Abstract
Although many researches have been made in the construction and fabrication of electrode materials for supercapacitors, it remains a difficult task to realize the application of the supercapacitor with high energy density. The binary MnCo2O4@NiO hierarchical electrode has been successfully fabricated on nickel foam (NF) without any binder or additives by simple hydrothermal methods and corresponding annealing processes. By virtue of constructing hierarchical core–shell structure with different morphologies and forming additive-free electrode, the composite electrode not only possesses abundant surface active sites for electrochemical reaction as well as large buffering space for volume deformation in the charging-discharging processes, but also shows the improved contacting area and electric conductivity between active substance and metal collector. The synthetic MnCo2O4@NiO/NF electrode presented higher specific capacitance (1186 F g−1 under 1 A g−1) and long cycle lifespan (maintain 91% of the original capacitance over 5000 cycles) under a high current density of 10 A g−1. Further, the asymmetric supercapacitor with MnCo2O4@NiO/NF electrode shows higher energy density of 42.2 Wh kg−1 under a power density of 748.2 W kg−1. These results demonstrate that the binary MnCo2O4@NiO hierarchical structure is the hopeful candidate material for high performance supercapacitor electrode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With rapidly developing new forms of clean power such as wind power, tidal power and solar power, energy storage and utilization put forward higher requirements to energy storage devices. Supercapacitor as a novel energy storage device has drawn increasing attention on intelligent power distribution system, transportation and miniaturized electronic devices due to excellent performance such as faster charging and discharging, long cycle lifespan, broad operating temperature range, environment friendly [1,2,3,4]. However, the ordinary energy density of supercapacitors limits further application [5]. For higher energy density, two strategies were proposed to improve energy storage performance according to the energy storage formula [6]. On one hand, employing organic electrolyte with wide voltage range and assembling asymmetric supercapacitors (ASC) can improve the potential window and thus enhance the energy density. Nevertheless, low ionic conductivity and high cost, especially chemical safety, limit the development of organic electrolyte [7]. On the other hand, making use of active materials with high specific capacity is a more direct and efficient way to promote the energy density. Therefore, designing and constructing high specific capacitance electrode materials and assembling ASCs are safe and efficient ways to enhance the energy density of supercapacitor.
Among various potential electrode materials, metal oxide has been extensively studied because of higher specific capacitance than carbon materials and more stable structure than polymer materials [2]. However, the poor conductivity of the mono-metallic oxide prevents the charge from being transferred rapidly in the electrochemical reaction and limits its electrochemical performance [8]. Therefore, a general method is to combine mono-metallic oxides with graphene, carbon nanotubes, reduced graphene oxide and other materials with better conductivity [9]. In addition, bimetallic oxides formed by new metal elements introduced into mono-metallic oxides can not only enhance electrical conductivity but also supply more redox reaction activity [10]. Typically, Co element is introduced into Mn3O4 to form Mn2.6Co0.4O4, the specific capacitance of bimetallic oxides is increased from 250 to 400 mAh g−1 compared with mono-metallic oxides [11]. The increase is attributable to that the presence of mixed valence of Co and Mn enhances the conductivity of Mn3O4 and promotes the electrochemical reaction. In recent studies, polymetallic oxides have become the potential supercapacitor materials due to the higher conductivity than mono-metallic oxides. The binary or ternary metal oxides get an increasing number of researches such as MnCo2O4 [12], ZnCo2O4 [13], CoMn2O4 [14], ZnMn2O4 [15], ZnFe2O4 [16], NiCo2O4 [17], CoMoO4 [18], Mn-Ni-Co ternary oxide [19], etc.
In particular, MnCo2O4 demonstrates excellent electrochemical behavior, because Co possesses high oxidation potential, and Mn assumes more oxidation states. MnCo2O4 is considered as the potential electroactive material because of environmentally friendly, low cost, high theoretical specific capacitance [20, 21]. In addition, MnCo2O4 presents a larger lattice parameter compared with other binary metal oxides, which demonstrates a faster charge-transfer process in the electrode material interface due to the enhanced ion diffusion coefficient [22, 23]. Nonetheless, the lower specific capacitance and inferior structure stability of MnCo2O4 hinder its development as high performance electrode material [24]. A reasonable way is to design the hybrid nanostructure composite materials by combing MnCo2O4 with other electroactive materials of high theoretical specific capacitance. NiO is a potential reinforcement material because of its excellent pseudocapacitance behavior, high theoretical specific capacitance, stable structure and low cost [25]. The NiO-MnCo2O4 porous microspheres fabricated with the one-pot spray demonstrate better electrochemical performance for lithium ion batteries [26]. Further, MnCo2O4@NiO porous nanosheet electrodes have been produced with poly(vinylidene fluoride) (PVDF) binder and acetylene black conductor, which demonstrate a specific capacitance of 508.3 F g−1 under 2 A g−1 and have capacitance retention ratio of 67.7% with the current density improved from 0.5 A g−1 to 6 A g−1 [27]. In spite of realizing some enhanced electrochemical performances of NiO-MnCo2O4 complexes, the agglomeration of electroactive material due to the randomly stacking and irregular distribution is prone to decrease the contacting surface area for conducting electrochemical reaction, which greatly limits efficient utilization of electroactive materials [28]. Besides, NiO grows on MnCo2O4 in a similar morphological structure (two mixed nanoparticles), which may result in low surface area. Thus, this structure greatly weakens the electrochemical performance of MnCo2O4 and NiO composites [29]. Furthermore, the poor combination and low conductivity between electroactive substance and current collector are caused by using PVDF binder, which is a great obstacle to give full play to the performance of active materials [30]. Therefore, designing a composite electrode with better matching structure and no any binder or additives can effectively solve these problems and further enhance the electrochemical properties of electrode materials.
In our work, we successfully prepared NiO nanoflakes decorated needle-like MnCo2O4 hierarchical structure directly deposited on nickle foam (NF) as an additive-free and high performance supercapacitor electrode. The fabrication process of MnCo2O4@NiO/NF electrode is briefly illustrated in Fig. 1. On one hand, one-dimensional needle-like MnCo2O4 not only ensures high conductivity and specific capacitance but also provides sufficient sites for NiO growth on the whole side. On the other hand, NiO flakes grow in a different morphological structure from MnCo2O4 to form the hierarchical structure, which provides abundant surface active sites for Faraday reaction as well as large buffering space for volume deformation in the charging-discharging processes. What’s more, in situ synthesis without additives not only increases the contacting area and electric conductivity between electroactive substance and metal collector, but also supplies plenty of room for transporting electrolyte ions. Then, the electrochemical performance of the MnCo2O4/NF, NiO/NF and MnCo2O4@NiO/NF was compared by using 2 M KOH as the electrolyte. Through electrochemical impedance spectrum and diffusion coefficient analysis, the performance of composite electrode is further understood. Finally, the performance of the composite electrode in practical device is evaluated by assembling ASCs. The results present that the MnCo2O4@NiO/NF electrode is a hopeful electrode material for supercapacitor.
Experimental
Preparation of MnCo2O4/NF electrode
In a typical synthetic process of needle-like MnCo2O4 electrode: firstly, NF (1*2 cm2) was washed with ultrasound in acetone, ethanol and ultrapure water, respectively, and then dried in a vacuum stove. Secondly, 0.8 mmol Mn(NO3)2·4H2O, 1.6 mmol Co(NO3)2·6H2O, 4 mmol urea and 1.6 mmol NH4F were added into 40 ml ultrapure water and then vibrated magnetically for an hour to derive the uniform pink solution. Thirdly, transferring the solution and the clean NF into a 50 mL reactor, which was placed in the oven for a complete reaction of 7 h at 140 °C, and then naturally cooled to ambient temperature. After reaction, the samples were cleaned with ultrapure water about 5 times and subsequently dried in the oven at 60 °C for 12 h. Finally, dried sample was put in a tubular furnace, temperature of tubular furnace was increased from 22 °C to 400 °C in 200 min, keeping 400 °C for 3 h in air atmosphere, then down to room temperature naturally.
Preparation of MnCo2O4@NiO/NF electrode
MnCo2O4@NiO hierarchical structure was further prepared through the approach similar to the MnCo2O4. First, 0.291 g Ni(NO3)2·6H2O and 0.601 g urea were dissolved in 40 ml ultrapure water and then vibrated for half an hour to derive the light green solution. Next, the solution and MnCo2O4/NF were put to 50 mL reactor, which was placed in the oven for 8 h at 100 °C. The sample loaded on NF and the powder precipitation at the bottom of the reactor were cleaned with ultrapure water about 5 times, and subsequently dried in the oven under 60 °C for 12 h. Finally, these samples were placed in the tube furnace, and temperature of tubular furnace was increased from 22 °C to 300 °C in 200 min, keeping 300 °C and anneal 3 h under air atmosphere, then down to room temperature naturally. The mass loading of the MnCo2O4@NiO composites on the NF is about 0.6 mg cm−2.
Characterization of prepared samples
The composition and structure of materials were detected with powder X-ray diffraction (XRD, Bruker D8 ADVANCE). The surface chemical state of materials was studied with X-ray photoelectron spectrometer (XPS, VG ESCA Lab220I-XL). Morphologies of materials were distinguished by scanning electron microscope (SEM, Carl Zeiss SIGMA) and transmission electron microscope (TEM, FEI Tecnai G2 F20).
Electrochemical measurements
All electrochemical tests of the synthesized materials were conducted on electrochemical workstations (CHI 660D, Chenhua, Shanghai). The electrochemical properties of the materials were researched via three-electrode setup which consists of as-fabricated electrode as the work electrode, Pt plate as the counter electrode, the Hg/HgO as the reference electrode and 2 M KOH as the electrolyte.
The performance of ASC device was tested by two-electrode system which is made up of positive electrode of MnCo2O4@NiO/NF, negative electrode of activated carbon (AC) and 2 M KOH as the electrolyte. Due to easy comparison with the results of other researches, AC as the usual negative electrode material was selected for the ASC device. Based on the formula of charge balance theory (details in Supporting Information), the active mass loading of the AC electrode in ASC device could be computed by the voltage range and specific capacitance of the two-electrodes as well as the active mass of positive electrode. Therefore, the active mass loading of the AC electrode in the ASC device is around 3.6 mg cm−2. The AC-based electrode was compounded by the following steps: (1) blending the AC, acetylene black and polytetrafluoroethylene in a weight ratio of 8:1:1 in ethanol; (2) Coating the well-mixed paste to NF substrate; (3) The coated electrode was then dried under 80 °C for 6 h.
Results and discussion
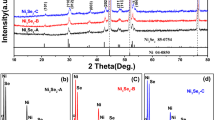
For the sake of determining the structure and composition of the prepared materials, the XRD test of samples was executed. Figure 2 is the XRD curves of three different electrode materials produced during the experiment. The red, black and blue curves are the MnCo2O4 powder of the first step hydrothermal reaction, the NiO powder of the second step hydrothermal reaction and the MnCo2O4@NiO/NF electrode. For the red curve, diffraction peaks appear in 18.5°, 30.5°, 36°, 37.6°, 43.8°, 54.3°, 57.9° and 66.8°, which is consistent with (1 1 1), (2 2 0), (3 1 1), (2 2 2), (4 0 0), (4 2 2), (5 1 1) and (5 3 1) crystal faces of the MnCo2O4 (JCPDS card no. 23–1237). Further, there are no other peaks in the red curve, which proves that the prepared sample is of high purity. Diffraction peaks of the black curve appear in 37.2°, 43.3°, 62.85°, 62.9°, 75.4° and 79.4°, which corresponds to (1 0 1), (0 1 2), (1 1 0), (1 0 4), (1 1 3) and (2 0 2) crystal faces of the NiO (JCPDS card no. 44-1159). The clear peak shape implies that the purity of the fabricated material is relatively high. The XRD result of the MnCo2O4@NiO/NF is presented in the blue curve. It can be seen in the curve that the strength of three peaks at 44.5°, 51.8° and 76.4° is far higher than that of other peaks, which is related to (1 1 1), (2 0 0) and (2 2 0) crystal faces of the Ni (JCPDS card no. 04-0850) in NF. Besides, there are only peaks from the MnCo2O4 and NiO without peaks of impurities and other phases. Therefore, the structure and composition of the MnCo2O4@NiO/NF sample were well confirmed.
In addition to XRD, the XPS is conducted to further understand the chemical state of the sample elements. A wide range of XPS was measured from 0 to 1100 eV, and the result was shown in Fig. 3a. In this figure, the peaks of the five identifiers represent the elements that appear during testing. Among them, C 1 s peak comes from the test environment such as air pollution. The O 1 s, Mn 2p, Co 2p and Ni 2p peaks are derived from the sample. In the Fig. 3b, the peaks at 855.0 eV and 872.1 eV are, respectively, originated from Ni 2p3/2 and Ni 2p1/2, and the peaks at 861.5 eV, and 880.0 eV are satellite peaks [31]. Further, both Ni 2p3/2 and Ni 2p1/2 were divided into two chemical states, which presents the coexistence of chemical states of Ni2+ and Ni3+ in materials [32]. Figure 3c shows four peaks associated with Co element. In particular, 780.4 eV and 795.7 eV are in accordance with Co 2p3/2 and Co 2p1/2 spin orbital peaks, respectively [33]. These two peaks can be fitted with four weak peaks, including 779.8 eV, 780.9 eV, 795.3 eV and 796.6 eV, which imply the presence of valence state of Co3+ and Co2+ [34]. The Mn 2p3/2 and Mn 2p1/2 peaks were observed at 642.5 eV and 653.9 eV in Fig. 3d, respectively. The splitting of each peak declares the Mn element exists in divalent and trivalent state [35]. All of these XPS spectra further confirm that the MnCo2O4 and NiO are present in materials.
For clearly observing the morphology of samples, SEM measurements of prepared samples were conducted, and the results were displayed in Fig. 4. According to Fig. 4a, needle-like MnCo2O4 was successfully grown on the NF substrate, and the appearance is dense and uniform. Through SEM diagram of high magnification in Fig. 4b, it could be found that interlaced needle-like MnCo2O4 grows on the substrate and the diameter of the needle-like MnCo2O4 is about 200 nm. For the powder deposited at the bottom of the reactor, MnCo2O4 agglomerates into a spherical with a diameter of about 10 μm due to the absence of basal support during the first hydrothermal process. And it is clear that the spherical MnCo2O4 presents actually a sea urchin structure formed by the polymerization of a large number of needle-like MnCo2O4 (displayed in Fig. S1 (a,b) of Supporting Information). The low magnification SEM picture of MnCo2O4@NiO is presented as Fig. 4c. The MnCo2O4@NiO with hierarchical structure is independent of each other, and there is no obvious collapse and agglomeration. In Fig. 4d, high magnification SEM image of MnCo2O4@NiO suggests that the NiO flakes grow uniformly and densely on the surface of MnCo2O4 skeleton and completely envelop the needle-like MnCo2O4. Moreover, the NiO flakes are interconnected with each other to form a continuous open network. After the growth of NiO, the diameter of the whole MnCo2O4@NiO hierarchical structure becomes about 1.2 μm. For the powder deposited at the bottom of the reactor, NiO aggregates into balls of different sizes during the second hydrothermal process. Furthermore, the ball consists of many NiO flakes agglomerated into a hydrangea shape (shown in Fig. S1 (c,d) of Supporting Information). To further confirm our samples, we selected part of the MnCo2O4@NiO for energy dispersive spectrometer (EDS), The selected region and EDS results are shown in Fig. 4e, f, respectively. The EDS demonstrates that Co, Mn, Ni and O elements are present in the prepared core–shell structure. These elements uniformly cover the entire selected area, and the proportion of oxygen atoms is significantly higher than that of other elements, which is consistent with a higher proportion of oxygen in the MnCo2O4@NiO. The SEM images show that the synthesized material has a hierarchical structure.
The detailed structure characteristics of the samples were further identified by TEM. The darker central region and the lighter peripheral region can be clearly distinguished in a low resolution TEM (Fig. 5a). The darker central region has a pronounced needle-like boundary, which is corresponding to the needle-like MnCo2O4. On the contrary, the boundaries of the lighter peripheral region are irregular, which can be understood as the overlapping result of NiO flakes in the lateral perspective. The high resolution TEM of the region marked by b and c in Fig. 5a is clearly displayed in Fig. 5b, c. Figure 5b shows crystalline interplanar distance of 0.29 nm and 0.25 nm, which is related to the (220) and (311) crystalline interplanar distance of MnCo2O4, respectively, and confirmed further by the selected area electron diffraction (SAED) pattern (Fig. S2 (b) of Supporting Information). Figure 5c presents the crystalline interplanar distance of 0.21 nm, which is corresponding to the (012) crystalline interplanar distance of NiO. The NiO nanoflakes display the polycrystalline structure, as proved by the related SAED pattern (Fig. S2 (c) of Supporting Information). The structural characteristics obtained by TEM well confirm the XRD results.
The electrochemical performance of the prepared materials was evaluated by the three-electrode setup with 2 M KOH aqueous solution as the electrolyte, Pt foil as the counter electrode and Hg/HgO electrode as the reference electrode. Figure 6a is the CV plots acquired by testing the MnCo2O4@NiO/NF electrode under the scan rate of 2–100 mV s−1. On the basis of CV plots, there are a couple of evident redox peaks, and the CV plot deviates from the rectangle, which reveals the capacitance is primarily caused by the Faraday reaction [36]. At the lower scan rates, the upward and downward peaks were related to the oxidation and reduction processes, respectively, which is connected with the following faradaic redox equations [37, 38]:
a CV plots of MnCo2O4@NiO/NF under various scan rates. b GCD plots of MnCo2O4@NiO/NF under various current densities. c CV plots at the scan rate of 50 mV s−1. d GCD plots under the current density of 1 A g−1. e Specific capacitances under various current densities. f Cycle performance of NiO/NF, MnCo2O4/NF and MnCo2O4@NiO/NF electrodes under 10 A g−1
As the scan rate increases, the response current and the integral area of CV plot improve gradually because of the rapid conduction of ions and electrons, which implies the ideal capacitive behavior and good rate capability [39]. Furthermore, the oxidation peak shift toward the positive side, and reduction peak shift toward the negative side because of the increasing internal diffusion resistance in the pseudocapacitance material with the improving scan rate [40]. Figure 6b displays the GCD plots under different current densities. The tendency is similar to a regular triangle except for the platform that corresponding to the redox reaction. The discharging time becomes shorter and shorter with the increasing of current density. On the basis of formula:
The specific capacitance under various current densities could be computed. Where, I (A), t (s), m (g) and V (V) are the magnitude of current in the galvanostatic charging-discharging process, the mass of the active substance, the discharging time and the potential window in the charging and discharging process, respectively. The MnCo2O4@NiO/NF electrode displays high specific capacitance of 1186, 1112, 1062, 970, 912 and 880 F g−1 under 1, 2, 3, 5, 8 and 10 A g−1, respectively. We compared the CV plots of MnCo2O4@NiO/NF, MnCo2O4/NF and NiO/NF electrodes under 50 mV s−1, as represented in Fig. 6c. Under the same scan rate, the integral region of the MnCo2O4@NiO/NF composite electrode is broader than that of the individual MnCo2O4/NF and single NiO/NF electrodes, which demonstrates that the MnCo2O4@NiO/NF electrode has higher capacitance than MnCo2O4/NF and NiO/NF electrodes. Figure 6d represents the GCD plots of MnCo2O4@NiO/NF, MnCo2O4/NF and NiO/NF electrodes under 1 A g−1. For the same current density, the MnCo2O4@NiO/NF composite electrode presents more discharge time than the MnCo2O4/NF and NiO/NF electrodes. By the calculation, the specific capacitance of MnCo2O4@NiO/NF composite electrode (1186 F g−1) is larger than that of MnCo2O4/NF electrode (607 F g−1) and NiO/NF electrode (350 F g−1) (the detailed electrochemical performance of the MnCo2O4/NF and NiO/NF electrodes are displayed in Fig. S3 and Table S1 of Supporting Information).
The rate capability of the three electrodes was further calculated as shown in the Fig. 6e. On one hand, it is clearly seen that the specific capacitance of MnCo2O4@NiO/NF electrode is higher than that of the MnCo2O4/NF and NiO/NF electrodes under the current density ranges from 1 to 10 A g−1. On the other hand, the composite electrode displays excellent rate capability over a single material. While the current density augments from 1 A g−1 to 10 A g−1, the capacitance retention ratio of the MnCo2O4@NiO/NF electrode is 74%, which is better than that of MnCo2O4/NF electrode (50%) and NiO/NF electrode (20%). The cycle ability of the electrode is a key standard to determine whether the electrode performance is reliable. Therefore, MnCo2O4@NiO/NF, MnCo2O4/NF and NiO/NF electrode materials were measured over 5000 cycles under 10 A g−1, as displayed in Fig. 6f. The cycle curve of NiO/NF electrode is kept flat during the whole cycle and shows good cycling stability. However, its specific capacity is only about 70 F g−1. It can be found from the cycle plot of the MnCo2O4/NF electrode that the capacitance value of the electrode augments in the initial 500 cycles, which is due to the activating process of the electrode material at the beginning of the cycle test. In the subsequent cycles, the capacitance value of the electrode remains basically stable. After 5000 cycles, the specific capacity decreased to 308 F g−1, which is approximately 85% of the original specific capacitance. For the cycle plot of MnCo2O4@NiO/NF electrode, its specific capacitance value remains approximately 91% of the original capacitance value over 5000 cycles, which proves that the MnCo2O4@NiO/NF electrode has better cyclic stability than the MnCo2O4/NF electrode. Therefore, compared with MnCo2O4/NF and NiO/NF electrodes, the MnCo2O4@NiO electrode has significantly improved electrochemical performance. In addition, the MnCo2O4@NiO/NF electrode exhibited superior specific capacitance, compared with most of MnCo2O4- and NiO-based supercapacitor electrode materials reported previously (as illustrated in Table S2 of Supporting Information) such as MnCo2O4@NiO porous nanosheets (604.5 F g−1 under 0.5 A g−1) [27], MnCo2O4 nanorods and microcubes (408.33 F g−1 under 5 A g−1) [41], MnCo2O4 nanorods (845.6 F g−1 under 1 A g−1) [42], MnCo2O4@N-CNF meso-microporous structure (871.5 F g−1 under 0.5 A g−1) [43], MnCo2O4@MnO2 (858 F g−1 under 1 A g−1) [44] and CNT@NiO (996 F g−1 under 1 A g−1) [45].
For further analysis of the electrochemical behaviors of the electrodes, electrochemical impedance spectroscopy (EIS) of the three samples was detected under the frequency interval of 0.01–100 kHz and 5 mV amplitude, as displayed in Fig. 7. At low frequencies, an approximately vertical line indicates the typical capacitive behavior. In the higher frequency zone, the intercept of the real axis and semicircular radius of Nyquist plots are connected with the series resistance (Rs) and charge-transferring resistance (Rct), respectively [46]. Further, the simulation of Z-view software is used to quantitatively fit the equivalent resistance Rs and Rct. The equivalent circuit is shown in the illustration, in which the Zw and Cdl correspond to Warburg impedance and the double layer capacitance, respectively. The Rs of MnCo2O4@NiO/NF, MnCo2O4/NF and NiO/NF electrodes was fitted to be 0.15 Ω, 0.21 Ω and 0.94 Ω, respectively. Meanwhile, the Rct of MnCo2O4@NiO/NF, MnCo2O4/NF and NiO/NF electrodes was determined to be 1.20 Ω, 2.21 Ω and 7.20 Ω, respectively. The MnCo2O4@NiO/NF electrode exhibits lower series resistance and charge-transfer resistance than MnCo2O4/NF and NiO/NF electrodes. The lower resistance is due to that the better designed hierarchical structure with needle-like MnCo2O4 and NiO flakes effectively shortened the distance of ion diffusion path [29]. Consequently, the diffusion and migration of ions and electrons are greatly promoted during the fast charging-discharging process, which helps to understand the better electrochemical properties of MnCo2O4@NiO/NF electrode than MnCo2O4/NF and NiO/NF electrodes. After 5000 cycles, as shown in Fig. S4 of Supporting Information, the Rs and Rct of MnCo2O4@NiO/NF electrode increase a little to 0.17 Ω and 1.67 Ω, respectively, which further demonstrates a small resistance change and superior cyclic stability.
From another point of view, we also fitted the peak current of the sample under various sweep rates with the square root of the sweep rate, as displayed in Fig. 8. The three straight lines in fitting results show that the redox reaction in the electrode material is the diffusion-dominated process. The diffusion coefficient of OH− in the three electrochemical processes can be calculated by Randles–Sevcik equation. The equation is described as follows [47]:
where ip (A), A (cm2), v (V/s), D (cm2 s−1) and C are the peak current, the area electrode, the sweep rate, the diffusion coefficient and the concentration of OH− in the solution, respectively. n is the number of electrons transferred in the electrochemical process, which could be determined by the two equations mentioned above (Eq. (1) and (2)). The ratio of diffusion coefficient between MnCo2O4@NiO and MnCo2O4 is calculated as:
The results show that OH− has a high diffusion coefficient in the MnCo2O4@NiO composite electrode. In particular, NiO exhibits the lowest diffusion coefficient due to the coating process. The results of diffusion coefficient analysis are consistent with the impedance analysis that the MnCo2O4@NiO/NF electrode exhibits lower charge-transfer resistance. The MnCo2O4@NiO/NF electrode has a higher diffusion coefficient, suggesting that there is weaker ion transport obstruction during the reaction. All of these are contributed to a better understanding of the superior electrochemical performance of MnCo2O4@NiO/NF composite electrode.
For the sake of exploring the potential application of MnCo2O4@NiO electrode materials in supercapacitor devices, ASC device based on MnCo2O4@NiO/NF electrode and AC/NF electrode is assembled as displayed in the Fig. 9a. In this ASC device, fiberglass filter paper and 2 M KOH are used for the membrane and electrolyte, respectively. Subsequent measurements of the electrochemical properties of the ASC devices are based on this configuration. It is necessary to confirm the proper working potential range for studying the properties of the device. Figure 9b exhibited the CV plots under 5 mV s−1 of AC/NF electrode in the potential window of −1 V–0 V and MnCo2O4@NiO/NF electrode in the voltage window of 0 V–0.5 V. The CV plots imply that two-electrode materials work stably within their test voltage range. Based on this result, the stable operating potential range of the ASC device could be expanded to the aggregation of the positive and negative electrode voltage ranges (1.5 V).
a Assembly structure diagram of MnCo2O4@NiO/NF-based ASC device. b CV measurements for the positive and negative electrodes under 5 mV s−1. c CV plots of the ASC device under various scan rates. d GCD plots under various current densities. e The specific capacitance under various current densities. f Ragone plot of energy density and power density for the as-prepared ASC devices
The CV plots of ASC device under various scan rates were represented as Fig. 9c. The profile of CV plots gradually deviates from the rectangle along with scan rate augmenting. Besides, the peak current augments with scan rate. For the GCD test of the ASC device under various current densities, the applied current is computed by multiplying the sum of the two-electrode active mass with the related current density. Figure 9d, e display the GCD plots and the related specific capacitances under various current densities. The specific capacitances for the ASC device at 1 A g−1, 2 A g−1, 3 A g−1, 5 A g−1, 8 A g−1 and 10 A g−1 are 135 F g−1, 101 F g−1, 90 F g−1, 83 F g−1, 74 F g−1 and 73 F g−1, respectively. In order to better estimate the energy storage ability of the ASC, the relationship on energy density and power density was drawn in Fig. 9f. The energy density and power density could be calculated as follows [6]:
where C is specific capacitance, ∆V is voltage window, and ∆t is discharging time. The computed results are summarized in the Fig. 9f. A energy density of the ASC is computed to be 42.2 Wh kg−1 under 748.2 W kg−1. Further, while the power density augments to 7499.7 W kg−1, the ASC device remains the energy density of 22.9 Wh kg−1. The energy storage ability of MnCo2O4@NiO/NF-based ASC device is better than that of most of manganese and nickel oxides-based ASC devices reported previously, such as NiO (12.4 Wh kg−1 under 985 W kg−1) [48], NiCo2O4@NiO (31.5 Wh kg−1 under 215.2 W kg−1) [49], NiCo2O4@MnO2 (35 Wh kg−1 under 163 W kg−1) [50], MnCo2O4@CoMoO4 (37.5 Wh kg−1 under 527.8 W kg−1) [51] and MnCo2O4@NiCo2O4 (40 Wh kg−1 under 248.7 W kg−1) [52]. Furthermore, the capacitance retention of the MnCo2O4@NiO/NF-based ASC device has an acceptable deterioration with conservation of 79.4% over 5000 cycles under 5A g−1, which reveals the ASC device has a good stability (displayed in Fig. S5 of Supporting Information). The related Coulombic efficiency of the ASC device is over 94.4% during the 5000 cycles (Fig. S6 of Supporting Information), showing the highly reversible electrochemical reactions.
Conclusion
The NiO@MnCo2O4 hierarchical structures were directly deposited on NF as the additive-free supercapacitor electrode with simple hydrothermal method and annealing processes. The MnCo2O4@NiO/NF electrode presented higher specific capacitance (1186 F g−1) than MnCo2O4 (607 F g−1) and NiO (350 F g−1) electrodes under 1 A g−1. The MnCo2O4@NiO/NF electrode also exhibited excellent cycling performance (remaining 91% of the original capacitance over 5000 cycles under 10 A g−1) and super rate capability. Based on the analysis of EIS and diffusion coefficient, we find that the construction of hierarchical structure in the MnCo2O4@NiO/NF electrode not only greatly promotes the diffusion and migration of ions and electrons in rapid reaction, but also greatly reduces the volume deformation and structure collapsing of the electrode materials during the charge–discharge processes, thus achieving improved electrochemical performance. In addition, an ASC device with high energy density was assembled with the MnCo2O4@NiO/NF and AC/NF as the positive and negative electrodes, respectively. The assembled ASC manifested higher energy density of 42.2 Wh kg−1 under 748.2 W kg−1 and still hold the energy density of 22.9 Wh kg−1 while the power density increased tenfold to 7499.7 W kg−1. Especially, the ASC device preserved 79.4% specific capability over 5000 cycles. Therefore, the MnCo2O4@NiO/NF with hierarchical structure is proved to be the promising material for enhanced performance energy storage device.
References
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7(11):845–854
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828
Zhang LL, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38(9):2520–2531
Kötz R, Carlen M (2000) Principles and applications of electrochemical capacitors. Electrochim Acta 45(15–16):2483–2498
Liu C, Yu Z, Neff D, Zhamu A, Jang BZ (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10(12):4863–4868
Fan Z, Yan J, Wei T, Zhi L, Ning G, Li T, Wei F (2011) Asymmetric supercapacitors based on graphene/mno2 and activated carbon nanofiber electrodes with high power and energy density. Adv Funct Mater 21(12):2366–2375
Béguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26(14):2219–2251
Yu G, Hu L, Liu N, Wang H, Vosgueritchian M, Yang Y, Cui Y, Bao Z (2011) Enhancing the Supercapacitor Performance of Graphene/MnO2 Nanostructured Electrodes by Conductive Wrapping. Nano Lett 11(10):4438–4442
Zhi M, Xiang C, Li J, Li M, Wu N (2013) Nanostructured carbon-metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5(1):72–88
Zhang Y, Li L, Su H, Huang W, Dong X (2015) Binary metal oxide: advanced energy storage materials in supercapacitors. J Mater Chem A 3(1):43–59
Pasero D, Reeves N, West A (2005) Co-doped MnO: a possible anode material for lithium batteries. J Power Sources 141(1):156–158
Hui KN, Hui KS, Tang Z, Jadhav VV, Xia QX (2016) Hierarchical chestnut-like MnCo2O4 nanoneedles grown on nickel foam as binder-free electrode for high energy density asymmetric supercapacitors. J Power Sources 330:195–203
Song D, Zhu J, Li J, Pu T, Huang B, Zhao C, Xie L, Chen L (2017) Free-standing two-dimensional mesoporous ZnCo2O4 thin sheets consisting of 3D ultrathin nanoflake array frameworks for high performance asymmetric supercapacitor. Electrochim Acta 257:455–464
Vigneshwaran P, Kandiban M, Senthil Kumar N, Venkatachalam V, Jayavel R, Vetha Potheher I (2016) A study on the synthesis and characterization of CoMn2O4 electrode material for supercapacitor applications. J Mater Sci-Mater Electron 27(5):4653–4658
Zeng J, Ren Y, Wang S, Hao Y, Wu H, Zhang S, Xing Y (2017) Hierarchical porous ZnMn2O4 microspheres assembled by nanosheets for high performance anodes of lithium ion batteries. Inorg Chem Front 4(10):1730–1736
Vadiyar MM, Kolekar SS, Deshpande NG, Chang J-Y, Kashale AA, Ghule AV (2016) Binder-free chemical synthesis of ZnFe2O4 thin films for asymmetric supercapacitor with improved performance. Ionics 23(3):741–749
Lv J, Liang T, Yang M, Ken S, Hideo M (2017) Performance comparison of NiCo2O4 and NiCo2S4 formed on Ni foam for supercapacitor. Compos Pt B-Eng 123:28–33
Lv J, Guo W, Liang T (2016) Synthesis of Co3O4@CoMoO4 core-shell architectures nanocomposites as high-performance supercapacitor electrode. J Electroanal Chem 783:250–257
Li L, Zhang Y, Shi F, Zhang Y, Zhang J, Gu C, Wang X, Tu J (2014) Spinel manganese-nickel-cobalt ternary oxide nanowire array for high-performance electrochemical capacitor applications. ACS Appl Mater Interfaces 6(20):18040–18047
Mondal AK, Su D, Chen S, Ung A, Kim H-S, Wang G (2015) Mesoporous MnCo2O4 with a flake-like structure as advanced electrode materials for lithium-ion batteries and supercapacitors. Chem-Eur J 21(4):1526–1532
Liu S, Hui KS, Hui KN (2015) 1 D hierarchical MnCo2O4 nanowire@MnO2 sheet core-shell arrays on graphite paper as superior electrodes for asymmetric supercapacitors. Chem Nano Mat 1(8):593–602
Liu S, Hui KS, Hui KN, Yun JM, Kim KH (2016) Vertically Stacked bilayer CuCo2O4/MnCo2O4 heterostructures on functionalized graphite paper for high-performance electrochemical capacitors. J Mater Chem A 4(21):8061–8071
Liu S, Ni D, Li HF, Jun SC, Hui KN, Ouyang CY (2018) Effect of cation substitution on pseudocapacitive performance of spinel cobaltite MCo2O4 (M = Mn, Ni, Cu, and Co). J Mater Chem A 6(23):10674–10685
Zhao Y, Hu L, Zhao S, Wu L (2016) Preparation of MnCo2O4@Ni(OH)2 core-shell flowers for asymmetric supercapacitor materials with ultrahigh specific capacitance. Adv Funct Mater 26(23):4085–4093
Kim S-I, Lee J-S, Ahn H-J, Song H-K, Jang J-H (2013) Facile route to an efficient NiO supercapacitor with a three-dimensional nanonetwork morphology. ACS Appl Mater Interfaces 5(5):1596–1603
Li T, Li X, Wang Z, Guo H, Hu Q, Peng W (2016) Robust synthesis of hierarchical mesoporous hybrid NiO-MnCo2O4 microspheres and their application in Lithium-ion batteries. Electrochim Acta 191:392–400
Cheng B, Zhang W, Yang M, Zhang Y, Meng F (2019) Preparation and study of porous MnCo2O4@NiO nanosheets for high-performance supercapacitor. Ceram Int 45(16):20451–20457
Jiang L, Yuan X, Liang J, Zhang J, Wang H, Zeng G (2016) Nanostructured core-shell electrode materials for electrochemical capacitors. J Power Sources 331:408–425
Ho K-C, Lin L-Y (2019) A review of electrode materials based on core-shell nanostructures for electrochemical supercapacitors. J Mater Chem A 7(8):3516–3530
Shinde NM, Yun JM, Mane RS, Mathur S, Kim KH (2018) An Overview of Self-Grown Nanostructured Electrode Materials in Electrochemical Supercapacitors. J Korean Ceram Soc 55(5):407–418
Cheng J, Zhao B, Zhang W, Shi F, Zheng G, Zhang D, Yang J (2015) High-performance supercapacitor applications of NiO-nanoparticle-decorated millimeter-long vertically aligned carbon nanotube arrays via an effective supercritical CO2-assisted method. Adv Funct Mater 25(47):7381–7391
Sahoo S, Shim J-J (2016) Facile synthesis of three-dimensional ternary ZnCo2O4/reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain Chem Eng 5(1):241–251
Wu Z, Ren W, Wen L, Gao L, Zhao J, Chen Z, Zhou G, Li F, Cheng H (2010) Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 6(4):3187–3194
Chuang TJ, Brundle CR, Rice DW (1976) Interpretation of the x-ray photoemission spectra of cobalt oxides and cobalt oxide surfaces. Surf Sci 59(2):413–429
Li L, He F, Gai S, Zhang S, Gao P, Zhang M, Chen Y, Yang P (2014) Hollow structured and flower-like C@MnCo2O4 composite for high electrochemical performance in a supercapacitor. Cryst Eng Comm 16(42):9873–9881
Wu Z-S, Wang D-W, Ren W, Zhao J, Zhou G, Li F, Cheng H-M (2010) Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors. Adv Funct Mater 20(20):3595–3602
Tholkappiyan R, Naveen AN, Sumithra S, Vishista K (2015) Investigation on spinel MnCo2O4 electrode material prepared via controlled and uncontrolled synthesis route for supercapacitor application. J Mater Sci 50(17):5833–5843
Yuan C, Zhang X, Su L, Gao B, Shen L (2009) Facile synthesis and self-assembly of hierarchical porous NiO nano/micro spherical superstructures for high performance supercapacitors. J Mater Chem 19(32):5772–5777
Lu X, Zhai T, Zhang X, Shen Y, Yuan L, Hu B, Gong L, Chen J, Gao Y, Zhou J, Tong Y, Wang ZL (2012) WO3-x@Au@MnO2 core-shell nanowires on carbon fabric for high-performance flexible supercapacitors. Adv Mater 24(7):938–944
Ji J, Zhang LL, Ji H, Li Y, Ruoff RS (2013) Nanoporous Ni(OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor. ACS Nano 7(7):6237–6243
Shi X, Liu Z, Zheng Y, Zhou G (2017) Controllable synthesis and electrochemical properties of MnCo2O4 nanorods and microcubes. Coll Surf A-Physicochem Eng Asp 522:525–535
Xu J, Sun Y, Lu M, Wang L, Zhang J, Tao E, Qian J, Liu X (2018) Fabrication of the porous MnCo2O4 nanorod arrays on Ni foam as an advanced electrode for asymmetric supercapacitors. Acta Mater 152:162–174
Cai N, Fu J, Chan V, Liu M, Chen W, Wang J, Zeng H, Yu F (2019) MnCo2O4@nitrogen-doped carbon nanofiber composites with meso-microporous structure for high-performance symmetric supercapacitors. J Alloy Compd 782:251–262
Zheng X, Ye Y, Yang Q, Geng B, Zhang X (2016) Hierarchical structures composed of MnCo2O4@MnO2 core-shell nanowire arrays with enhanced supercapacitor properties. Dalton Trans 45(2):572–578
Yi H, Wang H, Jing Y, Peng T, Wang X (2015) Asymmetric supercapacitors based on carbon nanotubes@NiO ultrathin nanosheets core-shell composites and MOF-derived porous carbon polyhedrons with super-long cycle life. J Power Sources 285:281–290
Zhao P, Li W, Wang G, Yu B, Li X, Bai J, Ren Z (2014) Facile hydrothermal fabrication of nitrogen-doped graphene/Fe2O3 composites as high performance electrode materials for supercapacitor. J Alloy Compd 604:87–93
Ren X, Zhao Q, McCulloch WD, Wu Y (2017) MoS2 as a long-life host material for potassium ion intercalation. Nano Res 10(4):1313–1321
Zhang S, Yin B, Wang Z, Peter F (2016) Super long-life all solid-state asymmetric supercapacitor based on NiO nanosheets and α-Fe2O3 nanorods. Chem Eng J 306:193–203
Liu X, Liu J, Sun X (2015) NiCo2O4@NiO hybrid arrays with improved electrochemical performance for pseudocapacitors. J Mater Chem A 3(26):13900–13905
Xu K, Li W, Liu Q, Li B, Liu X, An L, Chen Z, Zou R, Hu J (2014) Hierarchical mesoporous NiCo2O4@MnO2 core-shell nanowire arrays on nickel foam for aqueous asymmetric supercapacitors. J Mater Chem A 2(13):4795–4802
Feng Y, Liu W, Sun L, Zhu Y, Chen Y, Meng M, Li J, Yang J, Zhang Y, Liu K (2018) Hierarchical MnCo2O4@CoMoO4 core-shell nanowire arrays supported on Ni foam for supercapacitor. J Alloy Compd 753:761–770
Lee H-M, V. V. Muralee Gopi C, Rana PJS, Vinodh R, Kim S, Padma R, Kim H-J, (2018) Hierarchical nanostructured MnCo2O4-NiCo2O4 composites as innovative electrodes for supercapacitor applications. New J Chem 42(21):17190–17194
Acknowledgements
The work is financially supported by the National Natural Science Foundation of China (Grant No. 51572218), and the Key Project of Research & Development of Shaanxi Province in China (No. 2018ZDCXL-GY-08-05).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, M., Cao, L., Li, W. et al. NiO nanoflakes decorated needle-like MnCo2O4 hierarchical structure on nickle foam as an additive-free and high performance supercapacitor electrode. J Mater Sci 56, 8613–8626 (2021). https://doi.org/10.1007/s10853-021-05810-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-05810-8