Abstract
With the development of society, industry, agriculture, and other production activities are changing with each passing day. The primary mode of transportation in international trade is by ship. Due to the complex environment and biodiversity of the sea, ship surfaces are often corroded due to microorganisms, algae, shellfish, and other factors. The hydraulic conditions during the operation of the ship are also affected, increasing energy consumption. Thus, ships need to stop regularly for surface cleaning to prevent greater losses, but there are economic losses and human and material resource consumption that occur during these shutdown periods. To better solve the problem of corrosion, ship surfaces can be treated by antifouling coatings. This review discusses current popular and new marine antifouling coatings from the aspects of biofouling: microbial biofouling, conditioned film biofouling, algae attachment biofouling, shellfish attachment biofouling, and marine environmental impacts. This review also discusses the characteristics, formation mechanisms, and preparation processes of nanocomposite coatings, amphiphilic antifouling coatings, photocatalytic coatings, self-healing antifouling coatings, and self-polishing coatings. The new marine antifouling coatings are compared with traditional coatings in terms of hydraulic properties, such as water contact angle and antifouling performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ocean occupies approximately 71% of the total area of Earth. For social and economic development, the transportation industry is indispensable, and ocean transportation is an essential component of the transportation industry. The ocean covers a large area, and the current understanding of the ocean is not complete. Through further exploration of the ocean, we found that the energy contained in the ocean is vast, so research on the ocean is currently a popular topic [1,2,3,4].
Due to the vast ocean area, shipping has become one of the important methods of international trade. For the current marine transportation industry, the formation of ship biofouling during marine transportation is confounding. Due to the complexity of the marine environment and the variety of organisms in the ocean, ships traveling in the ocean for a long time will inevitably be affected. For example, the hull surface is made of metal. If it is not protected, it is easy for the water in the ocean to form an electrolytic cell, which will cause corrosion [5, 6]. There are many kinds of organisms in the ocean, including microorganisms. Many organisms will attach to metal surfaces due to their physiological structures and grow in large areas on hull surfaces over time. Microorganisms, such as fungi, protozoa, and viruses, easily form biofilms in large areas after adhering to hull surfaces, thus causing the erosion of hull surfaces. Moreover, due to this biological attachment, the hull weight and surface roughness change, affecting fuel consumption during operation and causing considerable financial losses [5,6,7,8].
To deal with such problems, hull surfaces can be cleaned regularly, but due to the high frequency of cleaning, the labour and physical costs during the cleaning period are high, and the ship cannot work during the cleaning period, so the financial losses are enormous. To reduce marine biofouling, one of the important methods is employing antifouling coatings, which were developed in ancient times. [9, 10] Traditional marine antifouling coatings include chemically toxic antifouling coatings and fouling/foul-release antifouling coatings.
Since the release of tributyltin (TBT) severely impacts the marine environment and endangers the survival of marine organisms, marine coatings with TBT and cuprous oxide as raw materials are prohibited [11,12,13,14]. Traditional marine antifouling coatings have indeed solved some of the problems encountered by ships during marine operation. However, their performance has not yet been optimized, and some traditional coatings have specific impacts on the environment [4, 9, 10]. Traditional antifouling or anticorrosion coatings with a single function cannot meet the current requirements [15].
To compensate for the shortcomings of traditional coatings, research on new marine coatings, such as new nanocomposite antifouling coatings, amphiphilic antifouling coatings, photocatalytic antifouling coatings, self-healing antifouling coatings, and self-polishing antifouling coatings, has gradually increased in recent years. The new marine antifouling coatings are an improvement on traditional coatings in terms of resisting different sources of biofouling formation in the sea. By selecting environmentally friendly raw materials or modifying the raw materials while improving the antifouling ability of the coating itself, some functional combinations can be facilitated to increase film suitability for the complex environment of the ocean and prevent pollution [16,17,18,19,20].
Through this review, first, we will analyse the causes of some biofouling, such as microbial biofouling, biofilm, algae attachment biofouling, shellfish biofouling, and marine environment biofouling [9, 21], as shown in Fig. 2. Second, we discuss the impacts of new marine antifouling coatings, such as nanocomposite coatings, on improving the hydrophobic capacity and the impacts of amphiphilic coatings on improving the microbial attachment capacity. Third, we discuss the impacts of photocatalytic coatings on improving the degradation ability of organic pollutants, the recovery ability of self-healing coatings with respect to damaged coatings and the use of self-polishing coatings for the removal of corrosion. We also explore the characterization of the water contact angle (WCA), the comparison of the hull surface before and after coating, and the detection of the content of pollutants [22,23,24,25,26].
Marine biofouling attached to the hull surface (reprinted with permission, ref. [5], copyright 2019, Springer Nature)
Compared with traditional coatings, new marine antifouling coatings have better hydraulic properties, which yield better economic benefits [10, 22]. The new marine antifouling coatings also have more post-damage functionalities, such as self-cleaning and self-healing abilities. In addition to their antifouling ability, the new marine antifouling coatings are developed with respect to the impact of the coating on the marine environment because the components of some traditional antifouling coatings were found to affect the original marine ecological environment. Hence, more research on environmentally friendly coatings is desired [17,18,19]. This review will further explore these aspects.
Formation of marine biofouling
The primary sources of hull surface contamination include microbial contamination, the formation of a conditioned film, the adhesion of macroalgae, the adhesion of shellfish, and the corrosion of hull surfaces caused by harsh ocean conditions.
Formation of microbial fouling
The growth of marine microorganisms in the ocean requires seawater, and these microorganisms can continue to live in an environment with poor nutrition and deplorable ecological conditions, including eukaryotic microorganisms (fungi, protozoa), prokaryotic microorganisms (marine bacteria, marine actinomycetes, marine cyanobacteria), and acellular organisms (viruses).
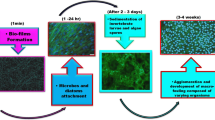
One study found that microorganisms in the ocean can corrode or accelerate the corrosion of metals [5,6,7,8, 27]. A majority of the damage to the external surfaces of ships is caused by microbial corrosion, which accounts for up to 20% of the total damage [28]. Microbial biofouling is mainly due to the formation of biofilms on the ship surface by a combination of anaerobic sulfate-reducing bacteria (SRB) and aerobic iron-oxidizing bacteria (IOB). For example, Fig. 3 shows that planktonic microorganisms adhere to the biofilm surface and become fixed microorganisms [29,30,31]. The metabolism of fixed microorganisms forms a stable biofilm, which accelerates corrosion [32]. With the passage of time, microbial erosion on the ship surface becomes increasingly serious, resulting in corrosion biofouling.
Formation process of biofilm and Mic (reprinted with permission, ref. [27], copyright 2019, Elsevier)
Conditioning film
After organic matter dissolves in the ocean, a film can form on the surface of a ship, which is a conditioned film. Conditioned films affect bacterial adsorption onto ship surfaces and are mainly composed of glycoproteins, humic acids, proteins, aromatic amino acids, carbohydrates, uronic acids, and unspecified macromolecules [33,34,35,36]. The adhesion of the conditional membrane mainly depends on van der Waals forces, electrostatic forces, and hydrogen bonds. Within 24 h after formation, bacteria, diatoms, and other microorganisms will attach to the regulating membrane. Floating bacteria will resist the surface of the regulating membrane and secrete new extracellular polymers (EPS), improving the adhesion of the regulating membrane, thus forming microfouling composed of water, organic matter and extracellular secretions. This process is irreversible. Subsequent microfouling provides nutrition and a suitable environment for large fouling organisms, which will lead to the attachment of large fouling organisms to the hull, thus accelerating the corrosion of the hull surface. This process takes only 1–2 months, and the resulting macro-biofouling will last many years. In addition, the conditional membrane will also affect surface tension and hydrophobicity. After strengthening the adsorption of bacteria, the increase in the number of bacteria will also accelerate the corrosion of the ship's surface, resulting in biofouling [16, 34,35,36].
Formation of algae attachment biofouling
There are abundant algae in the ocean. In addition to the algae initially suspended on the ocean surface, some algae in the depths of the ocean detach and float to the sea surface due to aging, collision, friction, and other reasons. When the ship passes, because the algae can adhere to the dirt-free surface, the algae will adhere to the surface of the ship. This algae can reproduce rapidly on a large scale in a short time, and the aforementioned conditioned film also provides favourable conditions for the attachment and reproduction of algae, and the algal growth will continue to expand over time [16, 37]. The attachment of algae will accelerate the local corrosion rate of the ship’s surface. As an invasive organism, a large number of algal attachments will not only increase the ship's weight but also roughen the ship’s surface, seriously affecting the hydraulic conditions of the ship during operation. In addition, some decayed algae are easily brought into other sea areas by attachment to the surfaces of ships or propellers and invade other sea areas. The eutrophication caused by algal growth has an inevitable impact on the marine water body itself as well as fish and other organisms living in the ocean, resulting in a different type of biofouling of the marine environment [6, 7, 38].
Formation of shellfish attachment biofouling
There are a large number of shellfish in the ocean. The mucus produced by shellfish (e.g. mussels and barnacles) can help them firmly adhere to the hull surface, and because of their rapid growth rate, shellfish can cover most of the hull surface in a short time [32, 39, 40]. A study found that when the shellfish attached to a ship surface, they were thickly covered with surface dirt since their mucus can adhere to the soil in the water. When the shellfish coverage reached 75%, the resistance increased by 10–20%. Therefore, it can be found that a large degree of shellfish attachment will reduce the operating efficiency of ships and increase energy consumption during driving [5,6,7,8, 16, 41]. In addition, Fig. 4 shows other hazards. For example, the mucus of shellfish is acidic, which will directly corrode the hull surface and reduce the service life of ships. Moreover, if shellfish attach to the propeller, this will directly affect the running power of the ship and will increase the safety hazards of the hull during operation, resulting in substantial economic losses. Furthermore, in the marine environment, some shellfish organisms contain toxins. Shellfish can travel on a boat to reach various sea areas, and their toxicity will affect the living conditions of organisms in seawater [41].
Impact of biological attachment on vessel operation (reprinted with permission, ref. [16], copyright 2020, MDPI)
Formation of marine environment biofouling on ship hulls
Ship surface coatings can be eroded by seawater, rain, and the atmosphere. Due to the high salinity of marine water bodies, seawater contains a large number of free chloride ions, which easily form an electrolytic cell with the metal surface of ship hulls, improving the corrosion activity of the metal and easily destroying the original passive film on the metal surface. Oxygen can attract electrons, and there is more oxygen above the water surface and less underwater, so the metal close to the water surface attracts electrons and becomes cathodic. The underwater part that loses electrons becomes anodic, which leads to corrosion. If cracks or gaps form, this will accelerate the degree of local corrosion and eventually form rust pits or rust penetrations [2, 3]. The biofouling of the hull by marine environment is caused by mechanical corrosion, including impact corrosion, which is caused by liquid turbulence or impact; cavitation corrosion, whereby high-speed flowing liquid, due to irregular flow, produces cavitation and produces the "water hammer effect", which often destroys the protective film on the metal surface and accelerates corrosion on ship surfaces, such as those of propellers and pump shafts; microvibration abrasion corrosion, which is abrasion caused by the mutual vibration of two adjacent surfaces; and stress corrosion cracking, which is the corrosion damage of metal under the action of tensile stress and corrosive media, and the occurrence of intergranular or transgranular cracks in the metal [2, 3]. In summary, the marine environment will also damage the structure on the hull surface [42], thereby reducing the service life of ships.
New marine antifouling coatings
Compared with traditional coatings, the new marine antifouling coatings have better antifouling effects and better hydraulic properties and environmental protection.
Nanocomposite coatings
Nanocomposite coatings combine nanoparticles with other materials and have other functions as well as the advantages of nanocoatings.
Characteristics of nanocomposite coatings
Coating materials composed of nanoparticles have elicit a small size effect, surface effect, quantum size effect, and macroquantum tunnelling effect. They have good performance in terms of mechanics and electricity. Due to the special physical properties of nanomaterials, nanocomposite coatings are compounded with other materials through nanoparticles to achieve more functionalities, such as superhydrophobicity and self-polishing. Therefore, nanocomposite coatings have good adhesion between the coating and the hull, do not easily fall off, and have multiple functionalities. Their performance can better overcome corrosion and wear problems, equipment failure, and life decline [43].
Antifouling mechanism of nanocomposite coatings
Nanomaterials can form a layer of thick film on a metal surface compared with ordinary coatings due to their remarkable physical properties [44]. Ordinary coatings will inevitably have leaves holes exposing the metal surface, and there will always be seawater immersion through the holes or cracks of the coating surface that corrodes the hull. However, nanocomposite coatings can be applied without the formation of holes due to the small ionic radii of nanomaterials [43, 45,46,47]. Therefore, using a nanocomposite coating on the metal surface of a hull can eliminate the existence of holes. Jin et al. [15] showed that nanocomposites’ inherent bacterial and photocatalytic activities can inhibit bacterial organism growth and that nanomaterials such as graphene can also inhibit corrosion due to their barrier and lubricating effects. In short, nanocomposite coatings can isolate almost all corrosive substances, such as microorganisms, algae, and shells, so that these cannot contact the surface of the ship and thus cannot corrode the surface of the hull [48, 49].
Second, due to the relatively large specific surface area of nanoparticles, the activation energy of their ionic surfaces can be increased. On the one hand, it was found that algae, mussels, and other substances easily adhere to a base surface with low surface energy, and improving the surface activity energy can reduce the adhesion of algae and other substances [6, 7]. On the other hand, improving the active energy of the surface protects the adhesion strength between the hull surface and the coating [50]. Compared with the van der Waals force and hydrogen bond adsorption of traditional coatings, the molecular adsorption and chemical bonding of nanomaterials are better [43], and nanocomposite coatings do not easily fall off, which can better protect the surface of the ship, improve the life of the coating itself, and improve the service life of the ship [51, 52].
Preparation of nanocomposite coatings
Nanocomposite coatings play a significant role in marine antifouling. Selim et al. prepared a light-induced silicone/TiO2 nanocomposite coating [24]. Figure 5a shows the composite mechanism of ZnO nanorods (NR) with organosilicon (PDMS) to form a composite coating of organosilicon/ZnO nanorods. The results of XRD and DLS tests are shown in Fig. 5b. When 5 wt% ZnO was added, the nanocomposite coating showed the best hydrophobic properties, with superhydrophobic properties. The maximum water contact angle (wcas) was 158°, and the minimum free energy was 11.25 mn/m. The surface distribution was good, which improved the ability of the coating to prevent dirt adhesion, and the resistance towards bacteria and fungi was increased, which resulted in a good self-cleaning effect.
a Superhydrophobic and self-cleaning behaviour of the prepared PDMS/ZnO (0.5 wt%) NR composite preventing the mechanism of dirt adhesion. b XRD analysis and DLS test of ZnO NRs. The illustration shows the droplet shape on the prepared superhydrophobic ZnO NRS composite (reprinted with permission, ref. [24], copyright 2018, Elsevier)
Gu et al. prepared graphene@cuprous oxide nanocomposites by an in situ reaction with graphene oxide, CuSO4, NaOH, and L-ascorbic acid as raw materials (rGO@Cu2O). The rGO@Cu2O nanocomposites were evenly distributed, and the size of Cu2O nanoparticles adsorbed on graphene sheets was quite uniform (2.3 nm). rGO@Cu2O was compounded with acrylic resin and prepared with a functional surface rGO@Cu2O/acrylic resin marine antifouling coating [23]. Gu tested it and found that the WCA of the coating was as high as 113°, and the average adhesion was 3.69 MPa. After a whole exposed panel was immersed in seawater, marine organisms grew in large numbers within 90 days, and the rGO@Cu2O coating surface was almost free from marine biofouling within 365 days. This study showed that rGO@Cu2O in situ syntheses are a potential tin-free alternative for inhibiting biofouling, and the rGO@Cu2O/acrylic resin marine antifouling coating has good performance in terms of hydrophobicity and self-cleaning [23].
Mohamed S. Selim et al. prepared superhydrophobic polydimethylsiloxane/Ag@SiO2 core–shell nanocomposites as antifouling coating materials [24]. An average size of 60 nm was facilitated by a simple solvothermal method and an improved StöBER method to produce Ag@SiO2 core–shell nanospheres with controllable shell thickness. Through solution casting technology, Ag@SiO2, the core–shell nanofiller, was inserted into the surface of silicone composites. A simple hydrosilylation curing mechanism was used to cure the surface coating. Nanofillers with different concentrations were mixed into a silicone (PDMS) matrix to study the structure–property relationship. Water contact angle (WCA) and surface free energy measurements, atomic force microscopy, and scanning electron microscopy were used to study the surface self-cleaning properties of the nanocomposites. The well-distributed organosilicon/Ag@SiO2 (0.5 wt%) core–shell nanocomposites showed good hydrophobicity and self-cleaning, with a WCA of 156° and surface free energy (SFE) of 11.15 Mn/m [24].
The WCA of the nanocomposite antifouling coating after compounding different substances with nanomaterials showed that it has good performance in terms of hydrophobicity and antifouling and has good research prospects in the future.
Amphiphilic antifouling coatings
An amphiphilic antifouling coating is a coating that has the two characteristics of hydrophilicity and hydrophobicity through its synergistic effect, allowing control of pollutant attachment.
Characteristics of amphiphilic antifouling coatings
Amphiphilic antifouling coatings are characterized by hydrophilicity and hydrophobicity. Hydrophobic films, such as PDMS films, can reduce the adhesion of hydrophilic polymers due to their low surface energy [53]. However, these films will be attached by diatoms, which easily form biofilms on the surface with other bacteria or microalgae [54]. Once the biofilm is formed, it will provide favourable conditions for the production of barnacles, bryozoans, and other large-scale biofouling [55]. Hydrophilic films form a hydrated layer that strongly binds water molecules to the surface so that other molecules and dirt organisms cannot adhere [15], which has good effects on reducing the adhesion of diatoms and inhibiting the adsorption of proteins [56]. However, the disadvantage is that the inorganic material is covered, and the extended immersion time will affect the antifouling properties of the hydrophilic film [15]. Therefore, a combination of hydrophilicity and hydrophobicity, through synergistic effects, for controlling the generation and release of biofouling has become the focus of research.
Antifouling mechanism of amphiphilic antifouling coatings
Previously, the synthesis of amphiphilic materials was mainly studied by combining polyethylene glycol (PEG) and hyperbranched fluoropolymer (HBFP). The hyperbranched fluoropolymer (HBFP)-poly(ethylene glycol) (PEG) coating found by Gudipati et al. [55] and Krishnan et al. mixed a surface-active fluoropolymer with PEG [57], and it was found that the surface had increased biofouling resistance to diatoms. However, it has been found that PEG materials are prone to oxidation and are not entirely suitable for the marine environment [58, 59]. At present, more studies use amphiphilic polymer molecules as new raw materials. For example, Lin et al. used amphiphilic polymers coated with hydrophobic inorganic nanoparticles as hydrophobic side chains, in which charged groups connect (generally –COO−) water-soluble hydrophilic main chains and can also connect functional molecules to hydrophobic side chains [60]. Moreover, M. Barretta introduced hydrophobic groups through a solgel reaction containing fluoroalkyl silane (FTSi) to form a new amphiphilic material [61]. Zhang et al. [62] used atom transfer radical polymerization (ATRP) to compound sulfobetaine meth-acrylate (SBMA) and carboxyl betaine methacrylate (CBMA) to produce ultralow dirt polymer brushes. Compared with the original PEG, the amphiphilic polymers of these methods show better hydration, have the same antifouling ability towards diatoms and proteins, and yield better antifouling effects in the marine environment.
Preparation of amphiphilic antifouling coatings
Great attention has been given to research on amphiphilic antifouling coatings. Taking Florian Koshitzk’s research as an example, a small amount of amphoteric carboxyl betaine methacrylate (CBMA) was mixed with hydrophobic ethylene glycol dicyclopentadiene ether acrylate (DCPEA) to synthesize a group of new copolymers with different amphiphilicities. When only 5 wt% CBMA was added, it was enough to form a hydrophilic interface quickly, thereby significantly reducing the amount of dirt. With the increase in CBMA, the water antennae decreased, indicating that the hydrophilic characteristics were displayed and relatively uniform. Through dynamic microfluidic determination, it was found that the number of diatoms decreased greatly, indicating that the antifouling ability was greatly improved [63]. The importance of hydrophobic bases was proposed, which facilitates more methods for subsequent research.
Lu et al. prepared two biobased hydrophilic gel coatings with excellent antifouling and mechanical properties [64]. The coatings were produced by forming hydrophobic and hydrophilic interpenetrating polymer networks (IPNs) in situ. Biobased epoxy monomers containing siloxane chains were synthesized from bioeugenol and further cross-linked by isophorone diamine (IPDA) to produce hydrophobic biobased epoxy networks. Moreover, a mercapto-containing hydrophilic polymer (PNIPAM-SH) was synthesized and mixed with silver trifluoromethanesulfonate to form a hydrophilic nanosilver (AgNP) hydrogel network, which penetrated into the biological epoxy network. The hydrophobic part of the silicone-containing epoxy resin improved the tensile strength and adhesion, which is conducive to the mechanical properties of the coating to achieve excellent antifouling performance. Figure 6 shows the antifouling mechanism of this layer. The hydrophilic hydrogel part cross-linked by hydrophilic polymers and nanosilver (AgNPs) had excellent antifouling performance and could resist proteins, bacteria, algae, and other marine organisms. The antifouling performance of the coating was evaluated by the adhesion of protein (BSA-FITC), bacteria (E. coli and Bacillus subtilis), and algae. The results showed that the two biobased hydrophilic gel coatings gave the surface a better ability to reduce the adhesion biofouling of proteins, bacteria, and microorganisms [64]. In addition, Lu et al. conducted a field test in the East China Sea for more than a month and evaluated the amphiphilic coating's overall antifouling and mechanical properties. The biobased hydrophilic gel coating was found to be intact, with almost no marine organisms attached. This work provides a new strategy for the manufacture of biobased and high-performance antifouling coatings.
Antifouling mechanism of two biobased hydrophilic gel coatings (reprinted with permission, ref. [64], copyright 2021, Elsevier)
Guo et al. synthesized a novel amphiphilic polymer by combining hydrophilic polyvinylpyrrolidone (PVP) with hydrophobic tetrafluoroethylene perfluoroxy ether copolymer perfluoroalkoxy (PFA) and polydimethylsiloxane (PDMS) [65]. Amphiphilic marine antifouling coatings of hydrophilic polyvinylpyrrolidone and hydrophobic fluorosilicone block copolymers with high antifouling performance were prepared by mixing an amphiphilic copolymer (PVP-PFA-PDMS) into a crosslinked PDMS matrix. The coating combined the resistance to the settling of biological dirt attributed to hydrophilic PVP segments with the reduced adhesion strength attributed to the low surface energy of the fluorine-silicon segments. Compared to the original PDMS coating, PVP migrated to the surface after three days in water, showing excellent antifouling performance by reducing bacterial adhesion by 98.1%, marine single-cell Navicula Parva diatom adhesion by 98.5%, and barnacle adhesion by 84.3% [65]. In addition, due to the long-term stability of PVP, the coating exhibited higher and more durable resistance to biological dirt adhesion. The nontoxic antifouling coating developed in this paper has the potential to be applied in various marine industrial facilities.
Research on the functionalization of new marine antifouling coatings
To deal with the complex environment, new marine antifouling coatings need to have better characteristics, so their functionality is receiving increasing attention.
Photocatalytic antifouling coatings
Photocatalytic coatings use a photocatalyst in the material to decompose the organic matter attached to the hull surface, mainly through the photocatalytic efficiency and utilization rate of the photocatalyst.
Mechanism of photocatalytic coatings
Photocatalysis, as an advanced oxidation technology, is based on the redox ability of semiconductor photocatalysts under light conditions, which can stimulate the generation of active oxidation substances to decompose or transform pollutants [66,67,68,69,70] and can decompose organic matter into H2O and CO2. Previous photocatalytic coatings were mainly based on titanium oxide (TiO2) and zinc oxide (ZnO), which showed good chemical stability and antifouling performance under ultraviolet conditions. However, it was found that the visible-light utilization of these coatings was low [71]. Currently, research on photocatalytic coatings mainly focuses on improving their photocatalytic activity and utilization rate of visible light [72, 73]. Li et al. used silver oxide coating by stone-grinding nitrogen carbide to form a Ag2O/g–C3N4 special-shaped structure with an improved photocatalytic activity [74]. Liu et al. synthesized a dynamic photocatalytic coating of ZnIn2S4 with high visible-light utilization by a relatively simple hydrothermal method [75]. Zhu et al. synthesized multifunctional GO/TiO2 photocatalytic thin films by combining UV treatment with vacuum filtration to reduce photocatalyst separation and reduce the damage by membrane biofouling [76]. Therefore, as an efficient, safe, and environmentally friendly technology, photocatalysis also plays an essential role in marine biofouling prevention and has excellent prospects.
Preparation process
Taking the photocatalytic antifouling coating based on carbon nitride and dynamic acrylate borofluorinated polymer prepared by Zhang et al. as an example, a series of environmentally friendly carbon nitride (C3N4) photocatalytic coatings (CNPs) were prepared by mixing C3N4 into self-polishing acrylate boron-fluorinated polymer (ABFP) through ultrasonic dispersion [77]. Figure 7 shows the antifouling mechanism of the CNP coating, indicating that the layered structure of CNPs has an improved utilization rate of visible light. When C3N4 was incorporated at 3–7 wt%, the antibacterial rates of Escherichia coli and Staphylococcus aureus reached 98.10% and 96.94%, respectively. In addition, chemical oxygen demand (COD) was tested, and it was found that the COD value of the CNP film with C3N4 was significantly reduced, indicating that the organic pollutants released during driving were significantly reduced. In addition to the dynamic surface of the CNP itself, the diphenylborides in the ABFP structure also inhibited the adhesion of dirt and could self-strip dirt organisms as the ship moved [77]. In summary, as a new environmentally friendly antifouling coating, the photocatalytic thin film inspires more ideas for our follow-up research.
Antifouling mechanism of CNP photocatalytic coatings (reproduced with permission, ref. [77], copyright 2020, ROYAL SOCIETY OF CHEMISTRY)
Zhu et al. found that Ag2WO4 is an effective photocatalytic material and bacteriostatic agent. However, due to its lack of optical stability and low quantum yield, its application scope is limited, and further development of this material is needed. A study was performed in which a new type of composite antifouling coating was prepared by modifying PEVE with a g-C3N4/Ag2WO4 composite catalyst [78]. PEVE has a high C–F bond content and excellent weather resistance, acid and alkali resistance, corrosion resistance, and durability [78,79,80]. The experimental results showed that due to the narrow band gap of g-C3N4, the multiple junctions formed by g-C3N4 and Ag2WO4 showed good photocatalytic activity, and the modified coating showed excellent antibacterial ability and cyclic stability under visible light. The construction of a stepped heterojunction between g-C3N4 and Ag2WO4 inhibited the recombination of photoelectron–hole pairs in the photocatalyst, which not only improved the photocatalytic activity of Ag2WO4 but also increased the stability of Ag2WO4 under light. Moreover, the presence of g-C3N4 contributed to the formation of β(β)-Ag2WO4. The g-C3N4/Ag2WO4 composite catalyst significantly improved the antibacterial ability of the composite coating. The stepped heterojunction formed by g-C3N4 and Ag2WO4 improved the electron transfer efficiency. It reduced the photocorrosion of Ag2WO4 [81, 82]. Zhu also soaked the coating in seawater with a fixed concentration of E. coli in this experiment and found that a 7CN/Ag2WO4 film composed of 7 wt% C3N4 had the best bacteriostatic performance, and its bactericidal efficiency reached over 90% after 1 h of illumination. Therefore, the photocatalytic film prepared by Zhu et al. achieved good antifouling performance and corrosion resistance [78].
Self-healing antifouling coatings
A self-healing coating refers to a coating that can quickly recover after damage through the nature of its internal structure. At present, four generations of these coatings have been developed.
Mechanism of self-healing coatings
In recent years, research on self-healing antifouling coatings has also increased steadily. Self-healing performance refers to the existence of factors such as hydrogen bonds and van der Waals forces between the internal structure molecules of the coating after the coating is damaged [83,84,85,86,87], making the original large molecular gap formed by the damage lesser so that the outer surface of the overall coating is restored to an undamaged state. Most of these coating are microcapsule coatings [83, 88, 89], and their self-repair and thermal stability require trigger conditions, including mechanical induction, ion concentration, triggering groups, and other aspects [90, 91]. As shown in Fig. 8, four generations of self-healing coatings have been developed thus far, with one to three generations showing defects in terms of time dependence, excitation conditions, and repair efficiency [92,93,94, 96]. For the fourth generation, Wong et al. proposed fluid materials (lubricants) with no trigger and a high repair rate that improved the overall performance of lubricants injected into the surface by improving the stability of the fluid coating on a flat substrate [95].
Development of self-healing materials from generation 1 to generation 4 (reprinted with permission, ref. [96], copyright 2021, Elsevier)
Preparation process
As a type of intelligent anti-corrosion coating, self-healing coatings have been developed into fourth-generation coatings. In the study of Wu et al., carbon nanotubes (CNTs) were added to a fluid matrix of epoxy resin (EP) or silicone oil (Oil), and perfluorooctane trethoxysilane (PTES) was added to premodify the CNTs, as shown in Fig. 9. A stable and fast self-healing coating was obtained on the metal matrix [96]. As a filler, CNTs can increase the fluid's viscosity and the adhesion between the fluid and the matrix. The special physical structure of CNTs can make them bounce back after being scratched. PTES can resist water infiltration in highly corrosive environments. This study proposed that when 0.56 wt% CNT and 0.12 wt% PTE were added to EP, the external 0.12 wt% PTE (denoted as EP/F@CNT2) had better self-healing ability and corrosion resistance. The antifouling mechanism shown in Fig. 9e, f was the same as expected [96]. This CNT-thickened liquid coating, as a self-healing coating, showed a high self-healing efficiency (recovery in tens of seconds) and high corrosion resistance, inspiring more ideas for our subsequent research.
Self-healing ability of fluid coating, a self-healing ability of EP/F@CNT in air, b microscopic self-healing ability of Oil/CNT2 in air, c, d self-healing ability of Oil/CNT2 in 1 M HCl solution and 1 M NaOH solution, e OCP of the composite coating during self-healing (immersion in 3.5 wt% NaCl solution), and f self-healing mechanism of scratched surface. (reproduced with permission, ref. [96], copyright 2021, Elsevier)
Zhang et al. designed a smooth organic gel coating (OG) using α,ω-aminopropyl terminal polydimethylsiloxane (APT-PDMS) and isophorone diisocyanate (IPDI) as raw materials and injected silicone oil into polydimethylsiloxane polyurea (PDMS-PUa) [97]. The self-healing properties of the polymer coating were attributed to the hydrogen bond breaking and reforming by the urea group between PDMS-PUa. Moreover, the self-generation of the lubricating oil layer was attributed to silicone oil infiltration into the damaged area through PDMS-PUa. The prepared OG had a lower water slip angle (< 10°). After 48 h of contact at room temperature, the broken coating could self-repair, and the self-repair efficiency increased with increasing temperature. Compared with bare glass (BG) and PDMS-PUa, the coating showed excellent antifouling performance in both static and dynamic environments after immersion in a culture medium inoculated with bacteria [97].
He et al. successfully prepared gallium-based liquid metal (GLM) nanodroplets modified by zwitterionic polymer (polyethylenimine-quaternized derivative, PEI)-based antifouling agents through ultrasound and Michael addition. The nanodroplets were added to a polydimethylsiloxane coating (PDMS) as a functional filler. A self-healing polydimethylsiloxane antifouling coating based on amphoteric ion polyethylene imine gallium functional nanodroplets was prepared [98]. Due to the synergistic bactericidal activity of Ga3+ from GLM nanodroplets and quaternary ammonium groups from amphoteric polymer PEIs, the PEI-functionalized GLM nanodroplets showed good antibacterial performance. The synergistic antibacterial performance of the PEI-GLM nanodroplets and the strong surface hydration of the zwitterionic polymer PEIs improved the antifouling performance of the filler. Subsequently, by cross-sectional observation, the prepared PEI-modified GLM nanodroplets showed good dispersion in the PDMS coating, which also ensured that the PEIS-GLM@PDMS coating had a satisfactory antifouling effect (more than 90% bacteria and more than 70% microalgae removal). In addition, the PDMS coating was endowed with self-healing properties through the exposed GLM that initiated polymerization under the impact of external water flow, the cross-linking of unreacted vinyl in PDMS, and the PEI-modified GLM nanodroplets [98]. In conclusion, the film prepared by He et al. had suitable antifouling and self-healing abilities, which also inspires ideas for our subsequent research.
Self-polishing antifouling coatings
A self-polishing coating can be self-polished in the process of ship operation through the removal of the attached biofouling, which exposes the new coating surface.
Mechanism of self-polishing coatings
A self-polishing coating (SPC) is a new type of marine antifouling coating, and its principle is that the coating can be self-polished after seawater scouring during ship operation to expose the new coating surface[99]. The antifouling ability and smoothness of self-polishing coatings are the same as those of the new coating. In addition to strengthening the antifouling ability, the smooth surface also provides better hydraulic properties, thus reducing the resistance encountered by the ship in the process of operation and reducing energy consumption [12,13,14]. The early self-polishing coatings were mostly composed of organic tin (such as tributyltin, TBT) and cuprous oxide, showing good self-polishing performance. Later, it was found that in this process, the coating releases toxic substances containing tin into the water, seriously affecting the marine environment [99,100,101,102,103,104]. Therefore, in recent years, new tin-free self-polishing coatings have been developed with improved antifouling and self-polishing properties that are more environmentally friendly [13, 14, 99,100,101].
Preparation process
The environmental friendliness of self-polishing coatings has always been a concern. In recent years, we have studied many materials (such as polyurethane) for replacing banned tributyltin (TBT). Tian et al. prepared a Cu/Ti composite self-polishing antifouling coating with a micron-scale Cu/Ti alternating-layer structure by plasma spraying mechanically mixed Cu/Ti powder [12]. The coating was designed to control the release of Cu ions by electrodissolving Cu laminates from Cu/Ti microgalvanic cells in an aqueous solution. The results showed that the significant antifouling efficiency of the Cu/Ti coating on bacterial survival and adhesion was as high as 100%. The Cu/Ti microgalvanic cells were formed in situ in the Cu/Ti coating and are responsible for the release of Cu ions. The continuous dissolution of Cu laminate leads to the formation of microchannels near the surface of the Ti laminate so that the reaction zone will extend to the adjacent bottom layer. The fresh Cu/Ti laminate will be exposed to seawater to continue the new galvanic reaction, which helps to control the slow release of Cu ions and the effect of self-polishing. Therefore, Cu/Ti coatings can achieve an environmentally friendly antifouling ability and an approximately 200% longer antifouling life than traditional organic antifouling coatings. On the other hand, compared with traditional organic antifouling coatings, Cu/Ti composite coatings show higher mechanical durability because of their strong adhesion, excellent mechanical properties and low wear rate. The aforementioned laminated Cu/Ti coating showed good antifouling and self-polishing performance, the coating efficiency was higher, and the coating life was longer [12]. The durable self-polishing antifouling Cu/Ti coating designed by the micron Cu/Ti laminated microstructure prepared by Tian et al. inspires many new ideas for the durability and environmental friendliness of new marine antifouling coatings.
Dai et al. prepared zinc-polyurethane (ZnX-PU) copolymer coatings in their study [105]. Figure 10 shows the corresponding preparation process. Through this preparation process, zinc ions existed in the form of polymerized salt, which can exchange with sodium ions in seawater, thus making zinc-polyurethane copolymer soluble in water and washed away by seawater. Figure 11a shows that the self-polishing rate of the coating can be controlled by the amount of 2,2-bis(hydroxymethyl)propionic acid (DMPA). The antifouling performance of the coating is mainly determined by the amount of 4,5-dichloro-N-octyl-4-isothiazoline-3-ketone (DCOIT) released in the coating. According to Fig. 11b, the release amount of DCOIT tended to be stable after the coating was soaked in seawater for 30 days, ensuring a good antifouling ability. In addition, it is observed from Fig. 12 that the zinc-polyurethane copolymer was well preserved within 12 months, which facilitates the prevention of marine biofouling. In addition, compared with traditional polyurethane, in which the main chain can only degrade, the new polyurethane can achieve side chain degradation through carboxyl groups to obtain better mechanical properties and polishing performance [105].
ZnX-PU synthesis scheme (reproduced with permission, ref. [105], copyright 2021, Springer Nature)
a Self-polishing rate of ZnX-PU film in artificial seawater, b DCOIT release rate of ZnX-PU antifouling coating (Reproduced with approval, ref. [105], copyright 2021, Springer Nature)
Typical pictures of galvanized polyurethane PVC board soaked in Xiamen Bay for 12 months: a Zn3-PU, b Zn6-PU, c Zn9-PU, d Zn12-PU, e Zn15-PU antifouling coatings and f uncoated. (reproduced with permission, ref. [105], copyright 2021, Springer Nature)
Conclusions
This review summarizes the formation of biofouling in the marine environment and the perspective of new marine antifouling coatings. To reduce fouling on ship surfaces, the characteristics, mechanisms, and preparation processes of nanocomposite antifouling coatings, amphiphilic antifouling coatings, photocatalytic antifouling coatings, self-healing antifouling coatings, and self-polishing antifouling coatings are introduced. Compared with traditional coatings, new marine antifouling coatings are improved in all aspects, showing better antifouling and anticorrosion capabilities. In the early 1980s, since TBT was found to cause irreversible harm to the ocean, it is forbidden to use TBT in antifouling coatings, which also focuses attention on the impacts of antifouling coatings on the environment. Therefore, most new marine antifouling coatings are receiving more attention because they are environmentally friendly. However, the influence of substances released by some new marine antifouling coatings in seawater on water bodies remains to be studied, and there is still room for improvement in the synthesis processes and subsequent performance of the coatings, which inspires the following new ideas for our subsequent research:
(1) The study of substances released from coatings after immersion, whether coatings will release substances that affect the environment, preventing secondary pollution to water bodies, and strictly preventing the occurrence of TBT events. (2) The study of the photoinduced organosilicon/TiO2 nanocomposite coating prepared by Selim et al., which not only has the advantages of being a nanocoating but also is superhydrophobic. Also, the study of functional combinations of the composites in nanocoatings. (3) Research on photocatalytic coatings that mainly focuses on the utilization of visible light, the catalytic efficiency, the utilization of light, and the stability of photocatalysts. (4) As self-healing and self-polishing have become popular research topics in recent years, the study of whether the repair time of self-healing coatings can be further reduced. PDMS can be used as the matrix, and other substances can be added to improve the repair efficiency in subsequent studies. Moreover, the study of self-polishing coatings can be used to further study side chain degradation, durability, and other aspects. On the other hand, it is worth noting that the substances entering water bodies during the self-polishing process should not cause secondary pollution to the water bodies. (5) For environmental protection, all coating research must focus on protecting the marine ecological environment while ensuring its antifouling and anticorrosion performance and functionality. (6) Further study is needed to optimize hydraulic properties in terms of reducing loss during operation, improving fuel efficiency and reducing operating costs.
References
Brodie RT, Ruckelshaus M, Swilling M, Allison EH, Osterblom H, Gelcich S, Mbatha P (2020) A transition to sustainable ocean governance. Nat Commun 11:1–14. https://doi.org/10.1038/s41467-020-17410-2
Shields MA, Woolf DK, Grist EPM, Kerr SA, Jackson AC, Harris RE, Bell MC, Beharie R et al (2011) Marine renewable energy: the ecological implications of altering the hydrodynamics of the marine environment. Ocean Coast Manag 54:2–9. https://doi.org/10.1016/j.ocecoaman.2010.10.036
Qu QZ, Tsai SB, Tang MX, Xu CJ, Dong WW (2016) Marine ecological environment management based on ecological compensation mechanisms. Sustainability-Basel 8:1267–1277. https://doi.org/10.3390/su8121267
Han C, Qu ZG (2021) A methodology for removing biofouling of the hull based on ultrasonic guided waves. J Phys Conf Ser 2031:012006–012013. https://doi.org/10.1088/1742-6596/2031/1/012006
Song CH, Cui WC (2020) Review of underwater ship hull cleaning technologies. J Mar Sci Appl 19:415–429. https://doi.org/10.1007/s11804-020-00157-z
Mineur F, Johnson MP, Maggs CA, Stegenga H (2006) Hull fouling on commercial ships as a vector of macroalgal introduction. Mar Biol 151:1299–1307. https://doi.org/10.1007/s00227-006-0567-y
Imchen T (2018) Marine macroalgae: prospective hitchhikers of ship ballast. ASEAN J Sci Technol Dev 35:43–47. https://doi.org/10.29037/ajstd.472
Banerjee I, Pangule RC, Kane RS (2011) Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater 23:690–718. https://doi.org/10.1002/adma.201001215
Pei X, Ye Q (2015) Development of marine antifouling coatings. Antifouling Surfaces Mater. https://doi.org/10.1007/978-3-662-45204-2_6
Rascio VJ (2000) Antifouling coatings: where do we go from here. Corros Rev 18:133–154. https://doi.org/10.1515/CORRREV.2000.18.2-3.133
Howell D, Behrends B (2006) A methodology for evaluating biocide release rate, surface roughness and leach layer formation in a TBT-free, self-polishing antifouling coating. Biofouling 22:303–315. https://doi.org/10.1080/08927010600924304
Tian JJ, Xu KW, Hu JH, Zhang SJ, Cao GQ, Shao GS (2021) Durable self-polishing antifouling Cu–Ti coating by a micron-scale Cu/Ti laminated microstructure design. J Mater Sci Technol 79:62–74. https://doi.org/10.1016/j.jmst.2020.11.038
Coneski PN, Weise NK, Fulmer PA, Wynne JH (2013) Development and evaluation of self-polishing urethane coatings with tethered quaternary ammonium biocides. Prog Org Coat 76:1376–1386. https://doi.org/10.1016/j.porgcoat.2013.04.012
Bressy C, Margaillan A, Faÿ F, Linossier I, Réhel K (2009) Tin-free self-polishing marine antifouling coatings. Adv Marine Antifouling Coat Technol. https://doi.org/10.1533/9781845696313.3.445
Jin HC, Wang JF, Tian LM, Gao MY, Zhao J, Ren LQ (2022) Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater Des 213:110307–110326. https://doi.org/10.1016/j.matdes.2021.110307
Gu Y, Yu L, Mou J, Wu D, Xu M, Zhou P, Ren Y (2020) Research strategies to develop environmentally friendly marine antifouling coatings. Mar Drugs 18:371–393. https://doi.org/10.3390/md18070371
Pascal B, Mariëlle W, Corné R, Zeger V (2012) A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J Coat Technol Res 10:29–36. https://doi.org/10.1007/s11998-012-9456-0
Xie C, Guo H, Zhao W, Zhang L (2020) Environmentally friendly marine antifouling coating based on a synergistic strategy. Langmuir 36:2396–2402. https://doi.org/10.1021/acs.langmuir.9b03764
Yebra DM, Kiil S, Dam JK (2004) Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog Org Coat 50:75–104. https://doi.org/10.1016/j.porgcoat.2003.06.001
He C, Gu Y, Zhang J, Ma L, Yan M, Mou J, Ren Y (2022) Preparation and modification technology analysis of ionic polymer-metal composites (IPMCs). Int J Mol Sci 23:3522–3540. https://doi.org/10.3390/ijms23073522
Yan H, Wu QS, Yu CM, Zhao TY, Liu MJ (2020) Recent progress of biomimetic antifouling surfaces in marine. Adv Mater Interfaces 7:2000966–2000984. https://doi.org/10.1002/admi.202000966
Liu MY, Li SN, Wang H, Jiang RJ, Zhou X (2021) Research progress of environmentally friendly marine antifouling coatings. Polym Chem 12:3702–3720. https://doi.org/10.1039/d1py00512j
Gu J, Li L, Huang DC, Jiang L, Liu L, Li FY, Pang AM, Guo X et al (2019) In situ synthesis of Graphene@cuprous oxide nanocomposite incorporated marine antifouling coating with elevated antifouling performance. Open J Org Polym Mater 09:47–62. https://doi.org/10.4236/ojopm.2019.93003
Selim MS, Yang H, Wang FQ, Fatthallah NA, Huang Y, Kuga S (2019) Silicone/ZnO nanorod composite coating as a marine antifouling surface. Appl Surf Sci 466:40–50. https://doi.org/10.1016/j.apsusc.2018.10.004
Gomathi SG, Sathya S, Sriyutha MP, Das A, Pandiyan R, Venugopalan VP, Doble M (2015) Polydimethyl siloxane nanocomposites: their antifouling efficacy in vitro and in marine conditions. Int Biodeterior Biodegradation 104:307–314. https://doi.org/10.1016/j.ibiod.2015.05.022
Gu YQ, Zhang JJ, Yu SW, Mou CQ, Li Z, He CD, Wu DH, Mou JG et al (2022) Unsteady numerical simulation method of hydrofoil surface cavitation. Int J Mech Sci 228:107490–107504. https://doi.org/10.1016/j.ijmecsci.2022.107490
Li Y, Ning C (2019) Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact Mater 4:189–195. https://doi.org/10.1016/j.bioactmat.2019.04.003
Liu HW, Gu TY, Asif M, Zhang GA, Liu HF (2017) The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros Sci 114:102–111. https://doi.org/10.1016/j.corsci.2016.10.025
Dong ZH, Liu T, Liu HF (2011) Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 27:487–495. https://doi.org/10.1080/08927014.2011.584369
Belkaid S, Ladjouzi MA, Hamdani S (2010) Effect of biofilm on naval steel corrosion in natural seawater. J Solid State Electrochem 15:525–537. https://doi.org/10.1007/s10008-010-1118-5
Yuan SJ, Liang B, Zhao Y, Pehkonen SO (2013) Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros Sci 74:353–366. https://doi.org/10.1016/j.corsci.2013.04.058
Lenhart TR, Duncan KE, Beech IB, Sunner JA, Smith W, Bonifay V, Biri B, Suflita JM (2014) Identification and characterization of microbial biofilm communities associated with corroded oil pipeline surfaces. Biofouling 30:823–835. https://doi.org/10.1080/08927014.2014.931379
Jain A, Bhosle NB (2009) Biochemical composition of the marine conditioning film: implications for bacterial adhesion. Biofouling 25:13–19. https://doi.org/10.1080/08927010802411969
Garg A, Jain A, Bhosle NB (2009) Chemical characterization of a marine conditioning film. Int Biodeterior Biodegrad 63:7–11. https://doi.org/10.1016/j.ibiod.2008.05.004
Schultz MP (2007) Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 23:331–341. https://doi.org/10.1080/08927010701461974
Long L, Wang R, Chiang HY, Ding W, Li YX, Chen F, Qian PY (2021) Discovery of antibiofilm activity of elasnin against marine biofilms and its application in the marine antifouling coatings. Mar Drugs 19:19–31. https://doi.org/10.3390/md19010019
Fletcher RL, Callow ME (1992) The settlement, attachment and establishment of marine algal spores. Br Phycol J 27:303–329. https://doi.org/10.1080/00071619200650281
Ma SH, Ye Q, Pei XW, Wang DA, Zhou F (2015) Antifouling on Gecko’s feet inspired fibrillar surfaces: evolving from land to marine and from liquid repellency to algae resistance. Adv Mater Interfaces 2:1600257–1600269. https://doi.org/10.1002/admi.201500257
Demirel YK, Uzun D, Zhang Y, Fang HC, Day AH, Turan O (2017) Effect of barnacle fouling on ship resistance and powering. Biofouling 33:819–834. https://doi.org/10.1080/08927014.2017.1373279
Uzun D, Ozyurt R, Demirel YK, Turan O (2020) Does the barnacle settlement pattern affect ship resistance and powering? Appl Ocean Res 95:102020–102039. https://doi.org/10.1016/j.apor.2019.102020
Dalsin JL, Hu B, Lee B, Messersmith PB (2003) Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc 125:4253–4258. https://doi.org/10.1021/ja0284963
Soares CG, Garbatov Y, Zayed A, Wang G (2009) Influence of environmental factors on corrosion of ship structures in marine atmosphere. Corros Sci 51:2014–2026. https://doi.org/10.1016/j.corsci.2009.05.028
Jiang YL, Liang XR, Wu SY (2011) Nanotechnology applications in the field of ship protection. Mater Sci Forum 694:239–243. https://doi.org/10.4028/www.scientific.net/MSF.694.239
Selim MS, El-Safty SA, El-Sockary MA, Hashem AI, Abo EOM, El-Saeed AM, Fatthallah NA (2016) Data on photo-nanofiller models for self-cleaning foul release coating of ship hulls. Data Brief 8:1357–1364. https://doi.org/10.1016/j.dib.2016.08.010
Smaradhana DF, Prabowo AR, Ganda ANF (2021) Exploring the potential of graphene materials in marine and shipping industries—a technical review for prospective application on ship operation and material-structure aspects. J Ocean Eng Sci 6:299–316. https://doi.org/10.1016/j.joes.2021.02.004
Miller RJ, Adeleye AS, Page HM, Kui L, Lenihan HS, Keller AA (2020) Nano and traditional copper and zinc antifouling coatings: metal release and impact on marine sessile invertebrate communities. J Nanopart Res 22:1–15. https://doi.org/10.1007/s11051-020-04875-x
Liu ZX, Tian S, Li Q, Wang JC, Pu JB, Wang G, Zhao WJ, Feng F et al (2020) Integrated dual-functional ORMOSIL coatings with AgNPs@rGO nanocomposite for corrosion resistance and antifouling applications. ACS Sustain Chem Eng 8:6786–6797. https://doi.org/10.1021/acssuschemeng.0c01294
Zhang JX, Pan MX, Luo CB, Chen XP, Kong JR, Zhou T (2016) A novel composite paint (TiO2/fluorinated acrylic nanocomposite) for antifouling application in marine environments. J Environ Chem Eng 4:2545–2555. https://doi.org/10.1016/j.jece.2016.05.002
Pourhashem S, Saba F, Duan JZ, Rashidi A, Guan F, Nezhad EG, Hou BR (2020) Polymer/inorganic nanocomposite coatings with superior corrosion protection performance: a review. J Ind Eng Chem 88:29–57. https://doi.org/10.1016/j.jiec.2020.04.029
Archana S, Sundaramoorthy B (2019) Review on biofouling prevention using nanotechnology. J Entomol Zool Stud 7:640–648. https://doi.org/10.22271/j.ento
Sathya S, Murthy PS, Das AS, Gomathi SG, Venkatnarayanan S, Pandian R, Sathyaseelan VS, Pandiyan V et al (2016) Marine antifouling property of PMMA nanocomposite films: results of laboratory and field assessment. Int Biodeterior Biodegrad 114:57–66. https://doi.org/10.1016/j.ibiod.2016.05.026
Fazli-Shokouhi S, Nasirpouri F, Khatamian M (2019) Polyaniline-modified graphene oxide nanocomposites in epoxy coatings for enhancing the anticorrosion and antifouling properties. J Coat Technol Res 16:983–997. https://doi.org/10.1007/s11998-018-00173-3
Pernak J, Kalewska J, Ksycińska H, Cybulski J (2001) Synthesis and anti-microbial activities of some pyridinium salts with alkoxymethyl hydrophobic group. Eur J Med Chem 36:899–907. https://doi.org/10.1016/S0223-5234(01)01280-6
Zecher K, Aitha VP, Heuer K, Ahlers H, Roland K, Fiedel M, Philipp B (2018) A multi-step approach for testing non-toxic amphiphilic antifouling coatings against marine microfouling at different levels of biological complexity. J Microbiol Methods 146:104–114. https://doi.org/10.1016/j.mimet.2018.02.009
Gudipati CS, Finlay JA, Callow JA, Callowet ME, Wooley KL (2005) The antifouling and fouling-release perfomance of hyperbranched fluoropolymer (HBFP)-poly(ethylene glycol) (PEG) composite coatings evaluated by adsorption of biomacromolecules and the green fouling alga Ulva. Langmuir 21:3044–3053. https://doi.org/10.1021/la048015o
Wen SF, Wang P, Wang L (2021) Preparation and antifouling performance evaluation of fluorine-containing amphiphilic silica nanoparticles. Colloids Surf A Physicochem Eng Asp 611:125823–125831. https://doi.org/10.1016/j.colsurfa.2020.125823
Krishnan S, Ayothi R, Hexemer A, Finlay JA, Sohn KE, Perry R, Ober CK, Kramer EJ et al (2006) Anti-biofouling properties of comblike block copolymers with amphiphilic side chains. Langmuir 22:5075–5086. https://doi.org/10.1021/la052978l
Galli G, Martinelli E (2017) Amphiphilic polymer platforms: surface engineering of films for marine antibiofouling. Macromol Rapid Commun 38:1600704–1600724. https://doi.org/10.1002/marc.201600704
Gudipati CS, Greenlief CM, Johnson JA, Prayongpan P, Wooley KL (2004) Hyperbranched fluoropolymer and linear poly(ethylene glycol) based amphiphilic crosslinked networks as efficient antifouling coatings: an insight into the surface compositions, topographies, and morphologies. J Polym Sci A Polym Chem 42:6193–6208. https://doi.org/10.1002/pola.20466
Lin CA, Sperling RA, Li JK, Yang TY, Li PY, Zanella M, Chang WH, Parak WJ (2008) Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small 4:334–341. https://doi.org/10.1002/smll.200700654
Barletta M, Aversa C, Pizzi E, Puopolo M, Vesco S (2018) Design, manufacturing and testing of anti-fouling/foul-release (AF/FR) amphiphilic coatings. Prog Org Coat 123:267–281. https://doi.org/10.1016/j.porgcoat.2018.07.016
Zhang Z, Chao T, Chen S, Jiang S (2006) Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 22:10072–10077. https://doi.org/10.1021/la062175d
Koschitzki F, Wanka R, Sobota L, Koc J, Gardner H, Hunsucker KZ, Swain GW, Rosenhahn A (2020) Amphiphilic dicyclopentenyl/carboxybetaine-containing copolymers for marine fouling-release applications. ACS Appl Mater Interfaces 12:34148–34160. https://doi.org/10.1021/acsami.0c07599
Lu GM, Tian S, Li JY, Xu YJ, Liu S, Pu JB (2021) Fabrication of bio-based amphiphilic hydrogel coating with excellent antifouling and mechanical properties. Chem Eng J 409:128134–128147. https://doi.org/10.1016/j.cej.2020.128134
Guo H, Chen P, Tian S, Ma Y, Li Q, Wen C, Yang J, Zhang L (2020) Amphiphilic marine antifouling coatings based on a hydrophilic polyvinylpyrrolidone and hydrophobic fluorine-silicon-containing block copolymer. Langmuir 36:14573–14581. https://doi.org/10.1021/acs.langmuir.0c02329
Kumar S, Ye F, Mazinani B, Dobretsov S, Dutta J (2021) Chitosan nanocomposite coatings containing chemically resistant ZnO–SnOx core-shell nanoparticles for photocatalytic antifouling. Int J Mol Sci 22:4513–4529. https://doi.org/10.3390/ijms22094513
Zhang HR, Mane AU, Yang XB, Xia ZJ, Barry EF, Luo JQ, Wan YH, Elam JW et al (2020) Visible-light-activated photocatalytic films toward self-cleaning membranes. Adv Funct Mater 30:2002874–2002882. https://doi.org/10.1002/adfm.202002847
Zhang X, Zhang J, Yu JQ, Zhang Y, Cui ZX, Sun Y, Hou BR (2018) Fabrication of InVO4/AgVO3 heterojunctions with enhanced photocatalytic antifouling efficiency under visible-light. Appl Catal B 220:57–66. https://doi.org/10.1016/j.apcatb.2017.07.074
Zhang L, Sha J, Chen R, Liu Q, Liu J, Yu J, Zhang H, Lin C et al (2020) Three-dimensional flower-like shaped Bi5O7I particles incorporation zwitterionic fluorinated polymers with synergistic hydration-photocatalytic for enhanced marine antifouling performance. J Hazard Mater 389:121854–121862. https://doi.org/10.1016/j.jhazmat.2019.121854
Zhu ZY, Zhou F, Zhan S, Tian Y, He QC (2018) Study on the bactericidal performance of graphene/TiO2 composite photocatalyst in the coating of PEVE. Appl Surf Sci 430:116–124. https://doi.org/10.1016/j.apsusc.2017.07.289
Scandura G, Ciriminna R, Xu YJ, Pagliaro M, Palmisano G (2016) Nanoflower-like Bi2WO6 encapsulated in ORMOSIL as a novel photocatalytic antifouling and foul-release coating. Chemistry 22:7063–7067. https://doi.org/10.1002/chem.201600831
Heng ZW, Chong WC, Pang YL, Sim LC, Koo CH (2021) Novel visible-light responsive NCQDs-TiO2/PAA/PES photocatalytic membrane with enhanced antifouling properties and self-cleaning performance. J Environ Chem Eng 9:105388–105399. https://doi.org/10.1016/j.jece.2021.105388
Wan J, Huang J, Yu H, Liu L, Shi Y, Liu C (2021) Fabrication of self-assembled 0D–2D Bi2MoO6-g-C3N4 photocatalytic composite membrane based on PDA intermediate coating with visible light self-cleaning performance. J Colloid Interface Sci 601:229–241. https://doi.org/10.1016/j.jcis.2021.05.038
Li YJ, Xue YJ, Tian J, Song XJ, Zhang XJ, Wang XZ, Cui HZ (2017) Silver oxide decorated graphitic carbon nitride for the realization of photocatalytic degradation over the full solar spectrum: from UV to NIR region. Sol Energy Mater Sol Cells 168:100–111. https://doi.org/10.1016/j.solmat.2017.04.031
Liu TT, Wang L, Liu X, Sun CX, Lv YT, Miao R, Wang XD (2020) Dynamic photocatalytic membrane coated with ZnIn2S4 for enhanced photocatalytic performance and antifouling property. Chem Eng J 379:122379–122390. https://doi.org/10.1016/j.cej.2019.122379
Zhu CY, Liu GG, Han K, Ye HQ, Wei SC, Zhou YH (2017) One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem Eng Process 120:20–26. https://doi.org/10.1016/j.cep.2017.06.012
Zhang ZX, Li YK, Chen RR, Liu Q, Liu JY, Yu J, Zhang HS, Song DL et al (2021) Photocatalytic antifouling coating based on carbon nitride with dynamic acrylate boron fluorinated polymers. New J Chem 45:780–787. https://doi.org/10.1039/d0nj05132b
Zhu ZY, Zhou F, Zhan S (2020) Enhanced antifouling property of fluorocarbon resin coating (PEVE) by the modification of g-C3N4/Ag2WO4 composite step-scheme photocatalyst. Appl Surf Sci 506:144934–144942. https://doi.org/10.1016/j.apsusc.2019.144934
Song YP, Zhou F (2020) Antifouling properties of PEVE coating modified by BiVO4/BiOIO3 composite photocatalyst. Appl Phys A 126:1–8. https://doi.org/10.1007/s00339-020-03717-w
Song YP, Zhou F, Chai YH, Zhan S (2021) Study on high antibacterial RGO/Bi2WO6 microspheres combined with PEVE coating for marine sterilization under visible light. Res Chem Intermed 47:2297–2310. https://doi.org/10.1007/s11164-021-04400-2
Di TM, Xu QL, Ho WK, Tang H, Xiang QJ, Yu JG (2019) Review on metal sulphide-based Z-scheme photocatalysts. ChemCatChem 11:1394–1411. https://doi.org/10.1002/cctc.201802024
Wang XF, Li SF, Ma YQ, Yu HG, Yu JG (2011) H2WO4·H2O/Ag/AgCl composite nanoplates: a plasmonic Z-scheme visible-light photocatalyst. J Phys Chem C 115:14648–14655. https://doi.org/10.1021/jp2037476
Wang W, Xu LK, Li XB, Lin ZF, Yang Y, An EP (2014) Self-healing mechanisms of water triggered smart coating in seawater. J Mater Chem A 2:1914–1921. https://doi.org/10.1039/c3ta13389c
Chang RX, Huang YF, Shan GR, Bao YZ, Yun XY, Dong TG, Pan PJ (2015) Alternating poly(lactic acid)/poly(ethylene-co-butylene) supramolecular multiblock copolymers with tunable shape memory and self-healing properties. Polym Chem 6:5899–5910. https://doi.org/10.1039/c5py00742a
Chuo TW, Wei TC, Liu YL (2013) Electrically driven self-healing polymers based on reversible guest-host complexation of β-cyclodextrin and ferrocene. J Polym Sci A Polym Chem 51:3395–3403. https://doi.org/10.1002/pola.26736
Hu P, Xie QY, Ma CF, Zhang GZ (2021) Fouling resistant silicone coating with self-healing induced by metal coordination. Chem Eng J 406:126870–126879. https://doi.org/10.1016/j.cej.2020.126870
Wang C, Wang T, Hu PD, Shen T, Xu JH, Ding CD, Fu JJ (2020) Dual-functional anti-biofouling coatings with intrinsic self-healing ability. Chem Eng J 389:123469–123507. https://doi.org/10.1016/j.cej.2019.123469
Ye YW, Chen H, Zou YJ, Ye Y, Zhao HC (2020) Corrosion protective mechanism of smart graphene-based self-healing coating on carbon steel. Corros Sci 174:108825–108860. https://doi.org/10.1016/j.corsci.2020.108825
Samadzadeh M, Boura SH, Peikari M, Kasiriha SM, Ashrafi A (2010) A review on self-healing coatings based on micro/nanocapsules. Prog Org Coat 68:159–164. https://doi.org/10.1016/j.porgcoat.2010.01.006
Tamate R, Hashimoto K, Horii T, Hirasawa M, Li X, Shibayama M, Watanabe M (2018) Self-healing micellar ion gels based on multiple hydrogen bonding. Adv Mater 30:1802792–1802798. https://doi.org/10.1002/adma.201802792
Zhao D, Feng M, Zhang L, He B, Chen X, Sun J (2021) Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr Polym 256:117580–117891. https://doi.org/10.1016/j.carbpol.2020.117580
Xu JH, Ye S, Ding CD, Tan LH, Fu JJ (2018) Autonomous self-healing supramolecular elastomer reinforced and toughened by graphitic carbon nitride nanosheets tailored for smart anticorrosion coating applications. J Mater Chem A 6:5887–5898. https://doi.org/10.1039/c7ta09841c
Chen Y, Kushner AM, Williams GA, Guan Z (2012) Multiphase design of autonomic self-healing thermoplastic elastomers. Nat Chem 4:467–472. https://doi.org/10.1038/nchem.1314
Yang MS, Sun YH, Chen GM, Wang GY, Lin SZ, Sun ZY (2020) Preparation of a self-healing silicone coating for inhibiting adhesion of benthic diatoms. Mater Lett 268:127496–127499. https://doi.org/10.1016/j.matlet.2020.127496
Wong TS, Kang SH, Tang SK, Smythe EJ, Hatton BD, Grinthal A, Aizenberg J (2011) Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477:443–447. https://doi.org/10.1038/nature10447
Wu Y, Zhao W, Ou J (2021) Stable, superfast and self-healing fluid coating with active corrosion resistance. Adv Colloid Interface Sci 295:102494–102506. https://doi.org/10.1016/j.cis.2021.102494
Zhang H, Liang YZ, Wang P, Zhang D (2019) Design of slippery organogel layer with room-temperature self-healing property for marine anti-fouling application. Prog Org Coat 132:132–138. https://doi.org/10.1016/j.porgcoat.2019.03.020
He BL, Du YX, Wang BW, Zhao XY, Liu SJ, Ye Q, Zhou F (2022) Self-healing polydimethylsiloxane antifouling coatings based on zwitterionic polyethylenimine-functionalized gallium nanodroplets. Chem Eng J 427:131019–131027. https://doi.org/10.1016/j.cej.2021.131019
Nwuzor IC, Idumah CI, Nwanonenyi SC, Ezeani OE (2021) Emerging trends in self-polishing anti-fouling coatings for marine environment. Saf Extreme Environ 3:9–25. https://doi.org/10.1007/s42797-021-00031-3
Liu C (2015) Development of anti-fouling coating using in marine environment. Int J Environ Monitor Anal 3:373–376. https://doi.org/10.11648/j.ijema.20150305.30
Chen YY, Liu ZX, Han S, Han J, Jiang DY (2016) Poly(propylene carbonate) polyurethane self-polishing coating for marine antifouling application. J Appl Polym Sci 133:43667–43675. https://doi.org/10.1002/app.43667
Xu G, Liu P, Pranantyo D, Neoh Koon G, Kang ET (2018) Dextran- and chitosan-based antifouling, antimicrobial adhesion, and self-polishing multilayer coatings from pH-responsive linkages-enabled layer-by-layer assembly. ACS Sustain Chem Eng 6:3916–3926. https://doi.org/10.1021/acssuschemeng.7b04286
Zhang JB, Liu YZ, Wang XW, Zhang CY, Liu H, Yang W, Cai MR, Pei XW et al (2020) Self-polishing emulsion platforms: Eco-friendly surface engineering of coatings toward water borne marine antifouling. Prog Org Coat 149:105945–105954. https://doi.org/10.1016/j.porgcoat.2020.105945
Jo JH, Ko Hyun C, Kim BW, Park H, Lee IW, Chun HH, Jo NJ (2016) Micellar core-shell-type acrylic-polyurethane hybrid materials with self-polishing property. Compos Interfaces 23:797–805. https://doi.org/10.1080/09276440.2016.1173473
Dai ZW, Cao M, Li SQ, Yao JH, Wu B, Wang YP, Wang HJ, Dong J et al (2021) A novel marine antifouling coating based on a self-polishing zinc-polyurethane copolymer. J Coat Technol Res 18:1333–1343. https://doi.org/10.1007/s11998-021-00496-8
Acknowledgements
This work was supported by the Foundation of Suzhou Science and Technology Project (Grant No. SYG201937) and by the Six-Talent Peak Project of Jiangsu Province (Grant No. XNYQC012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Zheng, X., Zhang, H. et al. Review on formation of biofouling in the marine environment and functionalization of new marine antifouling coatings. J Mater Sci 57, 18221–18242 (2022). https://doi.org/10.1007/s10853-022-07791-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07791-8