Abstract
A series of Sr1−x−yErxYbyMoO4 phosphor compositions codoped with Er3+ (x = 1 mol%) and varying Yb3+ (y = 0–9 mol%) dopants have been evaluated for luminescence-based thermometry. Sintered ceramics exhibit enhanced grain growth at an optimum Yb3+ content (y = 0.03) and exhibit improved upconversion luminescence and a relatively better thermometric performance over the phosphors in the powder form. Strong upconversion (UC) luminescence at ~ 529, ~ 552, and ~ 662 nm assisted by a 2-photon process, cooperative luminescence from Yb3+ ion pair at 495 nm, and weak UC emissions at ~ 380 and ~ 410 nm due to 3-photon process are identified. Luminescence quenching is observed in all the UC emission bands for Yb3+ content y > 3 mol%. Sensitization from Yb3+ to Er3+ and Yb3+–(MoO4)2− dimer to Er3+ ions results in selective enhancement of the green emission. Variation of UC emission intensity with increasing dopant concentration is analyzed using Dexter’s energy transfer formula, which supports the dipole–dipole interaction between Yb3+ and Er3+ ions. Changes in fluorescence intensity ratio (I529/I552) with temperature reveal the potential usefulness of the optimized Sr0.96Er0.01Yb0.03MoO4 phosphor composition in the ceramic form for non-contact optical thermometry and exhibits good repeatability for temperature sensing applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trivalent lanthanide ions (Ln3+) have shown remarkable adaptability in different host materials to up-convert near-infrared radiation into visible radiation with larger luminescence lifetimes and a high signal-to-noise ratio [1,2,3,4]. The applicability of the fluorescence intensity ratio method as a non-contact probe for temperature sensing has been recognized for spatial mapping of temperature fluctuations at the sub-micron scale in areas such as microscopic cellular interactions, biochemical processes, and medical diagnostics [5, 6]. Current research concentrates on a large number of host materials with different rare-earth ion combinations for achieving improved sensitivity over a wide temperature range, good linearity, and a fast response speed [7, 8].
For efficient UC emission, the non-radiative (NR) energy transfer (ET) process is either due to multipolar interaction or exchange interaction mechanism between the selected activator and the sensitizer ions at an appropriate distance between them [9]. The Er3+ ion acts as an activator due to its intra f-f transitions and Yb3+ as a sensitizer provides a large absorption cross section for the near-infrared radiation [10]. The stability of the host material is of crucial importance for achieving a controlled structural arrangement that favors efficient energy transfer and gives a controlled emission profile. The reported temperature sensing performance based on light upconversion in many host materials can be summarized as follows: (1) the sensitivity varies with temperature and reaches a maximum at a certain temperature, (2) different host materials exhibit maximum sensitivity at different temperatures, and (3) only a few host materials have been characterized for applicability over a wide temperature range [6, 8]. So far, tungstates and molybdates such as Er3+/Yb3+-codoped SrWO4 and SrMoO4 have revealed improved functional performance for high-temperature applications over a wide range (80–775 K), and Gd3Ga5O12 has exhibited an exceptionally high sensitivity in the cryogenic (4.2–300 K) range [11,12,13].
SrMoO4 is an attractive material due to its intense absorption near the UV region, low phonon energy (850 cm−1), and the strong polarization of Mo6+ ion which distorts the symmetry and enhances Stark splitting [2, 14]. Studies on RE ion-doped SrMoO4 in different forms including single crystals [15], nanocrystals/nanoparticles [16, 17], core–shell structures and nanopowders [18, 19] were reported. Synthesis of other Er3+/Yb3+-codoped CaMoO4, and CaWO4 phosphors prepared by different methods including sol–gel [20] and solid-state reaction [21] method has been attempted, and recently, the significant influence of Ce3+ and Sm3+ dopants in SrMoO4 has been reported [22, 23]. For temperature sensing applications, Du et al. [12] reported a stable performance over a wide temperature range (93–773 K) with a maximum sensitivity (0.0128 K−1) at 480 K using Er3+/Yb3+-codoped SrMoO4 powders. Soni et al. [24], showed optical heating effects and color tunability in co-precipitated Er3+/Yb3+-codoped SrMoO4 powders and reported a high sensitivity for optical thermometry over a limited range (300–543 K). Zhang et al. [25] emphasized the influence of large grain growth that was achieved in Er/Yb-codoped SrMoO4 phosphors which were further modified with aliovalent doping of Li+ and Ga3+. Strong upconversion luminescence, enhanced temperature sensitivity, and a high measurement accuracy with good repeatability were reported in the temperature range (300–600 K) prompting the usefulness of such molybdate phosphors for thermometric application.
Photonic materials in the form of bulk ceramics are also gaining lot of attention. Ceramics are robust and simple to make and allow superior control over their thermal and mechanical properties. They can be formed into different shapes and allow the chemical composition to be tailored for combining several functional properties with optical control. Comparison studies on glasses and glass-ceramics have revealed the beneficial influence of crystal field effects due to inherent microscopic crystallinity [26]. Recent studies have shown improvements in upconversion luminescence relating to crystallinity with the introduction of Bi3+ in rare-earth-codoped phosphors [27, 28]. Ceramics regardless of being opaque have shown interesting light upconversion properties (visible to ultraviolet) on their surface, and microstructural features including material thickness, increasing crystallite/grain size have shown a profound effect on the antimicrobial activity [29]. Motivated by the potential applicability of opaque ceramic surfaces, the present work is focused on the processing, microstructure, and light upconversion properties of Er/Yb-codoped SrMoO4 ceramics which have not been reported earlier. Effects of exchange interaction between the Er3+ and Yb3+ ions in the SrMoO4 lattice, cooperative luminescence from Yb3+ ion pair, and increased sensitization from Yb3+–(MoO4)2− dimer complex formation are analyzed. The present study compares the performance of the phosphors in powder and ceramic form and examines the influence of Er/Yb dopant concentration on the structural and thermometric properties. The performance of sintered ceramics is found to be superior with an optimum content of Yb3+ (3 mol%). The usefulness of the optimized composition for temperature sensing is evaluated over a wide range of temperature (90–775 K) in terms of linearity, sensitivity, repeatability, and measurement errors.
Experimental procedure
Modified Sr1−x−yErxYbyMoO4 ceramic compositions were prepared by reacting a mixture of oxide powders having a pre-set Er3+ content (x = 0.01) and varying Yb3+ content (y = 0.01, 0.03, 0.05, 0.07, 0.09). Fine powders of Er2O3, Yb2O3 (from Alfa Aesar), SrCO3, and MoO3 (from Sigma-Aldrich) of high purity (99.9%) in stoichiometric proportions were mixed with a motorized agate pestle and mortar for 6 h. The initial calcination step was carried out at 750 °C for 3 h, and the powder mixture was then pressed into pellets which were sintered at 950 °C for 5 h. BRUKER D8-X-ray diffraction equipment was used in the 2θ range (\(25^{^\circ } - 80^{^\circ }\)) and to collect the XRD data at a step-size of \(0.02^{^\circ }\). The light upconversion (UC) data were measured on a HORIBA PTI Quanta-master (8450–11) with 980 nm excitation produced by a continuous-wave (CW) solid-state laser. The lifetime measurements at room temperature (RT) were taken using a pulsed Xe light source. The UC spectra at different temperatures were recorded on a polished ceramic surface mounted on a programmable temperature-controlled stage (LinkamT95). The temperature of the sample was stabilized for 8 min before recording the UC emission, and a cooled photo-multiplicator (R928P Hamamatsu) was used.
Results and discussion
X-ray diffraction
The X-ray diffraction measurements revealed single phase formation in the sintered Sr1−x−yErxYbyMoO4 phosphors as shown in Fig. 1a. A good match with the reported reference data on SrMoO4 (JCPDS No. 00-008-0482) was achieved and found to correspond with the tetragonal structure of SrMoO4 with space group I41/a (No.88) [30, 31]. The well-reacted phosphors upon the initial calcination treatment at 750 °C/3 h itself showed single phase formation as shown in Fig. 1b. Further post-annealing treatment at 950 °C/5 h and the use of calcined powders for making ceramics which were sintered at 950 °C/5 h did not show any changes in the phase purity. No additional phases were observed confirming the solubility of Er3+ and Yb3+ in the host lattice. Table 1 presents the variations in the lattice constants a, c, and the unit-cell volume (V) with varying dopant concentration [x (Er3+) + y (Yb3+)]. A slight expansion in the lattice is noted with just the introduction of Er3+ (ESMO) dopant alone, and further addition of Yb3+ content showed lattice contraction. The changes in the lattice due to the incorporation of Er and Yb at the Sr2+ site are attributed to the variation of ionic radii, electronegativity, and atomic crystal structures of Sr, Er, and Yb, respectively. Sr and Yb atoms are known to exist with bcc structure, whereas Er exists in the hcp structure [32]. Considering the ionic radii differences of [Sr2+ (0.138 nm), Er3+ (0.103 nm Å), Yb3+ (0.1008 nm)], it is inferred that Er3+ is substituted at the Sr2+ site in hcp structure having a coordination number 12 CN. Therefore, substitution of hcp structured Er3+ in place of bcc structured Sr2+ results in the slight expansion of the lattice (Table 1) leading to increased unit-cell volume [33]. In all the other compositions (EYSMO) having a fixed 0.01 mol of Er3+ content, the substitution of Yb3+ is favored at the Sr2+ site as both the ions have a bcc structure, and both are in the same state with the same coordination number 8 CN.
Varying Yb3+ content is found to influence the grain growth in sintered ceramics as seen in the micrographs shown in Fig. 2a–f. An optimum Yb3+ content y = 0.03 produces a densely packed microstructure structure with a large grain size (~ 10 μm), and further increase in Yb3+ content increases the porosity and induces the development of non-uniform grain size in the ceramic microstructure.
Light upconversion and quenching
Comparison of phosphor powder and sintered ceramic
The upconversion luminescence in the developed phosphor Sr1−x−yErxYbyMoO4 with Er3+ (x = 0.01), and Yb3+ (y = 0.03) was compared in three different forms;
(1) Calcined powders, (2) calcined and annealed powder, and (3) dense ceramic pellets prepared from calcined powders. Although they were subject to varying heat treatment, the structural analysis (Fig. 1b) showed single phase formation without any significant changes in the crystallite size; however, they showed a significant change in the light upconversion spectra as shown in Fig. 3. The observed improvement in the upconverted light intensity is believed to arise from the remarkable increase in the grain size of the ceramics for the optimum concentration of Yb3+ (y = 0.03).
The observed dependence of UC emission intensity on grain size is in close agreement with the recent results of Zhang et al. [25], where enhanced upconversion luminescence, an increased FIR ratio, and improved temperature sensitivity were observed in Er/Yb-codoped SrMoO4 phosphors when further modified with Li+/Ga3+ dopants. Both Li+/Ga3+ were suggested as sintering aids which promoted enhanced grain growth in the developed phosphors. Motivated from this earlier study which was primarily concentrated on using additional dopants (Li+/Ga3+), we have rather concentrated on sintered bulk ceramics and observed a similar enhancement in the grain growth for an optimum Yb3+ concentration of y = 0.03 as seen from the scanning electron micrographs presented in Fig. 2c.
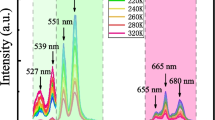
The light upconversion spectra measured on different Sr1−x−yErxYbyMoO4 ceramic compositions are shown in (Fig. 4a, b) when excited with 980 nm at a constant pump power (0.50 W). With single Er3+ dopant (x = 0.01, y = 0), only a weak UC emission in the green and red bands is observed as indicated by the black colored trace in (Fig. 4b). Addition of Yb3+ content (y = 0.01 to 0.09), along with the fixed Er3+ content (x = 0.01) reveals UC emissions at ~ 380, ~ 410 nm and ~ 495 nm due to the 4G11/2 → 4I15/2, 2H9/2 → 4I15/2, and cooperative luminescence of Yb3+ ion pair, respectively (Fig. 4a), in agreement with earlier reported studies [34,35,36,37]. The strong upconversion emissions in the green band at ~ 529 nm (2H11/2 → 4I15/2), and ~ 552 nm (4S3/2 → 4I15/2) are followed by the weak emission in the red band at 662 nm (4F9/2 → 4I15/2) as shown in Fig. 4b [25, 38,39,40].
The emission intensity at 552 nm being stronger than the emission at 529 nm (Fig. 4b) stems from the redistribution of energy transfer within the respective levels as they are coupled thermally. The upconverted emission intensity at ~ 380, ~ 410, ~ 495, ~ 529, ~ 552, and ~ 662 nm increases up to an optimum Yb3+ concentration y = 0.03. Further increase in Yb3+ dopant (y > 0.03) results in a sharp fall in the intensity (Fig. 4a, b) due to concentration quenching effect [12, 14, 16]. Beyond the quenching concentration (y = 0.03), the critical distance Rc between the Yb3+ and Er3+ ions is found to reduce considerably due to the shortened interatomic distance between these two ions and gives rise to non-radiative transitions. As the exchange interaction and multipole–multipole interaction have greater probability over radiative emission, the UC emission intensity thereby decreases [41]. It is noted that the presence of both the activator and the sensitizer ions play an important role in the calculation of the critical distance (Rc) for evaluating the quenching process [42],
where V is the unit-cell volume, \(X_{{\text{C}}}\) = (x + y) is the dopant concentration at which quenching is observed. Er3+ (x) and Yb3+ (y) is the dopant concentration and (Z = 4) represents the number of host cations per unit cell. For \({X}_{C}=0.04\), with x = 0.01 and y = 0.03, the critical distance in the presence of Yb3+ ion is ~ 16.08 Å which is smaller than the value 25.56 Å in the absence of the Yb3+ ions. The efficient transfer of energy from the Yb3+ sensitizer ion to the activator Er3+ ion occurs, when Rc in the presence of sensitizer is less than the value obtained in the absence of the sensitizer. Normally, the exchange interaction plays a key role for Rc < 5 Å for concentration quenching, and, alternately, the electric multipole interaction mechanism is dominant for Rc > 5 Å. For the optimum concentration of Yb3+ (y = 0.03), as the calculated value of Rc is 16.08 Å, it is implied that a multipolar interaction is accountable for the observed concentration quenching. This was further understood on the basis of Dexter’s energy transfer formula [43, 44] which governs the UC emission (I) and the concentration X = (x + y) of the sensitizer and the activator as:
The fitting parameter S = 6, 8, and 10 corresponds to (1) dipole–dipole, (2) dipole–quadrupole, or (3) quadrupole–quadrupole interactions, respectively, and “b” is a coefficient. The slope (S/3) obtained from a linear fit of the \(\mathrm{log}\left(I/X\right)\) versus \(\mathrm{log}\left(X\right)\) shown in the inset of Fig. 4b yields \(S\sim 6\) (5.83) and confirms the observed concentration quenching due to dipole–dipole interaction.
UC emission mechanism
The pathways for the energy transfer in the present Er3+/Yb3+: SrMoO4 system are depicted in Fig. 5 and show the sensitization from both (1) Yb3+ to Er3+ ions, and (2) Yb3+–(MoO4)2− dimer complex to Er3+ ions, and their combined influence is understood as follows:
Yb3+ to Er3+ ions
The luminescence mechanism triggered by Yb3+ to Er3+ is well known, where Er3+ levels (4G11/2 2H9/2, 2H11/2, 4S3/2, and 4F9/2) get populated, due to energy transfer from Yb3+ to Er3+ ion. The resultant sensitization due to successive energy transfers and relaxations gives rise to several UC emission bands at (380, 410, 495, and 662 nm) which remain weak despite the increase in Yb3+ concentration in contrast to the intense green emission bands at 529 and 552 nm as shown in Fig. 4a, b.
Primarily with the absorption of 980 nm photon, the population at the metastable level 4I11/2 of Er3+ increases via ground state absorption (GSA) and is supplemented by the matched resonant absorption from the (2F7/2–2F5/2) of Yb3+ and (4I15/2–4I11/2) levels of Er3+ [41, 45]. A non-radiative relaxation (NRR) process populates the 4I13/2 level. Yb3+ ions, which absorb energy from another photon actively transfer energy to nearby Er3+ ions at 4I13/2 and 4I11/2 levels and thereby populates the 4F9/2 and 4F7/2 levels via excited-state absorption (ESA).
As the energy gap between 4F7/2 and the underlying levels is small, the Er3+ ions rapidly de-excite non-radiatively via multiphonon relaxation (MPR) to the lower levels 2H11/2 and 4S3/2 which are thermally coupled. This leads to an instantaneous radiative relaxation from the thermally coupled levels to the 4I15/2 ground state and gives rise to the green emission bands at 529 and 552 nm, respectively. Likewise, relaxation from 4S3/2 to 4F9/2 and consequent de-excitation gives the red emission band at ~ 662 which is relatively weak due to the reduced possibility of transitions between 4S3/2 and 4F9/2 levels as the energy gap is large [18, 19, 41].
The Er3+ ions at the excited 2H11/2 or the 4S3/2 levels can get further excited to 4G7/2 by absorbing energy transferred from Yb3+ ions and via multiphonon non-radiative relaxation populate the 4G11/2 and 2H9/2 levels. Moreover, as the 4G11/2 level can also get populated by cross-relaxation: (Er3+) 4F9/2 + (Er3+) 2H11/2 → (Er3+) 4I13/2 + (Er3+) 4G11/2, the UC emissions at ~ 380 nm and ~ 410 are observed [46,47,48,49]. Cooperative luminescence from simultaneous radiative de-excitation of the Yb3+ ions from the virtual level V leads to the weak UC emission at ~ 495 nm in the blue band as explained in earlier reported studies [34,35,36] (Fig. 4a).
Yb3+–(MoO4)2− dimer formation
It is noted from Fig. 6 that an increasing Yb3+ concentration induces a selective increase in the intensity of the green band (I529) by nearly 36 times at the optimum Yb3+ content, whereas the enhancement in the other UC emission levels is very small. This could be understood by the additional sensitization provided by the Yb3+–(MoO4)2− dimer complex which is complementary and has a greater probability, because of the harmonizing match between the 4F7/2 level of Er3+ion and (|2F7/2, 3T2 >) of the dimer complex. Effectual energy transfer is expected because the high-excited-state-energy-transfer process (HESET) at the (|2F7/2, 3T2 >) state (T–IV) is relatively at a much higher level in comparison with the energy transfer levels (T–I, T–II, and T–III) available from Yb3+ to Er3+ (Fig. 5). As a result, the intensity ratio (I529 + I552)/I529 + I552 + I662)% tends to increase and reach a saturation at higher Yb3+ content (y = 0.09) indicating that Er/Yb-codoped SrMoO4 ceramics are essentially green phosphors (Fig. 6). The HESET from the |2F7/2, 3T2> state of the Yb3+–(MoO4)2− dimer effectively reduces the losses due to the lattice phonon quenching process and reinforces the UC green emission. These observations are in agreement with earlier studies on Er/Yb-codoped molybdates [16, 18], and Mo6+-doped oxides (TiO2, ZnO, Yb3Al5O12) which have highlighted the selective increase in the green emission due to additional sensitization effect arising from Yb3+–MoO42− dimer to Er3+ ions [50, 51]. In one of our recent papers, the selective enhancement in the green upconversion luminescence seen for Yb3+ concentration of y = 0.03 has been explained to originate directly from [Yb3+–MoO4]2− dimer complex and correlates with the increased degree of lattice distortion occurring at an optimum concentration of Yb3+ dopant concentration as evidenced through the changes in the lattice parameters and the unit-cell volume changes presented in Table 1 [52].
Pump power dependence
The Sr0.96Er0.01Yb0.03MoO4 composition with optimum concentration of Yb3+ ion (y = 0.03) was examined further at different power levels as shown (Fig. 7a, b). The upconverted intensity (\(I_{{{\text{uc}}}} )\) in all the observed emission bands increased with increasing pump power (P) (350–600 mW), and the number of incident photons absorbed in the light upconversion process is estimated from the following relation [53]:
The slope determined from the linear fit of the ln(I) versus ln(P) plots (inset of Fig. 7b) for each one of the UC emissions at ~ 380, ~ 410, ~ 495, ~ 529, ~ 552, and ~ 662 nm is found to be ~ 3.12, ~ 2.76, ~ 1.99, ~ 2.31, ~ 1.94, and ~ 1.96, respectively. A two-photon process is inferred for the green and red emission bands, and a three-photon process for the observed emission at ~ 380 and ~ 410 nm, as foreseen from the energy transfer process discussed in Fig. 5.
Decay curve analysis
Figure 8 compares the normalized decay profiles for the 4S3/2 → 4I15/2 transition (552 nm) transition in Er3+ doped (y = 0.00) and the Er3+ + Yb3+-codoped (y = 0.03) compositions under 380 nm excitation. Decay profile for y = 0 shows a good fit for the single exponential function; however, for y = 0.03 a close fit is not obtained, and the slight departure from the exponential behavior is observed for all other compositions with increasing Yb3+ content [54], and the respective decay profiles for all the other compositions are shown in Fig.S1 in the supplementary file. The time dependence of the emission intensity \(I(t)\) is expressed as [16]:
where A1 is a fitting parameter and Io is the offset parameter, and τ1 is an exponential component of lifetime.
UC emission intensity (λem = 552 nm) decay profiles for two different Sr1-x–y ErxYbyMoO4 compositions having fixed Er3+ (x = 0.01) and a varying Yb3+(y = 0.00 and 0.03) content. Measurements at room temperature under λex = 380 nm. Inset: Variation in the calculated lifetime for Sr1-x–y ErxYbyMoO4 compositions with varying Yb3+ (y) content
The estimated lifetime of Er3+ corresponding to 4S3/2 → 4I15/2 (552 nm) transition increases from 0.117 to 0.265 ms (inset of Fig. 8) for a change in Yb3+ content from y = 0 to 0.09 and suggests an increase of radiative emission. As the interatomic distance between the dopant species reduces with increasing Yb3+ concentration, the energy transfer is expedited more efficiently from Yb3+ to Er3+ [16, 55].
Noninvasive temperature sensing
The fluorescence intensity ratio method is used for studying the optimized Sr0.96Er0.01Yb0.03MoO4 composition by comparing the emission intensity of the thermally interacting (I529 & I552) levels. The intensity ratio between the thermally coupled electronic levels (FIRTCEL) is expressed as [11, 24, 56]:
where the intensities \({\mathrm{I}}_{529}\) and \({\mathrm{I}}_{552}\) relate with the respective 2H11/2 → 4I15/2 (~529 nm) and 4S3/2 → 4I15/2 (~ 552 nm) transitions, \(\Delta \mathrm{E}\) is the energy difference connecting the two thermally coupled levels, T, k, and D correspond to absolute temperature, Boltzmann constant, and an offset parameter, respectively. The pre-exponential coefficient C depends upon the degeneracy factor of the two levels and the relative probability of the transitions.
Using Eq. (5), a linear fit can be obtained between ln(FIR) versus (1/T) and the obtained thermometric parameter is used to determine the absolute and relative sensitivity, respectively. The temperature dependence of absolute sensitivity (Sa) was determined in accordance with relation defined commonly in the published literature [6, 57, 58]:
Alternately, the relative or comparative sensitivity (Sr) which is considered to be more informative for non-contact thermometry is defined as [6, 59]:
A comparison of the thermometric properties for the optimized phosphor composition in different forms (calcined + post-annealed powder (Fig. S2) and sintered ceramic pellets (Fig. S3)) shows a much higher sensitivity value for the sintered ceramic (Fig. 9a–d) in comparison with the powders as shown in Table 2,
a UC emission spectra recorded at different temperatures for optimum composition Sr1-x-yErxYbyMoO4: x = 0.01/y = 0.03 under 980 nm excitation; b temperature dependence of fluorescence intensity ratio FIR(I529/I552) for Sr0.96Er0.01Yb0.03MoO4 ceramic; c Ln (FIR) vs. temperature (T); d variation in the absolute (Sa) and relative (Sr) sensitivity with temperature for Sr0.96Er0.01Yb0.03MoO4 ceramic
In the present study, the impact of Yb3+ concentration has been studied on the thermometric properties of ceramic with varying Yb3+ content as shown in Fig. S3 (a-g) supplementary information, and the results are summarized in Table 3. It is noted that all the three compositions with different Yb3+ dopant concentrations show the maximum sensitivity around the same temperature 473 K, and an optimum content of Yb3+ ( y = 0.03) yields the maximum sensitivity.
The calculated sensitivity values are considered correct when the error \(\delta\) corresponding to the difference between \(\Delta E_{f}\) and \(\Delta E_{m}\) is low enough [25]. The energy difference \(\Delta E_{f}\) is calculated from fitting parameter \({\raise0.7ex\hbox{${\Delta E}$} \!\mathord{\left/ {\vphantom {{\Delta E} k}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$k$}}\) obtained from the linear fit of ln(FIR) versus (1/T) (Fig. 9c), and \(\Delta E_{m}\) is the energy difference obtained from the deconvolution of the UC emission spectra between the 2H11/2 and 4S3/2 levels. The values of \(\Delta E_{f}\), \(\Delta E_{m}\) and \(\delta = \frac{{\left| {\Delta E_{f} - \Delta E_{m} } \right|}}{{\Delta E_{m} }} \times 100\%\) for the three ceramics with varying Yb3+ content are given in Table 3. It is noted that an optimum Yb3+ content indicates the high values of C that corresponds to the high temperature sensitivity, and the low enough \(\delta\) value indicates the correctness of the determined sensitivity values.
Temperature sensing stability and repeatability
Since the FIR versus temperature data serve as the master data for calibration, it needs to be carefully measured by collecting much finer data at shorter temperature intervals and also demands special care towards the stabilization of the ceramic surface temperature before recording the upconversion luminescence for calculating the fluorescence intensity ratio (FIR). FIR values at different temperatures were determined at temperature intervals of 10 K, and the sample was stabilized for 7 min before recording the UC luminescence spectra (Fig. 9a) and it is presented in Fig. 9b.
The FIR value increased from 0.00224 to 5.78533 with increasing temperature in the range 93–773 K. The calculated FIR when plotted over the entire temperature range (93–773 K) revealed an “S-type” plot in the FIR vs. temperature T(K) graph (Fig. 9b) and is similar to (thermocouple voltage vs. temperature) plots obtained for standard thermocouple junctions (e.g., Type-J thermocouple) as shown in Fig. 9b. Such (S-type) variation has not been discussed so far in the reported literature due to the limited temperature range in the reported studies [11, 12, 24, 25]. The present observation of (S-type plot) in fact suggests that much more rigorous experimental data need to be examined in future studies with repeated measurements to establish a rich database for carrying out a reliable mathematical treatment and establish a good calibration curve. Alternately, the phosphor material composition also needs further improvisation to achieve a good linearization over a much wider temperature range for practical applications. In the present study, we have limited our analysis and utilized only that portion of (FIR vs. Temp.) plot which exhibits good linearization as shown in Fig. 9c and extends from 203 to 403 K.
In order to evaluate the temperature sensing performance and the stability, two observation points (273 and 373 K) were selected for the calibration study which correspond to the freezing and boiling temperatures of water in accordance with the standard practice followed for carrying out temperature sensor calibration.
The ceramic sample (0.5 mm thick) was fixed using a heat conducting grease (No.73174, Anton Paar, Germany) onto a temperature-controlled silver block in our temperature controller module which provides a temperature stability of < 0.1 °C. Upconversion emission spectra were logged at intervals of 1 min for an extended duration of ~ 100 mins, and the distribution in calculated FIR values is shown in Fig. 10a, c separately for the two temperatures (273 and 373 K). The temperature calibration curve in Fig. 9c was used to convert the FIR data into temperature as shown in Fig. 10b, d. The deviation in the measured temperature (ΔT) from FIR data and the holding temperature of the sample as maintained by the temperature controller is shown in Fig. 11a, b, respectively. The measurement accuracy (ΔT) at the two selected temperatures 273 and 373 K is found to be within − 0.2 to + 0.2 K with a standard deviation SD = 0.107 and 0.130 K respectively as shown in the histograms (Fig. 11b, d) and corresponds to the measurement uncertainty.
The reversibility test with heating and cooling cycles was driven at a rate of 100 °C/min, by cycling ceramic sensor temperature between 150 and 523 K as shown in Fig. 12. The FIR values remain unchanged, and the calculated relative standard deviation (RSD) values are found to be 1.362% and 0.73% at 150 K and 583 K, respectively.
The present investigations on the ceramic pellets over a wide temperature are in good agreement with the observations reported by Chai et al. [60] and Du et al. [12] on the high sensitivity of Er/Yb-codoped SrMoO4 and MgWO4 in the range 0.0093–0.0128 K−1. The close agreement in the high sensitivity values reported for Er/Yb: SrMoO4 prepared by different techniques establishes the reproducible performance of SrMoO4 host lattice for high-temperature applications. Therefore, the prepared Er/Yb-doped SrMoO4 ceramic composition may be used in wide range noninvasive temperature sensing applications.
Conclusions
Substitution of Er3+ at the Sr2+ sites in the singly doped SrMoO4 composition shows slight lattice expansion, and further addition of increasing Yb3+ content decreases the unit-cell volume and density. Sintered ceramics due to enhanced grain growth show superior performance over the corresponding phosphors in the powder form. An optimum Yb3+ content (y = 0.03) shows improved properties. An alternate energy transfer mechanism relating to Yb3+–(MoO4)2− dimer formation to Er3+ is seen to assist the selective increase in the UC green emission intensity. The luminescence quenching is found to occur beyond a critical Yb3+ content of y = 0.03. The occurrence of weak UC emission at ~ 380 and ~ 410 nm is confirmed in the codoped Er/Yb: SrMoO4 system due to a 3-photon process. The energy transfer between Yb3+ and Er3+ ions occurring due to dipole–dipole interaction is substantiated through Dexter’s energy transfer formula. The temperature sensing by fluorescence intensity ratio method shows a maximum sensitivity (0.0139 K−1) at 473 K for the optimized Sr0.96Er0.01Yb0.03MoO4 ceramic composition. Good linearity and high sensitivity are seen in the temperature range (203–403 K) with a measurement accuracy of − 0.2 to + 0.2 K, and thermometric performance shows good repeatability.
References
Runowski M, Bartkowiak A, Majewska M, Martin IR, Lis S (2018) Upconverting lanthanide doped fluoride NaLuF4:Yb3+-Er3+-Ho3+-optical sensor for multi-range fluorescence intensity ratio (FIR) thermometry in visible and NIR regions. J Lumin 201:104–109. https://doi.org/10.1016/j.jlumin.2018.04.040
Sinha S, Mahata MK, Swart HC, Kumar A, Kumar K (2017) Enhancement of upconversion, temperature sensing and cathodoluminescence in the K+/Na+ compensated CaMoO4:Er3+/Yb3+ nanophosphor. New J Chem 41:5362–5372. https://doi.org/10.1039/c7nj00086c
Hernández-Rodriguez MA, Lozano-Gorrín AD, Lavín V, Rodríguez-Mendoza UR, Martin IR, Manjón FJ (2018) Analysis of the upconversion emission of yttrium orthoaluminate nano-perovskite co-doped with Er3+/Yb3+ ions for thermal sensing applications. J Lumin 202:316–321. https://doi.org/10.1016/j.jlumin.2018.05.078
Lim CS, Aleksandrovsky A, Molokeev M, Oreshonkov A, Atuchin V (2015) Microwave sol-gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4: Er3+/Yb3+ phosphors with incommensurately modulated structure. J Solid State Chem 228:160–166. https://doi.org/10.1016/j.jssc.2015.04.032
Savchuk OA, Carvajal JJ, Brites CDS, Carlos LD, Aguilo M, Diaz F (2018) Upconversion thermometry: a new tool to measure the thermal resistance of nanoparticles. Nanoscale 10:6602–6610. https://doi.org/10.1039/c7nr08758f
Wang X, Liu Q, Bu Y, Liu CS, Liu T, Yan X (2015) Optical temperature sensing of rare-earth ion doped phosphors. RSC Adv 5:86219–86236. https://doi.org/10.1039/c5ra16986k
Senapati S, Nanda KK (2017) Red emitting Eu: ZnO nanorods for highly sensitive fluorescence intensity ratio based optical thermometry. J Mater Chem C 5:1074–1082. https://doi.org/10.1039/c6tc04296a
Zhao Y, Wang X, Zhang Y, Li Y, Yao X (2020) Optical temperature sensing of up-conversion luminescent materials: fundamentals and progress. J Alloys Compd 817:152691. https://doi.org/10.1016/j.jallcom.2019.152691
Du P, Luo L, Yu JS (2018) Energy back transfer induced colour controllable upconversion emissions in La2MoO6:Er3+/Yb3+ nanocrystals for versatile applications. Part Part Syst Char. https://doi.org/10.1002/ppsc.201700416
Zhang J, Hao Z, Li J, Zhang X, Luo Y, Pan G (2015) Observation of efficient population of the red-emitting state from the green state by non-multiphonon relaxation in the Er3+-Yb3+ system. Light Sci Appl 4:e239. https://doi.org/10.1038/lsa.2015.12
Pandey A, Rai VK, Kumar V, Kumar V, Swart HC (2015) Upconversion based temperature sensing ability of Er3+-Yb3+codoped SrWO4: an optical heating phosphor. Sens Actuators B Chem 209:352–358. https://doi.org/10.1016/j.snb.2014.11.126
Du P, Luo L, Yu JS (2015) Infrared-to-visible upconversion emission of Er3+/Yb3+-codoped SrMoO4 phosphors as wide-range temperature sensor. Curr Appl Phys 15:1576–1579. https://doi.org/10.1016/j.cap.2015.09.013
Zhang K, Tong L, Ma Y, Wang J, Xia Z, Han Y (2019) Modulated up-conversion luminescence and low-temperature sensing of Gd3Ga5O12:Yb3+/Er3+ by incorporation of Fe3+ ions. J Alloys Compd 781:467–472. https://doi.org/10.1016/j.jallcom.2018.12.147
Chung JH, Ryu JH, Eun JW, Lee JH, Lee SY, Heo TH, Choi BG, Shim KB (2012) Green upconversion luminescence from poly-crystalline Yb3+, Er3+ Co-doped CaMoO4. J Alloys Compd 522:30–34. https://doi.org/10.1016/j.jallcom.2012.01.059
Zhuang R, Wang G (2016) Sequential energy transfer up-conversion process in Yb3+/Er3+: SrMoO4 crystal. Opt Express 24:7543–7557. https://doi.org/10.1364/oe.24.007543
Li D, Huang Z, Nie Z, Zhang L, Bai Y, Zhang X, Song Y, Wang Y (2015) Anomalous upconversion luminescence of SrMoO4:Yb3+/Er3+ nanocrystals by high excited state energy transfer. J Alloys Compd 650:799–804. https://doi.org/10.1016/j.jallcom.2015.07.005
Li D, Wang Y, Zhang X, Shi G, Liu G, Song Y (2013) White upconversion emission in Yb3+/Tm3+/Ho3+ doped SrMoO4 nanocrystals by high excited state energy transfer. J Alloys Compd 550:509–513. https://doi.org/10.1016/j.jallcom.2012.10.142
Sun W, Chen Z, Zhou J, Li D, Huang Z, Jin X, Zhang Q, Li F, Li Q (2016) Ytterbium-erbium ion doped strontium molybdate (SrMoO4): synthesis, characterization, photophysical properties and application in solar cells. Phys Chem Chem Phys 18:33320–33328. https://doi.org/10.1039/c6cp06571f
Lim CS (2012) Cyclic MAM synthesis and UC photoluminescence of SrMoO4:Er3+ /Yb3+ nano-particles. J Ceram Process Res 13:565–570
Huang F, Gao Y, Zhou J, Xu J, Wang Y (2015) Yb3+/Er3+ co-doped CaMoO4: a promising green upconversion phosphor for optical temperature sensing. J Alloys Compd 639:325–329. https://doi.org/10.1016/j.jallcom.2015.02.228
Cheng X, Yang K, Wang J, Yang L, Cheng X (2016) Up-conversion luminescence and optical temperature sensing behaviour of Yb3+/Er3+ codoped CaWO4 material. Opt Mater 58:449–453. https://doi.org/10.1016/j.optmat.2016.06.029
Kaewnuam E, Wantana N, Saikaew S, Kaewkhao J (2020) The luminescence, optical, and structural properties of SrMoO4:Ce3+ phosphor for photonics material applications. J Met Mater Miner 30:73–79. https://doi.org/10.14456/jmmm.2020.8
Wang S, Zhang H, Wang T, Lv H, Zou X, Wei Y, Hu W, Su C (2020) Synthesis and luminescence properties of Sm3+ doped molybdate glass ceramic. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2020.153822
Soni AK, Rai VK (2017) Thermal and pump power effect in SrMoO4:Er3+-Yb3+ phosphor for thermometry and optical heating. Chem Phys Lett 667:226–232. https://doi.org/10.1016/j.cplett.2016.12.002
Zhang H, Zhao S, Wang X, Ren X, Ye J, Huang L, Xu S (2019) The enhanced photoluminescence and temperature sensing performance in rare earth doped SrMoO4 phosphors by aliovalent doping: from material design to device applications. J Mat Chem C. https://doi.org/10.1039/c9tc04965g
Hao Y, Lv S, Ma Z, Qiu J (2018) Understanding differences in Er3+-Yb3+ codoped glass and glass ceramic based on upconversion luminescence for optical thermometry. RSC Adv 8:12165–12172. https://doi.org/10.1039/C8RA01245H
Yadav RS, Dhoble SJ, Rai SB (2018) Improved photon upconversion photoluminescence and intrinsic optical bistability from a rare earth co-doped lanthanum oxide phosphor via Bi3+ doping. New J Chem 42:7272–7282
Yadav RS, Kumar D, Singh AK, Ekta R, Rai SB (2018) Effect of Bi3+ ion on up-conversion -based induced optical heating and temperature sensing characteristics in the Er3+/Yb3+ co-doped La2O3 nano-phosphor. RSC Adv 8:34699–34711
Cates SL, Cates EL, Cho M, Kim JH (2014) Synthesis and characterization of visible-to-UVC upconversion antimicrobial ceramics. Environ Sci Technol 48:2290–2297. https://doi.org/10.1021/es405229p
Zambonini F, Levi GR (1925) Richerche sull’isomor- fismo dei molibdati dei metalli delle terre rare con quello del calcio, dello stronzio, del bario e del piombo. II. Struttura dei molibdati di Ca, Sr, Ba, Pb, Rend, accad. Lincei 2:225–230
Swanson HE, Gilfrich NT, Marlene I (1957) Standard X-ray Diffraction Powder Patterns. Cook National Bureau of Standards Circular 539:50–51
Barrett CS, Massalski TB (1966) Structure of metals: crystallographic methods, principles, and data, 3rd edn. McGraw-Hill series in materials science and engineering. McGraw-Hill, New York
Yang J, Fei Y, Hu X, Greenberg E, Prakapenka VB (2019) Effect of carbon on the volume of solid iron at high pressure: implications for carbon substitution in iron structures and carbon content in the Earth’s inner core. Minerals. https://doi.org/10.3390/min9120720
Schaudel B, Goldner P, Prassas M, Auzel F (2000) Cooperative luminescence as a probe of clustering in Yb3+ doped glasses. J Alloys Compd 300:443–449. https://doi.org/10.1016/S0925-8388(99)00760-4
Nakazawa E, Shinoya S (1970) Cooperative luminescence in YbPO4. Phys Rev Lett 25:1710–1712. https://doi.org/10.1103/PhysRevLett.25.1710
Guan Y, Huang Y, Jin Seo H (2012) The blue cooperative up-conversion luminescence in Ca9Yb[VO4]7 ceramic. Mater Lett 89(2012):126–128. https://doi.org/10.1016/j.matlet.2012.08.056
Qin WP, Liu ZY, Sin CN, Wu CF, Qin GS, Chen Z, Zheng KZ (2014) Multi-ion cooperative processes in Yb3+ clusters. Light Sci Appl 3:e193. https://doi.org/10.1038/lsa.2014.74
Dong H, Sun LD, Yan CH (2013) Basic understanding of the lanthanide related upconversion emissions. Nanoscale 5:5703–5714. https://doi.org/10.1039/c3nr34069d
Auzel F (2004) Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev 104:139–173. https://doi.org/10.1021/cr020357g
Lim CS, Aleksandrovsky AS, Atuchin V, v., Molokeev M S, Oreshonkov A S, (2020) Microwave sol-gel synthesis, microstructural and spectroscopic properties of scheelite-type ternary molybdate upconversion phosphor NaPbLa(MoO4)3:Er3+/Yb3+. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2019.152095
Bokolia R, Thakur OP, Rai VK, Sharma SK, Sreenivas K (2016) Electrical properties and light up conversion effects in Bi3.79Er0.03Yb0.18Ti3-xWxO12 ferroelectric ceramics. Ceram Int 42:5718–5730. https://doi.org/10.1016/j.ceramint.2015.12.103
Blasse G, Grabmaier B C (1994), A General Introduction to Luminescent materials. In: Luminescent Materials. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-79017-1_1
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836–850. https://doi.org/10.1063/1.1699044
Dexter DL, Schulman JH (1954) Theory of concentration quenching in inorganic phosphors. J Chem Phys 22:1063–1070. https://doi.org/10.1063/1.1740265
Tu L, Liu X, Wu F, Zhang H (2015) Excitation energy migration dynamics in upconversion nanomaterials. Chem Soc Rev 44:1331–1345. https://doi.org/10.1039/c4cs00168k
Sinha S, Mahata MK, Kumar K, Tiwari SP, Rai VK (2017) Dualistic temperature sensing in Er3 +/Yb3 + doped CaMoO4 upconversion phosphor, spectrochim. Acta A 173:369–375. https://doi.org/10.1016/j.saa.2016.09.039
Singh BP, Parchur AK, Singh RK, Ansari AA, Singh P, Rai SB (2013) Structural and up-conversion properties of Er 3+ and Yb3+ co-doped Y2 Ti2 O7 phosphors. Phys Chem Chem Phys 15:3480–3489. https://doi.org/10.1039/c2cp44195k
Dong H, Sun LD, Yan CH (2015) Energy transfer in lanthanide upconversion studies for extended optical applications. Chem Soc Rev 44(2015):1608–1634. https://doi.org/10.1039/c4cs00188e
Mukhopadhyay L, Rai VK, Bokolia R, Sreenivas K (2017) 980 nm excited Er3+/Yb3+/Li+/Ba2+: NaZnPO4 upconverting phosphors in optical thermometry. J Lumin 187:368–377. https://doi.org/10.1016/j.jlumin.2017.03.035
He YY, Liu XL, Cao BS, Feng ZQ, Dong B (2013) A general approach for selective enhancement of green upconversion emissions in Er3+ doped oxides by Yb3+-MoO42- dimer sensitizing. J Sol-Gel Sci Technol 66:312–316. https://doi.org/10.1007/s10971-013-3010-0
Dong B, Cao B, He Y, Liu Z, Li Z, Feng Z (2012) Temperature sensing and in vivo imaging by molybdenum sensitized visible upconversion luminescence of rare-earth oxides Adv. Mater 24:1987–1993. https://doi.org/10.1002/adma.201200431
Shandilya A, Yadav RS, Gupta AK, Sreenivas K (2021) Effects of Yb3+ ion doping on lattice distortion, optical absorption and light upconversion in Er3+/Yb3+ co-doped SrMoO4 ceramics. Matr Chem Phys 264:124441. https://doi.org/10.1016/j.matchemphys.2021.124441
Pollnau M, Gamelin DR, Lü SR, Gü HU, Hehlen MP (2000) Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys Rev B 61:3337–3346. https://doi.org/10.1103/PhysRevB.61.3337
Desirena H, de La Rosa E, Díaz-Torres LA, Kumar GA (2006) Concentration effect of Er3+ ion on the spectroscopic properties of Er3+ and Yb3+/Er3+ co-doped phosphate glasses. Opt Mater 28:560–568. https://doi.org/10.1016/j.optmat.2005.04.002
Kang X, Lü W, Wang H, Ling D (2019) Multicolor-tunable up-conversion emissions of Yb3+, Er3+/Ho3+ co-doped Ba3Lu2Zn5O11: crystal structure, luminescence and energy transfer properties. Dalton Trans 48(2019):2917–2925. https://doi.org/10.1039/C8DT04577A
Wade SA, Collins SF, Baxter GW (2003) Fluorescence intensity ratio technique for optical fibre point temperature sensing. J Appl Phys 94:4743–4756. https://doi.org/10.1063/1.1606526
Brites CDS, Lima PP, Silva NJO, Millán A, Amaral VS, Palacio F, Carlos LD (2012) Thermometry at the nanoscale. Nanoscale 4:4799–4829. https://doi.org/10.1039/c2nr30663h
Bokolia R, Mondal M, Rai VK, Sreenivas K (2017) Enhanced infrared-to-visible up-conversion emission and temperature sensitivity in (Er3+, Yb3+, and W6+) tri-doped Bi4Ti3O12 ferroelectric oxide. J Appl Phys 121:084101. https://doi.org/10.1063/1.4977006
Ran W, Noh HM, Park SH, Choi BC, Kim JH, Jeong JH, Shi J (2019) Infrared excited Er3+ /Yb3+ codoped NaLaMgWO6 phosphors with intense green up-conversion luminescence and excellent temperature sensing performance. Dalton Trans 48:11382–11390. https://doi.org/10.1039/c9dt01970g
Chai X, Li J, Zhang Y, Wang X, Li Y, Yao X (2016) Bright dual-mode green emission and temperature sensing properties in Er3+/Yb3+ co-doped MgWO4 phosphor. RSC Adv 6:64072. https://doi.org/10.1039/c6ra09656e
Acknowledgements
The authors wish to thank the University Science Instrumentation Centre at the University of Delhi. One of the authors (Ankur Shandilya) is thankful to the Department of Science and Technology, Ministry of Science and Technology, India (DST), for providing financial assistance through the Inspire Junior Research Fellowship (IF160397) during (2016-2018) and up-gradation to senior research fellowship (SRF) for the period (2019-21).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Till Froemling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shandilya, A., Yadav, R.S., Gupta, A.K. et al. Temperature-dependent light upconversion and thermometric properties of Er3+/Yb3+-codoped SrMoO4 sintered ceramics. J Mater Sci 56, 12716–12731 (2021). https://doi.org/10.1007/s10853-021-06078-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06078-8