range of solid solution. In addition, Hsiao et al. measured the phase equilibria at the Zr-rich region as AlZr2 + AgZr2 + AgZr, AlZr2 + AgZr + Al2Zr3, and Al2Zr3 + AgZr + Al3Zr4, which differs from the results of Kotur et al. Earlier, Rieger [35] had reported that AlAg2Zr3 existed stably at 850 °C or 1100 °C, but the existence of an AlAg2Zr3 phase was not detected in the experiments by Kotur et al. and Hsiao et al. Because few studies exist on the Al–Ag–Zr ternary system, the thermodynamic data of the intermediate compounds are also very limited. Therefore, in this work, the isothermal sections of the Al–Ag–Zr system at 500 and 600 °C were further investigated using equilibrium alloys annealed for a longer time. The data on the crystallographic structure of each phase in the Al–Ag–Zr system are given in Table 1.
Experimental procedure
Alloy samples preparations
The isothermal sections of Al–Ag–Zr at 500 and 600 °C were extrapolated via the Thermo-Calc software [34] using databases [19, 20, 26] without any ternary parameters.
Using Al, Ag, and Zr of 99.99 wt% purity from Beijing Jinyu as the raw materials, fifteen individual 10 g alloys samples, were prepared via arc melting under argon protection. Table 2 lists their nominal compositions, which were chosen to place them in different phase regions. During alloy preparation, a titanium ball was used as an oxygen getter, and all alloys were turned six times after each melting round to ensure homogeneity. The as-melted samples were divided into two pieces, sealed in argon-filled quartz tubes, and placed in a diffusion furnace (KSL-1700X, Shenyang Kejin) at 500 °C for 120 days and 600 °C for 90 days, respectively.
Composition and phase equilibrium analysis
After annealing, the alloy samples were quenched in cold water and observed using a scanning electron microscope (SEM, FEI Quanta FEG 250) equipped with an energy dispersive X-ray spectrometer (EDS, AZtec X-Max 80) to investigate the alloy morphology. Electron probe microanalyzer (EPMA, JXA-8100 F) was performed at 20 kV and 20 nA using the pure elements as standards. X-ray diffraction (XRD, (Advance D8) was conducted on ground alloy powders under Cu-Kα radiation at 40 kV and 40 mA with a scan rate of 5°/min. The alloys were characterized using TEM (Tecnai G2 G20 ST) operating at 200 kV. The vacuum of the TEM was between 10−4 and 10−6 Torr.
Hardness testing
The hardness values of the τ1 and τ2 phases were measured on a nano-indenter (Anton-Paar NHT2) with a Berkovich diamond indenter, and the indentation depth was maintained at 1 μm.
Calculation methods
Thermodynamic modeling
The Gibbs energy of a pure element is dependent on temperature and pressure. The molar enthalpy of stable elements at 298.15 K and 1 bar is commonly used as the standard element reference (SER) state. The Gibbs energy for the element i (i = Ag, Al, Zr) can be expressed as follows:
The European Thermochemical Research Group (SGTE) has optimized the expressions of Gibbs energy as a function of temperature for all pure elements and established a corresponding database of lattice stability parameters. The Gibbs free energies for pure elements were retrieved from the Dinsdale compilation [43].
The solution phase φ (φ = Fcc_A1, Bcc_A2, Hcp_A3 and Liquid) is described using the substitutional solution model. Their Gibbs energy function is expressed by the following equation:
in which HSER is the abbreviation of xAl·H \(_{{{\text{Al}}}}^{{{\text{SER}}}}\) + xAg· H \(_{{{\text{Ag}}}}^{{{\text{SER}}}}\) + xZr· H \(_{{{\text{Zr}}}}^{{{\text{SER}}}}\), and xi is the mole fraction of component i (i = Al, Ag, Zr). \({}^{o}G_{i}^{\varphi }\) is the Gibbs energy for pure element i in the phase φ. R is the gas constant. \({}^{ex}G_{{}}^{\varphi }\) is the excess Gibbs energy of the phase φ, which is described by the Redlich–Kister polynomial [44]:
where \(L_{i,j}^{\varphi }\) (i, j = Al, Ag, Zr, and i ≠ j) represent the nth binary interaction parameters between i with j, \(L_{Ag,Al,Zr}^{\varphi }\) is the ternary interaction parameter to be evaluated in the present work.
The ternary intermetallic compound τ1 is modeled as a stoichiometric phase. The Gibbs energy of each compound is given by the following expression:
in which A and B are to be evaluated.
For the τ2 and τ3 phases, experimental results suggest that Ag may predominately replace Al. Therefore, the (Al, Ag)1.84(Ag)0.16(Zr)1 and (Al, Ag)6(Ag)1(Zr)6 thermodynamic models are employed to describe phases τ2 and τ3, respectively. The Gibbs energy of these phases can be expressed as follows:
where x, y, z denote the number of sites in each sublattice, and \(y_{Al}^{^{\prime}}\) and \(y_{Ag}^{^{\prime}}\) represent the site fractions of Al and Ag on the first sublattice. \(G_{Al:Ag:Zr}^{\varphi }\) and \(G_{Ag:Ag:Zr}^{\varphi }\) are the molar Gibbs energies of the end-members. \({}^{o}L_{Al,Ag:Ag:Zr}^{\varphi }\) represent the interaction parameters between Al and Ag in the first sublattice, expressed by the Redlich–Kister polynomials.
First-principles calculation
The formation enthalpies of ternary compounds τ1, τ2, τ3 and the binary compounds with solid solubility were computed by the Vienna ab-initio simulation package (VASP) [45], based on density functional theory (DFT). The computational details can be found in our previous work [14]. The formation enthalpy ΔHf(AlxZry) at 0 K could be expressed by the following equation:
where E(AlxZry) is the calculated total energy of the structure AgxZry, and E(Al) and E(Zr) denote the total energies of AlxZry, fcc-Al and hcp-Zr at 0 K, respectively. xi (i = Al or Zr) is the atomic fraction of the component i.
The enthalpy of formation for the ternary compound can be defined as:
in which, E(AlxAgyZrz), E(Al), E(Ag), E(Zr) are the total energies of AlxAgyZrz, fcc-Al, fcc-Ag and hcp-Zr, respectively, at 0 K.
Results and discussion
Establishing a thermodynamic database of the Al–Ag–Zr System
Tables 2 and 3 summarize the experimental results of phase composition measurements of alloy samples annealed at 500 °C for 120 days and 600 °C for 90 days. The isothermal sections of the Al–Ag–Zr system at 500 and 600 °C were obtained based on these experimental results, combined with the first-principles calculations of the formation enthalpy of τ1, τ2, τ3, and some metastable end-members phases. The thermodynamic optimization of Al–Ag–Zr system was performed using the PARROT module incorporated in Thermo-Calc software [34]. The optimized parameters are shown in Table 5.
Representative alloys located in different phase regions are discussed. As shown in Fig. 2a, two phases can be observed in 2#. According to the results of the EPMA and XRD, the dark and bright phases are AgZr2 and Zr, respectively. In addition, the eutectic structure of AgZr2 and Zr can be observed in this sample. Similarly, 7# and 8# exhibit a three-phase equilibrium of τ2 + τ3 + (Ag) and a two-phase equilibrium of Ag2Al + τ1. Some samples exhibit unevenly distributed black spots on the surface, which may be attributed to inherent pores in the samples or to water stains and contaminants introduced during the polishing and cleaning processes.
The microstructure of alloy 10# is shown in Fig. 3a. Notably, the dark τ2 phase is covered by a layer of τ1 phase, which is true for each grain. The microstructure of 10# is shown at higher magnification in Fig. 3c. The lighter phase is evidently encompassed by the darker phase, resulting in a structure similar to that of a core–shell. The line scan analysis in Fig. 3e exhibits significant changes in composition at the interface of two different contrasts. Combined with the XRD result, we determined that 10# is located in the three-phase region of (Ag) + τ1 + τ2, and this interesting phenomenon will be further studied in future.
As illustrated in Fig. 4a, 14# is in a three-phase equilibrium, where the plate-like phase is Al3Zr, the light phase is Ag2Al, and the darker phase is (Al). For 15#, as shown in Fig. 4c, the matrix phase is (Al), and the plate-like phase is Al3Zr. The XRD results of 14# and 15# confirm that they form phase equilibria as Ag2Al + (Al) + Al3Zr and Al3Zr + (Al), respectively.
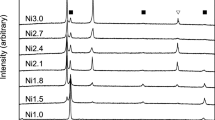
Figure 5 presents the XRD results of 3#, 5#, and 6#. The diffraction peaks of the samples match well with the corresponding phases. After annealing for an extended period, alloys 3#, 5#, and 6# form phase equilibria of AgZr + (Ag), (αZr) + Al3Zr + Ag2Zr, and (Ag) + τ2, respectively.
Table 2 summarizes the phase equilibria formed by alloy samples with different compositions after annealing at 500 °C for 120 days. Combined with the evaluation of the studies by Kotur et al. [17] and Hsiao et al. [18], the isothermal section of the Al–Ag–Zr system at 500 °C is constructed, as shown in Fig. 6. The experimental results reveal the presence of five three-phase regions: Al3Zr + Ag2Al + (Al), τ1 + τ2 + (Ag), τ2 + τ3 + (Ag), AlZr + τ2 + τ3, and (αZr) + AlZr3 + AgZr2 and six two-phase regions: Al3Zr + (Al), Ag2Al + τ1, τ2 + (Ag), τ3 + (Ag), AgZr + (Ag), and (Zr) + AgZr2.
Figure 7 shows the Backscattered electron image (BEI) and XRD results of 4#, 8# and 10#. The microstructure of 4# is shown in 4a, the dark lath-like phase in 4a is determined to be τ3 by EPMA and XRD whereas the light area corresponds to the (Ag) phase. For 8#, as shown in 4c, the dark phase with large area is τ1, and the light phase is Ag2Al, which is in good agreement with the XRD results. The results for 10# are similar to the result at 500 °C, located in the three-phase region of (Ag) + τ1 + τ2.
The XRD results of 2#, 3#, 5#, and 6# annealing at 600 °C for 90 days are shown in Fig. 8, and all diffraction peaks correspond well to the corresponding phases. These samples form the phase equilibria of (αZr) + AgZr2, AgZr + (Ag), (αZr) + Al3Zr + Ag2Zr, and (Al) + Al3Zr, respectively.
Similarly, we measured the isothermal section of the Al–Ag–Zr system at 600 °C, which include five three-phase regions and five two-phase regions, as shown in Fig. 9. Except for the appearance of an Al-rich end liquid phase, resulting from the melting of Al at 600 °C, the results elsewhere are similar to those at 500 °C.
Identification of the τ3 phase and determination of its equilibrium with neighboring phases
The microstructure of 4# after long-time annealing at 500 and 600 °C is shown in Fig. 10a and b. EPMA analysis determined that the light phase is (Ag), whereas the dark phase, with a composition of Al:Ag:Zr close to 0.33:0.17:0.50, corresponds to τ3. This conclusion is supported by the XRD patterns in Fig. 10c. The TEM bright-field phase of 4# after annealing at 600 °C for 90 days is presented in Fig. 10d, with the corresponding selected area electron diffraction (SAED) patterns shown in Fig. 10e and f for regions 1 and 2, respectively. After identification, the grain in the middle of Fig. 10d was determined to be τ3, while the grains on the left and right sides were determined to be (Ag). Therefore, we demonstrate that the phase with composition Al: Ag: Zr close to 0.33: 0.17: 0.50 is τ3, rather than Al3Zr4.
The correct phase relationship serves as the foundation for constructing a thermodynamic database, and a self-consistent thermodynamic database can guide material design. Therefore, we determined τ3 + AgZr + (Ag) three-phase equilibrium instead of Al3Zr4 + AgZr + (Ag) in the isothermal section. To prove that the three-phase region existing at 500 and 600 °C is τ3 + AgZr + (Ag) instead of Al3Zr4 + AgZr + (Ag) as proposed by Kotur et al. [17] and Hsiao et al. [18], we additionally prepared two samples with the same composition as 11# and 12# in the study by Hsiao et al. Figure 11a and c shows the as-cast microstructure of 16# and 17#, respectively, in which the dark strip phase correspond to τ3. The EPMA results are shown in Table 4. The XRD results in Fig. 11b and d also confirm that the specimens were located in the three-phase region of τ3 + AgZr + (Ag), which is consistent with our calculated results. In the report by Hsiao et al. [18], the XRD results of 10#, 11# and 12# were not given, and their EPMA results showed that Ag has a large solid solution in the Al3Zr4 phase, which is close to the composition of the τ3 phase we measured. Therefore, we consider the three-phase equilibrium to be τ3 + AgZr + (Ag).
Additionally, we prepared alloy sample 18# with a composition of Al0.42Ag0.06Zr0.52. Through XRD and EPMA analysis, the sample was found to be in the three-phase region of Al3Zr4 + τ3 + AlZr, directly confirming the existence of τ3 as a separate phase from Al3Zr4 and further validating the accuracy of our experimental phase diagram.
First-principles calculation and thermodynamic optimization
Through the analysis of the experimental results, we determined the solid solubility of some phase in the Al–Ag–Zr system and provided its corresponding thermodynamic models. First-principles calculations were incorporated to compute the zero-Kelvin energies of end-member phases in unstable structures, because experimental data are not available for these phases. The energies of the ternary phases τ1, τ2, and τ3, whose crystal structures are known, were calculated. The results of the first-principles calculations are shown in Table 5.
The constituent Al–Ag, Al–Zr, and Ag–Zr binary system were taken from the reports by Witusiewicz et al. [19], Fischer et al. [20], and Hsiao et al. [26], respectively. With the precise descriptions for the boundary binary systems, the phase equilibria and thermodynamic properties in the Al–Ag–Zr ternary system were computed via the standard procedure. The optimization procedures were conducted in the program PARROT module embedded in Thermo-Calc software [34] using the step-by-step optimization method reported by Du et al. [46]. Table 6 summarizes the optimized thermodynamic parameters of this ternary system.
As seen in Fig. 12a, the calculated results agree well with our experimental results and those of previous reports. Notably, we have confirmed that the three-phase equilibrium is τ3 + AgZr + (Ag) rather than Al3Zr4 + AgZr + (Ag). In Fig. 12b, the calculated isothermal section at 600 °C is also consistent with our experimental results, and all phase equilibria are accurately represented. It is worth noting that, in order to ensure the consistency between the calculated isothermal sections at 500 and 600 °C and the experimental data, we sacrificed the solid solubility of some phases, such as AgZr2 and AgZr, within a reasonable error range. We have therefore established a self-consistent and reliable Al–Ag–Zr thermodynamic database for alloy design.
The liquidus projection of the Al–Ag–Zr system is presented in Fig. 13, where all primary phases and isothermal lines are labeled. To better understand the solidification path and liquidus surface, we have calculated a partial Scheil reaction scheme for the Al–Ag–Zr system (Fig. 14) using the database obtained in this study. The scheme includes seven critical reactions.
High-strength ternary phase τ 2 in Al–Ag–Zr system
To investigate the influence on the properties of the alloy of the two ternary phases, τ1 and τ2, located at the Al-rich end in the Al–Ag–Zr system, the hardness of τ1 and τ2 was measured using a nanoindentation instrument. The microstructure of the sample is presented in Fig. 15a and b, and the residual imprints of the indentation are indicated with red circles in Fig. 15c and d. The indentation measurements performed on the single phases of τ1 and τ2, respectively, were repeated three times, and the average value of three consistent measurements was taken as the hardness of the single phase. The results are shown in Table 6. The load–displacement curves corresponding to τ1 and τ2 are shown in Fig. 15e.
According to the load–displacement curve, the average hardness values of τ1 and τ2 are 3.82 and 10.20 GPa, respectively. The hardness of τ2 was much higher than that of τ1 and exceeded that of the common strengthening Al9FeNi (7.71 GPa at room temperature) in Al alloys. Therefore, in the design of Al alloys, the mechanical properties of the material can be significantly improved by introducing the strengthening phase τ2 (Table 7).
Conclusions
In this work, we determined the isothermal sections of the Al–Ag–Zr system at 500 and 600 °C using the equilibrium alloy method. Six two-phase regions and five three-phase regions were identified at 500 °C, while five two-phase regions and five three-phase regions were identified at 600 °C. Additionally, we identified the uncertain τ3 phase using TEM, and determined that the three-phase equilibrium at 500 and 600 °C consists of τ3 + AgZr + (Ag), instead of Al3Zr4 + AgZr + (Ag). Based on our experimental results and first-principles calculations, we established a thermodynamic database for the Al–Ag–Zr system. Comparing it with previous literature data afforded, a good agreement between calculated and experimental results. Furthermore, we used nanoindentation to measure the hardness of the Al-rich ternary phases τ1 and τ2, which were found to have hardness values of 3.82 and 10.20 GPa, respectively. Therefore, introducing τ2 as a strengthening phase in the design of Al alloys can significantly enhance their mechanical performance.
Data availability
Data available on request from the authors.
References
Hu B, Quan BB, Li DJ, Wang XY, Li ZX, Zeng XQ (2021) Solid solution strengthening mechanism in high pressure die casting Al–Ce–Mg alloys. Mater Sci Eng A 812:141109. https://doi.org/10.1016/j.msea.2021.141109
De LA, DaN S, Dunand DC (2020) Mn and Mo additions to a dilute Al–Zr–Sc–Er–Si-based alloy to improve creep resistance through solid-solution- and precipitation-strengthening. Acta Mater 194:60–67. https://doi.org/10.1016/j.actamat.2020.04.022
Masuda T, Sauvage X, Hirosawa S, Horita Z (2020) Achieving highly strengthened Al–Cu–Mg alloy by grain refinement and grain boundary segregation. Mater Sci Eng A 793:139668. https://doi.org/10.1016/j.msea.2020.139668
Schliephake D, Lopes C, Eggeler YM, Chen H, Freudenberger J, Bayoumy D, Huang AJ, Kauffmann A (2022) Improved work hardening capability and ductility of an additively manufactured and deformed Al–Mn–Mg–Sc–Zr alloy. J Alloys Compd 924:166499. https://doi.org/10.1016/j.jallcom.2022.166499
Shaha SK, Czerwinski F, Kasprzak W, Chen DL (2014) Work hardening and texture during compression deformation of the Al–Si–Cu–Mg alloy modified with V, Zr and Ti. J Alloys Compd 593:290–299. https://doi.org/10.1016/j.jallcom.2013.12.081
Yang Z, Zhang ML, Fan LY, Bai JY, Chen XL, Zhang J, Li Z, Guan RG (2023) Towards high mechanical performance Al–Cu–Mg–Fe–Ni alloy: influence of composition, solution treatment and aged process on microstructural evolution and mechanical properties. J Mater Res Technol 23:2054–2064. https://doi.org/10.1016/j.jmrt.2023.01.166
Dar SM, Liao HC (2019) Creep behavior of heat resistant Al–Cu–Mn alloys strengthened by (θ′) and coarse (Al20Cu2Mn3) second phase particles. Mater Sci Eng A 763:138062. https://doi.org/10.1016/j.msea.2019.138062
Ekaputra CN, Rakhmonov JU, Weiss D, Mogonye JE, Dunand DC (2022) Microstructure and mechanical properties of cast Al–Ce–Sc–Zr–(Er) alloys strengthened by Al11Ce3 micro-platelets and L12 Al3(Sc, Zr, Er) nano-precipitates. Acta Mater 240:118354. https://doi.org/10.1016/j.actamat.2022.118354
Reich L, Murayama M, Hono K (1998) Evolution of Ω phase in an Al–Cu–Mg–Ag alloy—a three-dimensional atom probe study. Acta Mater 46:6053–6062
Ou YZ, Jiang Y, Wang YR, Liu ZQ, Lervik A, Holmestad R (2021) Vacancy and solute co-segregated η1 interface in over-aged Al–Zn–Mg alloys. Acta Mater 218:117082. https://doi.org/10.1016/j.actamat.2021.117082
Voorhees PW (2006) Scandium overtakes zirconium. Nat Mater 5:435–436
Elgallad EM, Shen P, Zhang Z, Chen XG (2014) Effects of heat treatment on the microstructure and mechanical properties of AA2618 DC cast alloy. Mater Des 61:133–140. https://doi.org/10.1016/j.matdes.2014.04.045
Shen ZJ, Liu CH, Ding QQ, Wang SB, Wei X, Chen L, Li JX, Zhang Z (2014) The structure determination of Al20Cu2Mn3 by near atomic resolution chemical mapping. J Alloys Compd 601:25–30. https://doi.org/10.1016/j.jallcom.2014.02.125
Deng ZX, Hu Q, Tian YY, Xue RH, Zhang LG, Liu LB (2023) Experimental investigation and thermodynamic assessment of the Al–Ag–Sc system. J Alloys Compd 934:167980. https://doi.org/10.1016/j.jallcom.2022.167980
De LA, Dunand DC, Seidman DN (2016) Mechanical properties and optimization of the aging of a dilute Al–Sc–Er–Zr–Si alloy with a high Zr/Sc ratio. Acta Mater 119:35–42. https://doi.org/10.1016/j.actamat.2016.08.018
Wang LY, Ye B, Bai Y, Zhao BB, Ding WJ (2021) Effect of Zr and Sc micro-additions on the microstructure and mechanical properties of as-cast Al–5Ce alloy. Mater Sci Eng A 822:141654. https://doi.org/10.1016/j.msea.2021.141654
Kotur B, Verbovytsky Y (2003) Phase equilibria in the {Zr, Hf}–Ag–Al systems at 500 °C and crystal structure of the ternary compounds. Prace Nauk WSP Chem 8:7
Hsiao HM, Lan YC, Hermana GN, Chen H, Yen YW (2018) Investigation of the phase equilibria at 773 K and metallic glass regions in the Ag–Al–Zr ternary system. MRS Commun 8:113–121. https://doi.org/10.1557/mrc.2017.134
Witusiewicz VT, Hecht U, Fries SG, Rex S (2004) The Ag–Al–Cu system: Part I: reassessment of the constituent binaries on the basis of new experimental data. J Alloys Compd 385:133–143. https://doi.org/10.1016/j.jallcom.2004.04.126
Fischer E, Colinet C (2015) An updated thermodynamic modeling of the Al–Zr system. J Phase Equilib Diffus 36:404–413. https://doi.org/10.1007/s11669-015-0398-y
McPherson DJ, Hansen M (1954) The system zirconium aluminum. Trans Am Soc Met 46:354–374
Peruzzi A (1992) Reinvestigation of the Zr-rich end of the Zr-Al equilibrium phase diagram. J Nucl Mater 186:89–99
Saunders N, Rivlin VG (1986) Thermodynamic characterization of Al–Cr, Al–Zr, and Al–Cr–Zr alloy systems. Mater Sci Technol 2:520–527
Saunders N (1989) Calculated stable and metastable phase equilibria in Al–Li–Zr Alloys/Berechnung der stabilen und metastabilen phasengleichgewichte in Al–Li–Zr-legierungen. Int J Mater Res 80:894–903
Wang T, Jin ZP, Zhao JC (2001) Thermodynamic assessment of the Al–Zr binary system. J Phase Equilib 22:544–551
Hsiao HM, Liang SM, Schmid-Fetzer R, Yen YW (2016) ‘Thermodynamic assessment of the Ag–Zr and Cu–Zr binary systems. Calphad 55:77–87. https://doi.org/10.1016/j.calphad.2016.08.001
Raub EV, Engel M (1948) Alloys of zirconium with copper, silver, and gold. Z Metallkd 39:172–177
Betterton JJ, Easton DS (1958) The silver-zirconium system. Trans Met Soc AIME, p. 212
Zhang KH, Zhao HZ, Zhou YH (1988) An investigation of the Ag–Zr phase diagram. J Less-Common Met 138:173–177
Karakaya I, Thompson WT (1992) The Ag–Zr (silver-zirconium) system. J Phase Equilib 13:143–146
Kang DH, Jung IH (2010) Critical thermodynamic evaluation and optimization of the Ag–Zr, Cu–Zr and Ag–Cu–Zr systems and its applications to amorphous Cu–Zr–Ag alloys. Intermetallics 18:815–833
Bale CW, Chartrand P, Degterov SA, Eriksson G, Hack K, Mahfoud RB, Melançon J, Pelton AD, Petersen S (2002) FactSage thermochemical software and databases. Calphad 26:189–228
Chen SL, Daniel S, Zhang F, Chang Y, Yan XY, Xie FY, Schmid-Fetzer R, Oates W (2002) The PANDAT software package and its applications. Calphad 26:175–188
Sundman B, Jansson B, Andersson JO (1985) The thermo-calc databank system. Calphad 9:153–190
Rieger W, Nowotny H, Benesovsky F (1964) Kristallchemische untersuchungen in systemen mit T-elementen,(Cu, Ag) und (Al, Ga). Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 95:1573–1576
Villars P, Calvert LD, Pearson WB (1985) Pearson’s handbook of crystallographic data for intermetallic phases. Acta Cryst 40:C444
Massalski TB (1990) Binary alloy phase diagrams, 2nd edn. Materials Park, Ohio, p 2705–2708
King HW (1981) Crystal structures of the elements at 25 °C. Bull Alloy Phase Diagr 2:401–402
Massalski TB, Murray J, Bennett L, Baker H (1986) Binary alloy phase diagrams. vol. i and ii. American Society for Metals, p. 2224
Murray J, Peruzzi A, Abriata J (1992) The Al–Zr (aluminum–zirconium) system. J Phase Equilib 13:277–291
Schmid-Fetzer R (2003) Al–Ti (Aluminium–Titanium)', MSIT binary evaluation program. MSIT Workplace
Endo N, Kameoka S, Tsai AP, Zou LL, Hirata T, Nishimura C (2010) Hydrogen absorption in the intermetallic compounds Zr2TM (TM = Cu, Ag and Au) with MoSi2-type structure (C11b). J Alloys Compd 490:L24–L27
Dinsdale AT (1991) SGTE data for pure elements. Calphad 15:317–425
Redlich O, Kister A (1948) Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem 40:345–348
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169
Du Y, Schmid-Fetzer R, Ohtani H (1997) Thermodynamic assessment of the VN system. Z Metallkd 88:545–556
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 51871248 and 52071339), and Natural Science Foundation of Hunan Province, China (Grant No. 2020JJ4739). We are grateful for resources from the High Performance Computing Center of Central South University.
Author information
Authors and Affiliations
Contributions
ZD contributed to experimental determination, data analysis, first-principles calculations, thermodynamic optimization, writing—original draft. YD contributed to experimental determination. PM contributed to writing—review and editing. KW contributed to sample preparation. LZ contributed to resources and funding acquisition. LL contributed to project administration and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: P. Nash.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, Z., Ding, Y., Masset, P.J. et al. Thermodynamic description of the Al–Ag–Zr system and discovery of the high-strength ternary phase Al1.84Ag0.16Zr. J Mater Sci 58, 10516–10538 (2023). https://doi.org/10.1007/s10853-023-08665-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08665-3