Abstract
The Cu2O/CuO/SnO2 transparent pn junction film device with Cu2+ self-oxidation transition layer is prepared via a simple thermal oxidation-sputtering method. There, the Cu2O is prepared via a simple sputtering method, the CuO transition layer is prepared via a thermal self-oxidation method on the surface of Cu2O film, and subsequently the SnO2 film is deposited via the sputtering method on the surface of CuO transition layer. As revealed, the as-prepared transparent photovoltaic device exhibits highly transparency of about ~ 75%, obvious photovoltaic conversion enhancement of about ~ 450 folds than unmodified device, decent stability during 10000 s’ cycle, which can be mainly ascribed to the Cu2+ self-oxidation transition layer; there, the appropriate Fermi level and nicer lattice matching can provide a decent channel for charge carrier transport, and the visible light response can improve photo-generated carrier excitation and injection. Additionally, the structure design of Cu2O/CuO/SnO2 can prevent the oxidation of Cu2O to increase photovoltaic stability.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the rapid consumption and non-renewability of fossil fuels, the energy crisis is becoming a more and more serious problem. At the same time, with the rapid industrial development, the greenhouse effect caused by fossil fuel combustion also brings more and more challenges for environment [1, 2]. So, the exploration of efficient alternatives for fossil fuels would be extremely urgent. There, the renewable energy is regarded as an efficient candidate for solving this problem [3,4,5,6], including water energy, wind energy, solar energy, biomass energy or tidal energy, etc. [7, 8]. Among these, the solar cells or photovoltaic devices [9,10,11,12], with the convenience, environmental friendly and can directly convert solar energy into electric energy, have attracted a great of attentions.
For traditional solar cells, most of them mainly focus on light response [13, 14], and the dark semiconductors would be primary candidates, so that which encounters prominent limitations in practical applications, including building walls or windows. Herein, the transparent solar cells or photovoltaic devices, with the balanced transmittance and photovoltaic conversion [15,16,17,18,19], are regarded as ideal solutions, such as ZnO-based device, TiO2-based device and so on.
Among these, the SnO2-based device, with a wide band gap (3.6 eV) and decent intrinsic highly conductivity [20,21,22], is reported as a hot topic, such as Cheema et al. have prepared transparent SnO2-based dye-sensitized solar cells with remarkable photon conversion performance [23], Jia et al. have reported decent perovskite solar cells with SnO2 transport layers [24], etc. In addition, the stale physical–chemical performances of SnO2 would be additional advantages [25, 26].
However, the transparent device is still a difficult topic, because that, the opportune and matched p-type semiconductor would be a great challenge for the fabrication of pn junction [27, 28], especially the low intrinsic mobility and carrier concentration would be the most significant issues [29,30,31], for example, SnO, CuAlO2, etc. Herein, as a typical p-type direct band gap semiconductor, the Cu2O [32], with easy preparation, low cost, non-toxic and decent transmittance (2.0–2.6 eV), is reported as an outstanding candidate [33, 34], such as Yun and Kim groups have reported the Cu2O/ZnO/AZO photovoltaic device for transparent applications [35], Osorio-Rivera et al. have prepared the Cd2SnO4/CdS/Cu2O/Ag solar cell with remarkable photovoltaic performance [36], Wong groups have used the metal oxide frameworks for transparent photovoltaic devices [37], etc. Additionally, the Cu vacancy and interstitial oxygen can induce the formation of excess holes charge carriers [38, 39], which is regarded as another advantage for improving the photovoltaic efficiency.
For all this, the pn junction device is still hard to be applied in windows, because that the low intrinsic charge carriers caused by p-type semiconductor and great energy band alignment from different materials would restrict the photovoltaic conversion significantly [40,41,42]. That the efficient hole carrier donor or band gap adjustment is regarded as effective approach [43,44,45], such as element doping and plasma injection. There, the transition layer modification [46], with easy preparation and sufficient candidates, is reported as an efficient method. Especially that, the appropriate transition layer [47, 48], can not only act as a donor to inject sufficient charge carriers, but also provide a decent channel for charge carriers transport, for instance, Qin et al. have prepared the Cu2O/SnO2 pn junction solar cell with MgO transition layer [49], Azizi groups have fabricated the Cu2O/ZnO pn junctions with ZnS transition layers [50], etc. For this Cu2O/SnO2 pn junction, the CuO [51], self-oxidation by the Cu2O, would obtain a unique advantage, because that, compared with other transition layers, due to the homogeneous oxidation, the CuO formed at the surface of Cu2O would cause minimal lattice distortion [52,53,54,55], which can improve the pn junction crystalline quality to increase the photovoltaic conversion [56, 57]. In addition, CuO, obtains a narrower band gap of 1.3–2.0 eV [58], can increase light response for increasing the charge carriers injection further, and the good physical–chemical stability would be another important advantage [59]. What’s more, the ultrathin thickness can shorten the transport time for restrain the photo-generated charge carrier recombination, which is also an important reason [60, 61].
In this work, we have fabricated the Cu2O/CuO/SnO2 transparent pn junction film device with Cu2+ self-oxidation transition layer via a simple thermal oxidation-sputtering method. The results indicate that the as-prepared Cu2O/CuO/SnO2 transparent pn junction film device obtains a highly transparency of about ~ 75% in visible light, an efficient photovoltaic conversion enhancement of about ~ 450 folds than unmodified device and a decent stability during 10000 s’ cycle. Further, the mechanism of photovoltaic enhancement of Cu2O/CuO/SnO2 transparent pn junction device is investigated via series of electrochemical characterizations.
Experiment
The Cu2O/CuO/SnO2 transparent pn junction film device with Cu2+ self-oxidation transition layer was prepared via a simple thermal oxidation-sputtering method. There, the Cu2O film was prepared via a sputtering method, and then the CuO ultrathin layer was prepared by thermal self-oxidation of Cu2O film surface in air at 180 °C. Subsequently, the SnO2 film was prepared via a sputtering method on surface of CuO film. The process is displayed as Fig. 1. Herein, the transparent pn junction devices with different oxidation time were labelled as Cu2O/SnO2-CuO-0 (0 min), Cu2O/SnO2-CuO-1 (15 min), Cu2O/SnO2-CuO-2 (30 min), Cu2O/SnO2-CuO-3 (45 min) and Cu2O/SnO2-CuO-4 (60 min), respectively. The detail of preparation and characterization is shown in ESI.
Results and discussions
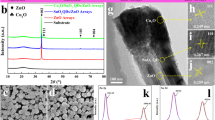
Figure 2 is the XRD of Cu2O/CuO/SnO2 transparent devices with different ratios of CuO transition layers. As displayed, due to the ultrathin thickness and lower crystallinity, the peaks of Cu2O and SnO2 could be hardly observed and would be characterized by the HRTEM and HRXPS. However, it’s interesting that, with the increased oxidation time, the weak diffraction peaks at 31.66° and 35.21° exhibit an increasing and are ascribed to the (110) and (002) planes of CuO(PDF#48-1548) [51, 52], respectively. Further, no other diffraction peaks could be observed, which manifest that the thermal oxidation process would not introduce other impurity.
Figure 3 is the SEM of Cu2O/CuO/SnO2 pn junction film device, including the cross section. As revealed, the Cu2O (Fig. 3a) prepared by sputtering obtains a smooth and uniform surface. Then, with the formation of CuO transition layer (Fig. 3b) by thermal oxidation, there is no obvious change could be observed, which can be ascribed to that the ultrathin layer and decent lattice matching of CuO. Figure 3c is the surface of SnO2 film, as shown, which is smooth and uniform and is beneficial for the fabrication of pn junction film device. Figure 3d is the cross section of as-prepared ternary pn junction film device. As demonstrated, the Cu2O/CuO/SnO2 device obtains a decent interfaces, such high-quality pn junction is beneficial for charge carrier transport. It’s significant that, restricted by the ultra-micro structure and distinguishability, the CuO transition layer could be hardly observed in SEM, and would be characterized by HRTEM. By calculation, the thicknesses of SnO2 and Cu2O films are about ~ 200 nm and ~ 100 nm, respectively.
Figure 4a is the TEM of Cu2O/CuO/SnO2 pn junction film device (Cu2O/SnO2-CuO-3) interface. As revealed, such pn junction obtains a clear and high-quality interface, which corresponds to SEM. As demonstrated in HRTEM, the lattice spaces of 0.264 nm (Fig. 4b) and 0.248 nm (Fig. 4d) are ascribed to the (101) plane of SnO2 [29] and (111) plane of Cu2O [33], respectively. It’s significant that, the CuO transition layer can be observed at the interface between Cu2O and SnO2 layers, by fitting, the corresponding lattice space of 0.254 nm (Fig. 4c) is ascribed to the (002) plane of CuO [51, 52], which is an reasonable evidence for the presence of CuO transition layer.
HRXPS reveals the different chemical valences of each component, especially the presence of CuO. As revealed in Fig. 5a, the peaks at 933.09 eV and 952.96 eV are ascribed to Cu 2p3/2 and Cu 2p1/2 of Cu+, and the peaks at 934.66 eV and 954.59 eV are ascribed to Cu 2p3/2 and Cu 2p1/2 of Cu2+ [29, 51, 52], such results indicate that the successful deposition of Cu2O film and formation of CuO transition layer. Figure 5b is the HRXPS of Sn 3d; there, the peaks at 486.92 eV and 494.92 eV are ascribed to Sn4+ [29], which manifest that the successful deposition of SnO2 film. All above indicate that the Cu2O/CuO/SnO2 pn junction film device with Cu2+ self-oxidation transition layer has been fabricated successfully.
As revealed, due to the wide band gap of Cu2O and SnO2, the as-prepared Cu2O/SnO2 pn junction exhibits a highly transparency at visible light (Fig. 6), and because the band gap of Cu2O is narrower than SnO2, the absorption edge mainly depends on Cu2O at about ~ 550 nm [29, 51, 52]. Further, with the formation of CuO self-oxidation transition layer, the transparency of modified pn junction device exhibits a certain decrease, which is ascribed to the narrow band gap of CuO [46, 47]. By calculation (ESI Fig. S1), the corresponding band gaps are 2.274 eV (Cu2O), 1.673 eV (CuO) and 3.626 eV (SnO2), respectively. However, most of Cu2O/CuO/SnO2 pn junction film devices with CuO transition layer still remain a highly transparency of about ~ 75% in visible light, which would not influence its application in windows and can be supported by the clear and transparent photo in insert.
Figure S2 (ESI) is the I–V curve of Cu2O/SnO2-CuO-3 under illumination, as shown, which manifest that the as-prepared transparent device is a typical pn junction [62]. Additionally, all of as-prepared Cu2O/CuO/SnO2 pn junction film devices exhibit similar open-circuit voltage of about ~ 150 mV, which is another important factor for photovoltaic conversion.
Herein, the photovoltaic conversion performances of different Cu2O/CuO/SnO2 pn junction film devices are demonstrated in Fig. 7. As displayed, the as-prepared pn junction device without CuO transition layer (Cu2O/SnO2-CuO-0) exhibits a weak intrinsic photocurrents of about ~ 6.62 × 10–7 A. Subsequently, with the formation of CuO transition layer, the photocurrents exhibit obvious enhancement to about ~ 4.40 × 10–5 A (Cu2O/SnO2-CuO-1), 1.16 × 10–4 A (Cu2O/SnO2-CuO-2), 2.92 × 10–4 A (Cu2O/SnO2-CuO-3) and 1.95 × 10–4 A (Cu2O/SnO2-CuO-4), respectively. By calculation, the Cu2O/CuO/SnO2 pn junction film device (Cu2O/SnO2-CuO-3) exhibits significant enhancement of about ~ 450 folds than unmodified device, and the PEC (photovoltaic conversion efficiency) is about 1.17%, that outclasses the increased absorption and can be regarded as a decent result in transparent device, which manifests that the charge carrier transport would play a more important role than absorption. It’s interesting that, the excess CuO would lead a decrease in photovoltaic conversion, because the CuO itself obtains weak conductivity and blocked up transition layer would lead an interface scattering for charge carrier, so that the photocurrent of Cu2O/SnO2-CuO-4 exhibits a decreasing. The response time can be fitted by the formulas [62] of \(\Delta\sigma =\Delta {\sigma }_{s}(1-{e}^{-\frac{t}{\tau }})\) and \(\Delta\sigma =\Delta {\sigma }_{s}{e}^{-\frac{t}{\tau }}\); there, the rise time is 68 ms, and decay time is 63 ms, respectively.
Subsequently, the photovoltaic stability is evaluated by the recycle I-T curve in Fig. 8. As revealed, during the 10000 s’ cycle, the as-prepared Cu2O/CuO/SnO2 pn junction film device (Cu2O/SnO2-CuO-3) exhibits a decent stability, including the photocurrent and response time, which indicate that this transparent pn junction device is promising to be applied in windows.
Additionally, the illumination depending photovoltaic response is another important factor for practical application and is revealed in Fig. 9. It’s interesting that with the decreased illumination of 0.1 solar, the photocurrent exhibits a nonlinear descent, by calculation, at the intensity of 0.1 solar, the transparent pn junction (Cu2O/SnO2-CuO-3) can still remain a higher photovoltaic conversion about 30% of standard value, which is regarded as a decent result.
All above indicate that the formation of self-oxidation CuO transition layer can improve the photovoltaic conversion efficiency efficiently, so the mechanism of photovoltaic enhancement would be an interesting issue.
As revealed in Fig. 10a, the decreased arcs indicate that, with the formation of CuO transition layer, the interface charge carrier transferring exhibits an obvious improving and obtains the optimal value at Cu2O/SnO2-CuO-3, which corresponds the photovoltaic performance and indicate the CuO transition layer can accelerate the charge carrier transport [42]. As demonstrated in Fig. 10b, fitted by the formula [62] of \(\tau_{n} = \frac{1}{{2\pi f_{m} }}\), the charge carrier lifetime exhibits prolonging with the formation of CuO transition layer and corresponds to the EIS (optimal value at Cu2O/SnO2-CuO-3). Such results also can be supported by the PL (ESI Fig. S3). As displayed, with the introducing of CuO transition layer, the fluorescence obtains significant decrease, which indicates that the interface charge carriers recombination is optimized and corresponds to photovoltaic performance. All above manifest that the interface charge carrier efficiency obtains an improving with the formation of CuO transition layer, including the transport, recombination and lifetime.
The charge carrier transport route would be another significant factor for photovoltaic conversion, which depends on the potential and can be evaluated by the Mott–Schottky plot via the formula [42, 62, 63] of
where the x-axis intercept is the Efb (band potential, corrected by AgCl, vs. 0.197 eV), and the slope denotes the type of semiconductor. As demonstrated, the negative slopes in Fig. 11a and b manifest the Cu2O and CuO are typical p-type semiconductors, and the positive slope in Fig. 11c manifests the SnO2 is typical n-type semiconductor. As displayed, the flat band potentials of different films are 0.723 eV (VB of Cu2O, vs. Ag/AgCl), 0.629 eV (VB of CuO, vs. Ag/AgCl) and -0.083 eV (CB of SnO2, vs. Ag/AgCl), respectively. By correction, the corresponding Fermi levels are 0.920 eV (VB of Cu2O, vs. NHE), 0.826 eV (VB of CuO, vs. NHE) and 0.114 eV (CB of SnO2, vs. NHE). Incorporation with the intrinsic band gap (ESI Fig. S1), the CB of the Cu2O and CuO is -1.354 eV (vs. NHE) and -0.847 eV (vs. NHE), and the VB of SnO2 is 3.740 eV (vs. NHE). It’s obviously, such Fermi levels are hard to form a decent channel for charge carrier transferring, so the Mott–Schottky plot of as-prepared transparent pn junction device (Cu2O/SnO2-CuO-3) is revealed in Fig. 11d. By correction, the corresponding Fermi levels of each component are 0.295 eV (VB of Cu2O, vs. NHE), 0.486 eV (VB of CuO, vs. NHE) and -0.045 eV (CB of SnO2, vs. NHE). Correspondingly, the CB of Cu2O and CuO is -1.979 eV (vs. NHE) and -1.187 eV (vs. NHE), and the VB of SnO2 is 3.581 eV (vs. NHE). It’s evident that the ECB-Cu2O < ECB-CuO < ECB-SnO2 and EVB-Cu2O < EVB-CuO < EVB-SnO2. It’s obvious that, with the presence of Cu2+ self-oxidation transition layer, the appropriate potential of CuO would make the photo-generated electron transport channel from Cu2O to CuO to SnO2 being achievable [42]. Correspondingly, the photo-generated hole would transfer along the channel of SnO2 to CuO to Cu2O [29, 62]. Above results manifest that the Cu2+ self-oxidation CuO transition layer with an appropriate potential can provide a decent channel for accelerating photo-generated carrier transport, which is beneficial for photovoltaic conversion.
Thus, the photovoltaic enhancement mechanism of the Cu2O/CuO/SnO2 transparent pn junction film device can be proposed as Fig. 12, which is mainly ascribed to that the appropriate potential of Cu2+ self-oxidation transition layer and excess visible light response of CuO.
As shown, the Cu2O/SnO2-CuO-3 obtains a significant photovoltaic enhancement (~ 450 folds than unmodified device), but a few decreased transparency (15%), which indicates that the charge carrier transport improved via Cu2+ self-oxidation transition layer would play a more important role than the visible light response during the photovoltaic conversion [42]. There, with the formation of Cu2O/SnO2 pn junction, the intrinsic potential structure is hard for photo-generated carriers transferring. That, with the formation of Cu2+ self-oxidation transition layer, the appropriate potential of CuO would fabricate a decent gradient [29, 51, 52], which would provide a favourable channel for charge carriers transferring, such process can improving the photo-generated carrier separation/recombination, transport and prolong the lifetime efficiently for improving photovoltaic conversion, and can be supported by the EIS, PL, Bode and Mott–Schottky plots. There, as depicted in Fig. 12, driven by self-photovoltage, the photo-generated electron would transfer along the route of Cu2O to CuO to SnO2; correspondingly, the photo-generated hole would transfer to Cu2O, this process is the core issue for this transparent pn junction device [51, 52, 62, 63]. In addition, the Cu2O and SnO2 with wider band gap exhibit weaker response in visible light, but the CuO with narrower band gap can absorption visible light to excite excess photo-generated charge carriers and inject into pn junction, which is also an important advantage [23,24,25, 51, 52]. Further, with the decent adjustment of thickness, such CuO transition layer would not influence the transparency for transparent device application. On the other hand, the decent lattice matching of Cu2+ self-oxidation transition layer would provide a decent channel for charge carrier transport, though the excess transition layer would lead an interface scattering [29, 51, 52], so that the Cu2O/SnO2-CuO-4 exhibits a decrease. What’s more, the structure design of Cu2O/CuO/SnO2 can prevent the oxidation of Cu2O film efficiently, which is an important reason for the decent photovoltaic stability.
Conclusion
We have prepared the Cu2O/CuO/SnO2 transparent pn junction film device with Cu2+ self-oxidation transition layer via a simple thermal oxidation-sputtering method. The as-prepared Cu2O/SnO2-CuO-3 obtains highly transparency of about ~ 75%, obvious photovoltaic conversion enhancement of about ~ 450 folds than unmodified device, decent stability during 10000 s’ cycle. There, the Cu2+ self-oxidation transition layer with appropriate Fermi level and decent lattice matching can provide a decent channel for charge carrier transport, and the visible light response can improve excess photo-generated carrier injection are regarded as the main reasons. In addition, the structure design of Cu2O/CuO/SnO2 can prevent the oxidation of Cu2O film to increase the photovoltaic stability.
Thus, this Cu2O/CuO/SnO2 transparent pn junction film device with balanced transparency and photovoltaic performance is expected to be a promising candidate in windows.
References
Patel M, Kim HS, Kim J, Yun JH, Kim SJ, Choi EH, Park HH (2017) Excitonic metal oxide heterojunction (NiO/ZnO) solar cells for all-transparent module integration. Sol Energy Mater Sol Cells 170:246–253
Lin S, Wang HY, Zhang XN, Wang D, Zu D, Song JN, Liu ZL, Huang Y, Huang K, Tao N, Li ZW, Bai XP, Li B, Lei M, Yu ZF, Hui Wu (2019) Direct spray-coating of highly robust and transparent ag nanowires for energy saving windows. Nano Energy 62:111–116
Zhong WW, Shen SJ, He M, Wang D, Wang ZP, Lin ZP, Tu WG, Yu JG (2019) The pulsed laser-induced Schottky junction via in-situ forming Cd clusters on CdS surfaces toward efficient visible light-driven photocatalytic hydrogen evolution. Appl Catal B Environ 258:117967
Jiang Q, Chu Z, Wang P, Yang X, Liu H, Wang Y, Yin Z, Wu J, Zhang X, You J (2017) Planar-structure Perovskite solar cells with efficiency beyond 21%. Adv Mater 29:1703852
Zhong WW, Wang ZP, Gao N, Huang LA, Lin ZP, Liu YP, Meng FQ, Deng J, Jin SF, Zhang QH, Gu L. Coupled vacancy pairs in Ni-doped CoSe for improved electrocatalytic hydrogen production through topochemical deintercalation. Angew Chem Int Ed. https://doi.org/10.1002/anie.202011378,
Tian QH, Yan JB, Yang L, Chen JZ (2018) Fabrication of three-dimensional carbon coating for SnO2/TiO2 hybrid anode material of lithium-ion batteries. Electrochim Acta 282:38–47
Zhong WW, Tu WG, Wang ZP, Lin ZP, Xu AJ, Ye XF, Chen DC, Xiao BB (2020) Ultralow-temperature assisted synthesis of single platinum atoms anchored on carbon nanotubes for effciently electrocatalytic acidic hydrogen evolution. J Energy Chem 51:280–284
Ma YW, Lu YF, Hai GT, Dong WJ, Li RJ, Liu JH, Wang G (2020) Bidentate carboxylate linked TiO2 with NH2-MIL-101(Fe) photocatalyst: a conjugation effect platform for high photocatalytic activity under visible light irradiation. Sci Bull 65:658–669
Sun H, Deng K, Jiang Y, Ni J, Xiong J, Li L (2020) Realizing stable artificial photon energy harvesting based on Perovskite solar cells for diverse applications. Small 16:1906681
Liu WJ, Liu ML, Chen X, Shen T, Lei M, Guo JG, Deng HX, Zhang W, Dai CQ, Zhang XF, Wei ZY (2020) Ultrafast photonics of two dimensional material AuTe2Se4/3 in fiber lasers. Commun Phys 3:15
Wang QT, Li HM, Zhuang J, Guo H, Liu XC, Guo ZL, Gong XL, Li HY (2020) Multifunctional molecules of surfactant to support enhanced efficiency and stability for perovskite solar cells. J Mater Sci 55:14761–14772. https://doi.org/10.1007/s10853-020-05059-7
Lin PS, Bai XP, Wang HY, Wang HL, Song JN, Huang K, Wang C, Wang N, Li B, Lei M, Wu H (2017) Roll-to-roll production of transparent silver nanofiber network electrode for flexible electrochromic smart windows. Adv Mater 29:1703238
Li G, Chang WH, Yang Y (2017) Low-bandgap conjugated polymers enabling solution-processable tandem solar cells. Nat Rev Mater 2:17043
Cheon S, Jeong DS, Park JK, Kim WM, Lee TS, Lee H, Kim I (2018) Enhanced blue responses in nanostructured Si solar cells by shallow doping. J Phys D Appl Phys 51:125102
Xue Q, Xia R, Brabec CJ, Yip HL (2018) Recent advances in semi-transparent polymer and perovskite solar cells for power generating window applications. Energy Environ Sci 11:1688–1709
Liu B, Niu MQ, Fu J, Xi ZY, Lei M, Quhe RG (2019) Negative Poisson’s ratio in puckered two-dimensional materials. Phys Rev Mater 3:054002
Yang C, Liu D, Bates M, Barr MC, Lunt RR (2019) How to accurately report transparent solar cells. Joule 3:1803–1809
Liu WJ, Liu ML, Liu XM, Wang XT, Deng HX, Lei M, Wei ZM, Wei ZY (2020) Recent advances of 2D materials in nonlinear photonics and fiber lasers. Adv Opt Mater 8:1901631
Lin S, Wang HY, Wu F, Wang QM, Bai XP, Zu D, Song JN, Wang D, Liu ZL, Li ZW, Tao N, Huang K, Lei M, Li B, Wu H (2019) Room-temperature production of silver-nanofiber film for large-area, transparent and flexible surface electromagnetic interference shielding, npj Flex. Electron 3:6
Yu J, Wang Y, Huang Y, Wang X, Guo J, Yang J, Zhao H (2020) Structural and electronic properties of SnO2 doped with non-metal elements. Beilstein J Nanotech 11:1321–1328
Wang Q, Peng C, Du L, Li H, Zhang W, Xie J, Qi H, Li Y, Tian L, Huang Y (2020) Enhanced performance of perovskite solar cells via low-temperature-processed mesoporous SnO2. Adv Mater Interfaces 7:1901866
Pan JQ, Li S, Ou W, Liu YY, Li HL, Wang JJ, Song CS, Zheng YY, Li CR (2020) The photovoltaic conversion enhancement of NiO/Tm:CeO2/SnO2 transparent p-n junction device with dual-functional Tm:CeO2 quantum dots. Chem Eng J 393:124802
Cheema H, Delcamp JH (2019) SnO2 transparent printing pastes from powders for photon conversion in SnO2-based dye-sensitized solar cells. Chem Eur J 25:14205–14213
Jia J, Dong J, Wu J, Wei H, Cao B (2020) Combustion procedure deposited SnO2 electron transport layers for high efficient perovskite solar cells. J Alloys Compd 844:156032
Noh YW, Jin IS, Kim KS, Park SH, Jung JW (2020) Reduced energy loss in SnO2/ZnO bilayer electron transport layer-based perovskite solar cells for achieving high efficiencies in outdoor/indoor environments. J Mater Chem A 8:17163–17173
Tian QH, Zhang F, Zhang W, Yang L (2019) Non-smooth carbon coating porous SnO2 quasi-nanocubes towards high lithium storage. Electrochim Acta 307:393–402
Shi B, Duan L, Zhao Y, Luo J, Zhang X (2020) Semitransparent perovskite solar cells: from materials and devices to applications. Adv Mater 32:1806474
Guillen C, Herrero J (2020) Comparing metal oxide thin films as transparent p-type conductive electrodes. Mater Res Express 7:016411
Pan JQ, Li S, Liu YY, Ou W, Li HL, Zhao WJ, Wang JJ, Song CS, Zheng YY, Li CR (2020) The flexible-transparent p-n junction film device of N-doped Cu2O/SnO2 orderly nanowire arrays towards highly photovoltaic conversion and stability. Chem Eng J 382:122813
Teng F, Hu K, Ouyang W, Fang X (2018) Photoelectric detectors based on inorganic p-type semiconductor materials. Adv Mater 30:1706262
Pan JQ, Li HL, Li S, Ou W, Liu YY, Wang JJ, Song CS, Zheng YY, Li CR (2020) The enhanced photocatalytic hydrogen production of nickel-cobaltbimetals sulfide synergistic modified CdS nanorods with active facets. Renew Energy 156:469–477
Stepniowski WJ, Misiolek WZ (2018) Review of fabrication methods, physical properties, and applications of nanostructured copper oxides formed via electrochemical oxidation. Nanomaterials 8:379
Pan J, Zhao C, Wang B, Dong Z, Jiang Z, Wang J, Song C, Zheng Y, Li C (2020) The transparent device of CdS quantum dots modified Cu2O/ZnO orderly nano array p-n junction towards the enhanced photovoltaic conversion. J Alloys Compds 827:154267
Wang YB, Cao SY, Huan YH, Nie TS, Ji Z, Bai ZM, Cheng XQ, Xi JH, Yan XQ (2020) The effect of composite catalyst on Cu2O/TiO2 heterojunction photocathodes for efficient water splitting. Appl Surf Sci 526:146700
Rana AK, Ban DK, Patel M, Yun JH, Kim J (2019) A transparent photovoltaic device based on Cu2O/ZnO/AZO for see-through applications. Mater Lett 255:126517
Osorio-Rivera D, Torres-Delgado G, Castanedo-Pérez R, Márquez-Marín J, Zelaya-Ángel O (2020) Cd2SnO4/CdS/Cu2O/Ag solar cell obtained by chemical techniques. Mater Res Bull 122:110669
Rana AK, Park JT, Kim J, Wong C-P (2019) See-through metal oxide frameworks for transparent photovoltaics and broadband photodetectors. Nano Energy 64:103952
Aggarwal G, Maurya SK, Singh AJ, Singh AK, Kavaipatti B (2019) Intrinsic Acceptor-like defects and their effect on carrier transport in polycrystalline Cu2O photocathodes. J Phys Chem C 123:26057–26064
Giraldo B, Yeh WC, Kobayashi NP (2020) Formation of single-crystal Cu2O strips in non-singlecrystal CuO thin films by continuous-wave laser diode with micro-chevron laser beam (mu-CLB). J Mater Sci 55:14105–14111. https://doi.org/10.1007/s10853-020-05013-7
Pan JQ, Liu YY, Ou W, Li S, Li HL, Wang JJ, Song CS, Zheng YY, Li CR (2020) The photocatalytic hydrogen evolution enhancement of the MoS2 lamellas modified g-C3N4/SrTiO3 core-shell heterojunction. Renew Energy 161:340–349
Wei J, Chu X, Chen JG, Xu K, Deng HX, Wei ZM, Lei M (2019) Machine learning in material science Infomat 1:338–358
Pan JQ, Dong ZJ, Jiang ZY, Zhao C, Wang BB, Zhao WJ, Wang JJ, Song CS, Zheng YY, Li CR (2019) MoS2 quantum dots modified black Ti3+-TiO2/g-C3N4 hollow nanosphere heterojunction toward photocatalytic hydrogen production enhancement. Solar RRL 3:1900337
Yan N, Zhao C, You S, Zhang Y, Li W (2020) Recent progress of thin-film photovoltaics for indoor application. Chin Chem Lett 31:643–653
Brus VV, Lee J, Luginbuhl BR, Ko S-J, Bazan GC, Nguyen TQ (2019) Solution-processed semitransparent organic photovoltaics: from molecular design to device performance. Adv Mater 31:1900904
Tian QH, Zhang F, Yang L (2019) Fabricating thin two-dimensional hollow tin dioxide/carbon nanocomposite for high-performance lithium-ion battery anode. Appl Surf Sci 481:1377–1384
Zuo C, Ding L (2015) Solution-processed Cu2O and CuO as hole transport materials for efficient perovskite solar cells. Small 11:5528–5532
Alajlani Y, Placido F, Chu HO, De Bold R, Fleming L, Gibson D (2017) Characterisation of Cu2O/CuO thin films produced by plasma-assisted DC sputtering for solar cell application. Thin Solid Films 642:45–50
Bhunia R, Dolai S, Dey R, Das S, Hussain S, Bhar R, Pal AK (2018) Fabrication and characterization of Cu/Cu2O/CuO/ZnO/Al-ZnO/Ag heterojunction solar cells. Semicond Sci Technol 33:105007
Qin C, Wang Y, Lou Z, Yue S, Niu W, Zhu L (2019) Surface modification and stoichiometry control of Cu2O/SnO2 heterojunction solar cell by an ultrathin MgO tunneling layer. J Alloys Compds 779:387–393
Bengas R, Lahmar H, Redha KM, Mentar L, Azizi A, Schmerber G, Dinia A (2019) Electrochemical synthesis of n-type ZnS layers on p-Cu2O/n-ZnO heterojunctions with different deposition temperatures. RSC Adv 9:29056–29069
Ji Y, Jung U, Xian Z, Kim D, Yu J, Park J (2020) Ultraviolet photodetectors using hollow p-CuO nanospheres/n-ZnO nanorods with a pn junction structure. Sensor Actuat A-Phys 304:111876
Izaki M, Fukazawa K, Sato K, Khoo PL, Kobayashi M, Takeuchi A, Uesugi K (2019) Defect structure and photovoltaic characteristics of internally stacked CuO/Cu2O photoactive layer prepared by electrodeposition and heating. ACS Appl Energy Mater 2:4833–4840
Lin S, Liu JC, Li WZ, Wang D, Huang Y, Jia C, Li ZW, Wang HY, Song JN, Liu ZL, Huang K, Zu D, Lei M, Hong B, Wu H (2019) A flexible, robust and gel-free electroencephalogram electrode for noninvasive brain-computer interfaces. Nano Lett 19:6853–6861
Maack B, Nilius N (2020) Morphological and kinetic insights into Cu2O-CuO oxidation. Phys Status Solidi B 257:1900365
Lin S, Liu JC, Wang QM, Zu D, Wang HY, Wu F, Bai XP, Song JN, Liu ZL, Li ZW, Huang K, Li B, Lei M, Wu H (2020) Roll-to-Roll production of highly robust 3D-metallized sponge for large-scale high-performance electromagnetic interference shielding. Adv Mater Tech 5:1900761
Wei B, Yang N, Pang F, Ge J (2018) Cu2O-CuO hollow nanospheres as a heterogeneous catalyst for synergetic oxidation of CO. J Phys Chem C 122:19524–19531
Wang ZP, Xiao BB, Lin ZP, Shen SJ, Xu AJ, Du ZX, Chen YC, Zhong WW (2021) In-situ surface decoration of RuO2 nanoparticles by laser ablation for improved oxygen evolution reaction activity in both acid and alkali solutions. J Energy Chem 54:510–518
Ayesh AI, Alyafei AA, Anjum RS, Mohamed RM, Abuharb MB, Salah B, El-Muraikhi M (2019) Production of sensitive gas sensors using CuO/SnO2 nanoparticles. Appl Phys A-Mater 125:550
Han J, Zong X, Zhou X, Li C (2015) Cu2O/CuO photocathode with improved stability for photoelectrochemical water reduction. RSC Adv 5:10790–10794
Quhe RG, Liu JC, Wu JX, Yang J, Wang YY, Li QH, Li TR, Yang JB, Peng HL, Lei M, Lu J (2019) High-performance sub-10 nm monolayer Bi2O2Se transistors. Nanoscale 11:532–540
Masudy-Panah S, Zhuk S, Tan HR, Gong X, Dalapati GK (2018) Palladium nanostructure incorporated cupric oxide thin film with strong optical absorption, compatible charge collection and low recombination loss for low cost solar cell applications. Nano Energy 46:158–167
Pan JQ, Wang PH, Wang PP, Yu Q, Wang JJ, Song CS, Zheng YY, Li CR (2021) The photocatalytic overall water splitting hydrogen production of g-C3N4/CdS hollow core–shell heterojunction via the HER/OER matching of Pt/MnOx. Chem Eng J 405:126622
Pan JQ, Dong ZJ, Wang BB, Jiang ZY, Chuang C, Wang JJ, Song CS, Zheng YY, Li CR (2019) The enhancement of photocatalytic hydrogen production via Ti3+ self-doping black TiO2/g-C3N4 hollow core-shell nano-heterojunction. Appl Catal B: Environ 242:92–99
Acknowledgements
This work was supported by the Natural Science Foundation of China (Nos. 51672249, 51802282 and 11804301) and the Zhejiang Provincial Natural Science Foundation of China (No. LQ17F040004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Kyle Brinkman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Q., Pan, J., Mei, J. et al. The Cu2O/CuO/SnO2 transparent pn junction film device towards photovoltaic enhancement with Cu2+ self-oxidation transition layer. J Mater Sci 56, 5736–5747 (2021). https://doi.org/10.1007/s10853-020-05704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05704-1