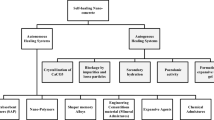
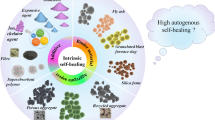
Abstract
Concrete is a very common material consisting of a mixture of aggregates (sand, gravel, crashed rock) and paste (cement, water and additives). The paste component, made up mainly of cement and water, degrades with time. Despite this shortcoming, research toward the next-generation cement and concrete materials has intensified in the past 10–15 years. Self-healing cementitious materials, in particular, are a research area that has attracted a great deal of attention. A number of novel formulations have demonstrated an increase in mechanical and chemical stability with respect to conventional Portland cement, through the addition of inorganic, organic, and even biological additives. This review reports on the latest developments in cement research related to the synthesis of cement and concrete materials with autogenous healing and/or self-healing capability. These include geopolymers, engineered cementitious materials, bacterial cement composites, microencapsulated self-healing materials, self-healing assisted by shape-memory alloys, and polymer–cement composites. This work describes the performance of each cementitious material and the mechanism responsible for healing, including a section on atomistic simulations and modeling of cementitious materials. A detailed understanding of various cement technologies with autogenous healing and self-healing properties, including their strengths and weaknesses, is critical to determine the areas where new development is needed to enable novel, energy-efficient, and environmentally responsible cement and concrete solutions. To this end, molecular simulations can play a significant role and have already demonstrated promise in achieving an atomic level view of interactions between cementitious materials and other add-on compounds, such as carbon nanotubes or polymers for enhanced reinforcement or autonomous healing properties.
Graphic abstract
Next-generation construction materials are led by self-healing cement and concrete. With a wide range of autonomous healing strategies based on smart polymers and alloys, biotechnology, and molecular modeling, advanced cementitious materials are becoming part of our daily lives. This work represents a comprehensive review on progress, challenges, and opportunities in the development of these materials for infrastructure and beyond.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coveted for its relatively low cost and strong mechanical properties, concrete is the most used man-made building material to date. Concrete is a product of cement, water and sand with the addition of coarse aggregates [1]. An adverse effect of cement and concrete’s popularity is its negative impact on our environment, specifically CO2 emissions. It is estimated that Portland cement alone contributes up to 7% of the global anthropogenic CO2 emissions [2]. This, in concert with the fact that concrete is very susceptible to cracking, results in high costs associated with structure’s maintenance and repair. Present-day cement has not advanced much since its discovery in the nineteenth century, which is why it is considered a low-tech building material.
Cementitious materials are used in bridges, roads, buildings, dams, and even in the subsurface for oil and gas recovery, geothermal energy production, and disposal of nuclear waste. The global cement and concrete market is estimated to grow by $332 billion between 2020 and 2024 alone [3]. The consistent use of cementitious materials on such different constructions calls for alternative materials to reduce the frequency of maintenance, repairs, and in some cases, complete failure of the structure. In Europe for example, it is estimated that 50% of the annual construction budget is exhausted on rehabilitation and repair of existing concrete structures [4]. In the USA, fixing concrete infrastructure represents a trillion dollar market [5]. For example, bridges alone have an estimated cost between $20 billion to $200 billion in reconstruction costs along with an average annual maintenance cost of $5.2 billion [6]. The self-repairing capability of cracks in built infrastructure will significantly reduce the carbon footprint and costs by reducing the frequency of required maintenance and extend the lifetime of the structures. However, at the time of modifying a cement and concrete formulation, researchers and engineers need to take into account that the cost of cement is very low ($0.05/lb) and introducing additives can dramatically increase its cost. Nevertheless, lifecycle savings are what need to be contemplated when proposing novel formulations toward increasing the durability of cementitious materials and concrete.
Concrete structures have a limited tensile strength making the material susceptible to cracking during its entire lifetime. These inevitable cracks pose a threat to the integrity of the structure as they provide a pathway for undesirable substances throughout the matrix [7]. The transport properties of concrete materials change dramatically when cracks are present, and so water and chloride permeation becomes a concern [8]. Two important transport mechanisms that can occur within concrete are advection and diffusion [9]. Aside from cracks, these mechanisms can also be greatly affected by the porosity, and more importantly, the permeability of the material. This is one of many properties that are of interest when developing next-generation cementitious materials [10]. Recently, there has been a great deal of research to convert cementitious materials from low-tech to high-tech/smart materials by incorporating self-healing properties [11,12,13,14]. When any of these novel technologies can be economically adopted, the cost associated with maintenance and, in some cases, replacement of cement and concrete structures can be significantly reduced.
Reinforced concrete uses supporting steel rods (rebar) to modify/improve the structural performance of the concrete. It is worth noting that while not designed for self-healing, reinforced concrete does have a superior mechanical performance and workability as a construction material with respect to concrete [15]. Reinforced concrete is a popular building material commonly seen in many structures such as bridges, buildings, viaducts, retaining walls, tunnels, tanks, and conduits [16]. Despite increasing the mechanical performance of the concrete, reinforced concrete does have significant drawbacks, the major one being steel corrosion. The corrosion of the rebar can lead to cracking, resulting in strength reduction, serviceability, and aesthetics of the entire structure [17,18,19]. Repair and maintenance of these structures involve enormous costs; therefore, introducing self-healing capability to reinforced concrete via chemical modification of the cement material would be of great interest.
Normal cementitious materials can undergo autogenous healing when small cracks are present, typically smaller than 50 μm for complete healing (partial healing has been observed on fractures up to 150 μm) [20], due to delayed hydration of residual cement reagents or the carbonation of dissolved calcium hydroxide [20, 21]. In order for this type of healing to occur, fluid has to be in contact with the fracture. One alternative method of autogenous healing observed in regular cement is swelling of the calcium–silicate–hydrate (C–S–H) products generated upon cement’s curing or the blocking of cracks by concrete particles or other fine particles present in water [22]. A variety of additives have been developed and implemented in pursuit of better performing cementitious materials, such as fly ash or other aluminosilicate source materials to promote silicon-oxo-aluminate chains and networks [23,24,25,26]. Fibers including carbon, cellulose, glass, polyethylene terephthalate (PET), among other plastic materials, have also been introduced in cement and concrete to reduce the crack size, leading to the development of engineered cementitious composites [8, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. These various healing paths are depicted in Fig. 1.
Different mechanisms of autogenous healing in normal cement. Reproduced with permission [22].
Autogenous healing behavior in conventional cement triggered research efforts toward introducing additives to produce cementitious materials with effective autonomic (manufactured) healing capability (also referred as self-healing) and improved mechanical properties [43,44,45]. A number of articles dedicated to self-healing cements exist in the open literature [11,12,13,14, 21, 43, 44, 46,47,48,49,50,51,52,53,54,55]. For example, healing agents have been encapsulated in glass or polymer beads designed to survive the mixing process, but also break and release the healing reagent in the presence of a crack [7, 56,57,58,59,60,61,62,63,64,65,66,67]. Furthermore, encapsulation and other protective carriers have been used to introduce bacteria that promote self-healing in cementitious materials [13, 44, 68,69,70,71,72,73,74,75,76,77,78,79,80,81]. Self-healing studies also included the incorporation of polymers into the cement matrix. Our group recently demonstrated that by introducing thermally stable and self-healing polymers to conventional cement, it is possible to generate a new cementitious material with multiple self-repairing cycles capability and enhanced mechanical properties such as a significant increase in ductility. This is the result of dynamic and reversible bonding interactions between the polymer and the cement matrix [82,83,84,85,86,87,88]. Multiple autogenous and autonomous healing behavior has also been observed on cementitious materials including cements modified with slag and fly ash additives [26, 89,90,91], cements modified with superabsorbent polymers (SAP) [92,93,94,95,96,97,98,99], and bacteria-based cement and concrete materials [13, 69,70,71,72,73,74,75,76,77,78, 100].
The present review provides the reader with an introduction to advanced cementitious materials, their applications and areas for development, followed by a brief description of the different methods and approaches to improve their mechanical stability with special focus on cement and concrete materials with self-healing capability. The review mainly focuses on experimental work but dedicates a section on molecular modeling as an important and complementary tool for the design of next-generation, longer-lasting, cementitious materials.
Self-healing cementitious materials
Application of self-healing cementitious materials
There has been a rising demand for superior cementitious materials for industrial applications such as construction, energy, and transportation [101]. However, the high cost of novel technologies is currently inhibiting their incorporation to wide spread industrial use [102] and as such the majority of information that can be gathered on alternative cementitious materials including self-healing cements and concrete is from research and development published literature only. Cement cracking shortens the life span of the product, especially in extreme environments including aquatic and marine environments as found with dams; deep wellbore environments including geothermal, carbon capture, and oil and gas production; and cementitious waste forms used for storage and disposal of radioactive waste.
Materials used for aquatic and marine environments include dams, levees, and canal linings. The specific method of repair depends on the size and nature of the damage. There are two main objectives when repairing cracks and holes: structural bonding and stopping water flow. To this end, one of the most recent developments in concrete repair is the use of synthetic materials for bonding and patching [102, 103]. Epoxy-resin compounds are used extensively because of their high bonding properties and great strength [104, 105]; however, they undergo degradation with aging and need to be replaced over the lifetime of the structure, like in the case of dams. Another barrier associated with repairs is the weak bonding between the original structural materials (aged concrete, rebar) and the repairing material [103,104,105]. For these types of structures, there have been promising reports of bacterial-based healing [74, 77], though this form of fracture healing is in some cases prohibitively slow (weeks to months) and healing methods such as epoxy resins are more appropriate due to the faster self-repairing rates (hours to days).
Wellbores are engineered for a variety of uses including water, geothermal, oil, and gas production. Cementing is used to fill the annulus between the geologic formation and the wellbore casing, to hydraulically isolate the production zones the wellbore penetrates [84, 88, 106,107,108]. Wellbore cement is injected often at great depths and can be exposed to extreme conditions such as high temperature (up to 400 °C) and chemically corrosive environments (hypersaline, CO2 rich, H2S rich), which further contribute to fracturing. Both inorganic and organic formulations have been developed for wellbore cement though some show poor strength and are vulnerable to fracturing [106, 108, 109]. There have been reports of self-healing cement for wellbore applications, but the self-healing capability can be limited to single fracturing events and is vulnerable to separation of cement from casing and rock formation [21, 51]. Due to the depths and extreme environments that wellbore cement is exposed to, the development of self-healing cementitious materials (SHCM) is, at the same time, critical and difficult to achieve.
Cementitious waste forms are used to encapsulate radioactive waste with over 90% of this waste being low-activity waste (such as 99Tc and 129I). These waste forms are arguably most vulnerable to crack formation during the early curing period [110]. When a crack develops within a waste form during its service life, an increase in the rate of release of contaminants may take place due to the resulting increase in surface area and the formation of large transport pathways. Development of a cracking-preventative technology for the early life of cementitious materials ensures the integrity of the waste forms through its entire life cycle (expected to be over 10,000 years). Factors that significantly affect long-term contaminant release from cementitious waste forms are the chemical reaction pathways that occur as the cementitious waste form cures, ages, and interacts with air and water in its disposal environment. These interactions are accelerated by the formation of cracks. For example, high sulfate content of a waste stream has shown to be deleterious to the structural integrity of baseline cementitious formulations, partially due to formation of ettringite beyond the initial curing phase that leads to expansion-associated cracking [111]. Therefore, if cracks and other fluid pathways can be self-repaired before water infiltration takes place, the lifetime of the waste forms and contaminant immobilization could be significantly enhanced.
Geopolymer cement
Geopolymer cement has received significant interest because of its excellent mechanical and physical properties, low cost, low energy consumption, and reduced “greenhouse emissions” during the elaboration process [112,113,114]. Several definitions of geopolymer cement exist in the open literature but all of them seem to converge in the following designation: cementitious materials consisting of tetrahedral frameworks linked by shared oxygens as poly(sialates) or poly(sialate–siloxo) or poly(sialate–disiloxo) via long-range covalent bonds produced by the reaction of SiO4 and AlO4 [115, 116]. Geopolymer cement consists then of a polymeric Si–O–Al framework, similar to zeolites though, unlike zeolites, geopolymer cement is mostly amorphous in nature. The term geopolymer cement has also been referred to cement made by mixing aluminosilicate source materials such as fly ash and ground granulated blast-furnace slag with an alkaline solution to form silicon-oxo-aluminate chains and networks [23].
According to the reaction process, the final geopolymer cement product is dependent on two main factors: the aluminosilicate and activator [117]. Properties of the solid aluminosilicate will directly affect the dissolution and the subsequent reaction processes, while the liquid activator will partially or completely dissolve the solid raw material. The activator will also determine break and recombination of the aluminosilicate structure, polycondensation and charge balance in the reaction system. Furthermore, the type of activator will dictate the properties of the hydration products of geopolymers, while the activator’s anion plays an important role in microstructural development [117]. Activators commonly used include sodium hydroxide, silicate, carbonate, and sulfate although potassium hydroxide and sodium carbonate have also been used as activating agents [118,119,120]. Geopolymer cement has also been shown to exhibit superior mechanical properties to those of ordinary Portland cement (OPC) as a result of the hardening of the alkali-activated aluminosilicate constituent of the binding system and of the action of the technologic conditions of the hardening [121,122,123]. Geopolymer cement exhibits a wide range of properties and characteristics that make it suitable for diverse applications, depending on the raw materials used in polymer synthesis [124]. For example, the starting raw materials play a vital role in the geopolymerization reactions and control the chemical composition and microstructure of the final product.
Ali et al. reported on the autogenous healing (although they called the process self-healing) of geopolymer cement materials prepared by combining a pore-forming agent with alkaline solutions sodium silicate solution and sodium hydroxide solution as the activating agents. Geopolymer concrete materials were also prepared by using nondisclosed fine aggregates in saturated surface dry conditions [125]. Twelve beams of geopolymer concrete were tested at the ages of 14, 28, and 56 days subsequently after the healing process. The results are illustrated in Fig. 2. Every age group of the tested beams shows a similar percentage of load capacity increment. However, this percentage of load increment reduced significantly with the increased aged of the tested specimens. The beams tested at the age of 14 days showed around 166% increment in load capacity than similar beams without the exposure of the healing process. However, the specimens tested at the age of 28 days have less increment in load-bearing capacity and recorded a maximum value of increment around 72%. As the materials were aged for longer times, a lower load increment was reported and the phenomenon explained by the availability of the nonreactive cement particles which continued to react when the beams were exposed to the healing process (autogenous healing). The more reactive particles the more geopolymeric gel is produced, with the latter being responsible for the increase in load capacity. The newly formed geopolymer paste causes the load capacity to improve with the age of specimens. This observation shows similarity to the well-known behavior of OPC and OPC-based concrete, which undergoes a continuous hydration process over time and hence gains strength over time until it reaches a stable state with no additional capability for (autogenous) healing.
Left: maximum load for 145 mm × 25 mm × 25 mm geopolymer cement samples before autogenous healing (prehealing) and after autogenous healing stages for different curing times. Top right: three-point bending testing configuration; bottom right: load–crack mouth opening displacement for healing and cracking stages at 28 days. Reproduced with permission [125]
On another report by Tae-ho Ahn et al. [126], autogenous healing in concrete prepared by incorporating geopolymer cement as a partial OPC replacement was investigated in terms of recrystallization on cracked concrete and the effects of various carbonates for the recrystallization and autogenous healing. To do so, they use a geopolymer containing 71.3% of SiO2 and 15.4% of Al2O3. XRD analysis post-reaction showed SiO2, sodium aluminum silicate hydroxide [Na0.6Al4.70Si7.32O20(OH)4] as the main hydration products together with montmorillonite, feldspar, and quartz. This type of geopolymer is known to swell about 15–18 times its dry size when wetted by water due mainly to the presence of montmorillonite. Indeed, it was found that the alkaline activation of this geopolymer in the presence of calcium hydroxide led to the formation of an amorphous calcium aluminosilicate between cracks, which had the same characteristics as a geopolymeric gel in a highly alkaline environment. Figure 3 shows the healing process in the presence of water on a 0.2 mm crack as a function of time. The crack was nearly completely healed after 200 days though rehydration products between the crack were clearly observed after 14 days [126].
Process of autogenous healing of a geopolymer-modified OPC sample with a water/cement ratio of 0.45. Reproduced with permission [126]
Summarizing, although geopolymer cement exhibits superior mechanical properties to those of Ordinary Portland Cement (OPC), the main mechanism for autogenous healing in geopolymer cements and their concrete mortars resembles that of OPC and OPC-based concrete. That is, the healing is a result of the continuous hydration reactions of unreacted precursors filling the cracks. This introduces a limitation of the healing capability over time, which is mainly controlled by the amount of unhydrated precursors left in the cured material.
Engineered cementitious composites
Engineered cementitious composites (ECCs) are a subset of high-performance reinforced cementitious composites (HPFRCC) consisting of fiber-containing cements known by their high tensile ductility with moderate fiber volume fraction (typically 2% by volume) [127]. ECCs were developed for the large material volume usage and cost-sensitive construction industry and are of interest due to their high ductility and damage tolerance under mechanical loading [128]. Their ability to deform in response to high tensile strains makes ECCs autogenous healing materials. When an ECC cracks due to strain, the fibers provide bridging properties alleviating the stress on the material and preventing crack propagation. As a result, this property can be tailored to limit crack width to values below 100 μm increasing, in this fashion, the prospect for autogenous healing event [8]. In other words, narrowing the crack width aids the filling/plugging of the fracture via hydration of unreacted cement precursors (autogenous healing) [129].
It has been reported that due to the autogenous healing capability of ECC, the permeability of single and complex cracks decreased drastically with time, and materials with the smallest cracks achieved the greatest and fastest decrease in permeability post-healing [8, 130, 131]. Since ECCs are more ductile than conventional cement and concrete, structural failure by fracture generation is less of a concern. Figure 4 illustrates an ECC material that, despite the large number of small cracks, exhibits strain-hardening characteristics making it very effective in handling tensile stress. Although the large number of cracks that can be formed in ECC-based concrete structures may render the material aesthetically unappealing, its autogenous healing capability and resistance to tensile stress minimize the probability of structure’s failure [8].
Progression of crack formation in an ECC sample as strain increases. Reproduced with permission [8].
Liu et al. performed permeability studies on ECC materials under different curing and healing conditions focusing on the effect of crack width on material’s healing performance. The permeability of the ECC samples was tested under tensile load while continuously exposing them to water to promote autogenous healing. The number of cracks formed and the individual crack’s widths were monitored, and the information helped describe the change in permeability of ECC due to autogenous healing [131]. These data coupled with the crack pattern and single crack permeability led to an analytical model for predicting the changes in permeability of ECC during autogenous healing. Once again, these studies suggest that crack width has a great impact on ECC material properties and their self-healing performance. Aside from the permeability decreasing drastically over time associated with the autogenous healing process, it was found that tighter cracks healed faster and more completely [128, 129].
Research on hybrid healing methodologies, where geopolymer concrete and ECC technologies are combined, has also been conducted. Fly ash and slag have been incorporated in ECCs to determine the effects of this new composite. The study by Sahmaran et al. [132] compared ECCs performance by incorporating Class-F fly ash, Class-C fly ash, and ground-granulated blast-furnace slag in different mixtures. The group cured these materials under different conditions including continuous air exposure, continuous water exposure, and freeze/thaw cycles. It was found that the slag–ECC combination had the lowest chloride permeability which was attributed to the fine particle size and lowest available alkali content of the slag materials. The authors also found that smaller pore sizes were a result of smaller particles size, reducing chloride permeability by densification of the cement matrix. Nevertheless, although the ECC–slag hybrid materials showed lower permeability than ECC–fly ash hybrids, the latter showed a higher ability for autogenous healing. The authors also reported that, although lower particle sizes in the dry mix resulted in a more compact cement matrix and therefore a material with lower permeability, larger particle sizes provided more interparticle space among unhydrated cementitious materials enabling autogenous healing from forthcoming hydration processes.
ECCs tend to develop small cracks under stress, which highly supports autogenous healing. The only foreseeable limitation of this type of cement is the fact that the autogenous healing capability is limited by the amount of unhydrated cement present in the cured matrix. These latent (autogenous) precursors in cured cement will be reduced every time a water-triggered healing event takes place, to the point where the material no longer possesses unhydrated particles available and, as a result, no healing capability [133].
Self-healing bacterial cements
The use of bacteria for ecological engineering purposes is on the rise, and the development of bacteria-induced self-healing is part of these advances [78]. The self-healing process evolves around microbial-induced calcium carbonate precipitation (MICP), in which calcium carbonate minerals are formed from calcium and carbonate ions, obstructing cracks [76]. Different types (families) of bacteria have been studied, as well as different approaches to introduce bacteria in cement materials. Initially, MICP piqued research interests on stone surfaces, and eventually methods to bring about MICP inside cements were explored, leading to the development of self-healing cementitious material [134]. Different bacteria’s metabolic routes have been explored to trigger crack sealing in concrete. These pathways include aerobic respiration of dissolved inorganic carbon, urea hydrolysis, and nitrate reduction [68].
Bacteria need to be alkaliphilic to survive pH environments between 11 and 13 found in cement. The genus Bacillus is a subdivision of rod-shape bacteria from the Bacillaceae family, and it is an aerobic alkaliphilic spore-forming bacteria used in cement. Pei et al. [76] showed that even the walls of bacterial cells can also accelerate carbonation of concrete. Other bacteria types have also shown similar carbonation processes under alkaline environments, such as Escherichia coli [76]. Pei’s group studied the carbonation activity of cell walls from B. subtilis, Gram-positive Micrococcus luteus, and Gram-negative bacteria E. coli in calcium hydroxide solution (0.94 mM) observing in vitro precipitation of calcium carbonate. Live and dead cells of the different bacteria were tested to learn about their ability to form calcium carbonate. It was observed that all bacteria cells facilitate the production of calcium carbonate and can be incorporated in cement to bring about bacterial-based self-healing capability (Fig. 5).
Precipitation of calcium carbonate by different bacteria’s cell walls and dead cells in alkaline environments. Reproduced with permission [76].
A study done by Jonkers et al. [78] showed that although bacterial spores can be directly incorporated in cement, some of them have a limited lifetime, such as 4 months in the case of alkali-resistant spore-forming bacteria related to the genus Bacillus. Ideally, a carrier is used to protect the bacteria and ensure its survival during concrete’s mixing. Clay particles, granular activated carbon particles, and even microcapsules have been evaluated as carriers to protect and transport bacteria and other cement additives [68]. Ersan et al. [68] looked at expanded clay particles and granular activated carbon particles with size ranges of 0.5–2 mm to carry Diaphorobacter nitroreducens and Pseudomonas aeruginosa. In these studies, nitrate reduction processes were incorporated to accelerate the precipitation of calcium carbonate. Nitrate reduction occurs under limited O2 conditions and is performed by bacteria with the ability to feed on nitrate. The study found that both protective carriers along with the two bacteria systems succeeded in healing cracks ranging from 70 to 750 μm in aperture. Figure 6 shows a time lapse of cracks evolution up to 56 days for two bacteria types incorporated with granular activated carbon particles and bacteria nutrients in cement. The figure also shows a reference mortar and an abiotic control consisting of the protective carrier along with the nutrients in the cement matrix in the absence of bacteria. The successful demonstration of self-healing promoted by nitrate reduction represents an important advance of this technology since the process is more environmentally friendly due to the lack of toxic by-products produced by other bacterial metabolic pathways such as ureolysis.
Self-healing of a reference mortar, b abiotic control, c mortar with Diaphorobacter nitroreducens in granular activated carbon particles, and d mortar with Pseudomonas aeruginosa in granular activated carbon particles. Reproduced with permission [68].
Although the addition of bacteria brings about self-healing properties to concrete, it can also negatively affect its mechanical properties. Of primary concern is the fact that the addition of bacteria including Bacillus pseudofirmus has a negative effect on compressive strength and tensile strength [78]. While there are other bacteria types, including S. Pasteurii and P. aeruginosa that will maintain or increase compressive strength and tensile strength of concrete, they do not bring about self-healing capability to the material [134]. Permeability may also increase with bacteria’s concentration [75]. Another limitation associated with bacterial concrete is bacteria’s lifetime as described earlier [78]. Bacteria have specific temperature ranges and chemical conditions in which they thrive and can die when exposed to harsh chemical environments. Furthermore, certain metabolic pathways, such as aerobic respiration and urea hydrolysis that promote carbonation and self-healing in some bacterial cements, are highly dependent on O2 availability. On the other hand, anaerobic bacteria need to be tolerant to O2 since the cement matrix is oxic due to ingress oxygen (diffusion through matrix capillaries) [78]. Bacterial-based self-healing cements are appropriate for applications aboveground, in structures such as buildings and bridges, though crack opening displacement rates have to be slow to enable proper autonomous healing which can take several weeks to a few months depending on fracture size/aperture.
Self-healing cements based on microencapsulation and hollow fiber networks
Microencapsulation of healing reagent
Encapsulation can provide healing agents protection from the severe chemical environments in cement and concrete and has the capability to release the healing agent upon crack formation, for several years [64]. Figure 7 shows a microcapsule incorporated within a cement matrix [135]. Microcapsules in cement must be mechanically strong to withstand the high shear stress of the mixing process as they are distributed into the cement and concrete matrix but also must be capable to open when a crack develops in the cement matrix to release the healing agent that will cure and block the crack.
Environmental scanning electron microscopy (ESEM) image of a single microcapsule. Reproduced with permission [135].
Initially, glass was proposed for the encapsulation of healing agents; however, glass capsules typically cannot endure the mortar mixing process. Microcapsules with different wall thicknesses were studied to improve the capsule’s mechanical stability during mixing of cement, as well as to provide protection of the microcapsules against water dissolution [136]. Araujo et al. [64] developed poly(methyl methacrylate) (PMMA) capsules specifically designed to endure the mixing process. In this research, polymeric cylindrical capsules made of PMMA with varying wall thickness (0.2 mm, 0.4 mm, and 0.7 mm) were generated. The importance of wall thickness was immediately evident, as the capsules with wall thickness of 0.2 mm and 0.4 mm did not survive the concrete mixing process. Therefore, only the 0.7-mm-thick PMMA microcapsules were tested alongside glass microcapsules to compare the mechanical resistance to mixing and healing ability of these two types of encapsulating materials. To do so, water absorption was performed on mortar samples containing the different microcapsules before and after a healing event. No significant difference was observed between the self-healing efficiency of glass and PMMA capsules as demonstrated by measuring water absorption of the cracked and subsequently healed samples. The water absorption coefficients of the post-healed samples containing the PMMA microcapsules and glass microcapsules were also significantly lower than those of the reference mortar samples in the absence of microcapsules. The self-healing capability was independent of the material (PMMA or glass) from which the microcapsules were made of. The study also shows that there is a less homogeneous distribution of PMMA microcapsules in the cement matrix as compared to glass capsules. It was hypothesized that the lower density of PMMA capsules was the main reason for heterogeneity in their distribution. Furthermore, it was also found that a surface treatment of the microcapsules was needed to promote their rupture and healing agent release when small cracks (< 100 μm) developed in the concrete.
Other encapsulation materials employed to protect and transport healing agents in cement have been explored, including natural fibers, gelatin capsules, paraffin to encapsulate water, wax and polyurethane [7]. Yang et al. [67] developed silica gel microcapsules with an oil core, which were tested in combination with carbon microfibers of 3 mm in length in the cement matrix. In this study, both a healing agent (methyl methacrylate) and catalyst (triethylborane) were encapsulated in order to initiate self-healing in the presence of a crack, as illustrated in Fig. 8. As discussed in section "Engineered cementitious composites", fibers have been successfully applied to control the width of a crack in cement and concrete. As expected, the material only developed small cracks due to the presence of fibers, and the permeability of the originally fractured material decreased with time due to the self-healing capability of the microcapsules-containing cement triggered by the reaction between MMA and triethylborane [67]. It was also found that crack resistance and toughness of the specimens under fatigue loading improved with the addition of the microcapsules in combination with the fibers.
Schematic illustration of self-healing mechanism based on microcapsules. In this example, a healing agent (methyl methacrylate) and catalyst (triethylborane) were encapsulated to initiate self-healing in concrete in the presence of a crack. Carbon microfibers were also added to the cement matrix to control the width of a crack in cement and concrete and aid the self-healing process. Reproduced with permission [67].
A number of healing agents incorporated inside capsules in microcapsule-modified cements have been explored. Epoxy compounds and amine-functionalized materials were studied, and while the cement does show self-healing properties, it also displays poor mechanical properties [136]. A study by Van Tittelboom et al. [137] used polyurethanes stored in ceramic capsules which reacted in the presence of a fracture producing a foam within the cement matrix. The permeability of the composite decreased due to the expansion of the foam; however, the mechanical strength was only partially recovered. As described in "Self-healing bacterial cements" section, bacteria have also been microencapsulated. For example, Wang et al. studied the self-healing capability of bacteria inside microcapsules in cement. The study shows that the cement healed itself in the presence of cracks as wide as 970 μm with a resulting reduction in permeability. Despite the advances in microencapsulation-based self-healing of cement and concrete, a huge impediment still remains: multiple self-healing events at the same location are limited to the availability of unreacted precursors after each healing event. This is due to the irreversible nature of the self-healing process (breaking of the capsule followed by mixing and reaction of the healing precursors). Nevertheless, recent research efforts focused on smart release of healing agents where capsules may be designed so that multiple healing cycles can be achieved [138]. However, there are a limited number of publications where clear evidence of multiple self-healing cycles in cement and concrete materials with microencapsulation technology is demonstrated. Thao et al. [139] demonstrated two healing cycles on a cement material containing exceptionally large tubular capsules (400 mm in length) with high storage capacity for a polymeric healing agent. In this study, the group used four-point bending tests to show 88% and 85% recovery of stiffness after the first and second healing events attributing the small decrease to reduction in healing agent [139]. Van Tittelboom et al. [137] also evaluated the effectiveness of healing under multiple loading cycles by incorporating healing agent (including epoxy resins, polyurethane, and methyl methacrylate) in tubes at predicted damage locations. They found that recovery of stiffness decreases under multiple cycles of loading with glass capsules performing best for recovery of strength while ceramic capsules being the material of choice for permeability reduction post-healing [137, 140].
Hollow fiber network
Similar to the microencapsulation approach, hollow fiber networks work as carriers for healing agents which are released when a crack is formed. Originally developed for polymer composites, the concept consists of inserting long hollow fibers filled with a healing agent into the cement matrix. The cement material is therefore protected against cracking provided the fibers are distributed in the entire matrix. As in the case of microencapsulation, a crack would simply open a fiber and release the healing agent in the composite [141, 142]. The fibers with healing agent resemble the cardiovascular system of an organism transporting and releasing the healing agent to where the damage occurs [143]. The self-healing efficiency of this method was demonstrated in an experiment using a UV fluorescent dye and UV mapping techniques [143]. In addition to demonstrate the release and infiltration of healing agent into the crack and subsequent healing of the fracture, it was shown that the healed material recovered its flexural strength up to 93% [144]. However, in this particular study, the healing capability dramatically decreased with time (weeks) due to degradation of the repairing resin [144].
An adhesive methyl methacrylate was also used as a healing agent in cement showing restoration of mechanical strength, increased ductility, and effective healing of cracks [142]. In this work, methyl methacrylate was mixed with cobalt neodecanoate as the healing agent and cumine hydroperoxide as the catalyst. This three-component adhesive system was later reduced to two components due to the potential explosion of cobalt neodecanoate and cumine hydroperoxide in the absence of methyl methacrylate. Although successful in bringing about self-healing capability to cement, like in the case of microcapsules, three conditions are required: a hollow fiber containing the healing agent, a separate hollow fiber containing the catalyst, and simultaneous breakage of the two. It was also found that the liquid components were too fluidic, so a single crack could exhaust a large quantity of healing agent. The overall concept and mechanism of hollow fibers are nearly identical to microcapsules, with the difference in larger or longer protective carriers. Regardless of the length of the hollow fibers and their concentration and distribution in the cement matrix, a crack needs to break at least one fiber (or two if a catalyst is required) in order for the polymer precursors to react and heal the fracture. Due to the close parallels between the two methods, both hollow fibers and microencapsulation-based self-healing approaches can be applied to the same type of concrete structures, including dams, underground structures, and bridges [137].
Self-healing assisted by shape-memory alloys
A different approach in SHCMs consists of physical contact followed by chemical bonding if at all possible. This method of healing is achieved by incorporating shape-memory alloy (SMA) bars as tendons in cementitious materials [145]. SMAs have high power density, solid-state actuation, high damping capacity, durability, and fatigue resistance [146, 147]. By heating the SMA, the SMA shrinks and as a result the crack closes. The compressive stress generated by the shrinkage of the SMA across the crack guarantees a long-lasting repair as illustrated in Fig. 9. Many types of shape-memory alloys have been explored, with the most commonly used being Nitinol (a nickel–titanium alloy) due to its superior thermomechanical and thermoelectric properties [148].
Schematic illustration of SMA healing method. PET is polyethylene terephthalate. Reproduced with permission [145].
This method of self-healing by physical contact is possible due to the combination of two properties in a single material: shape-memory effect and superelasticity. The shape-memory effect refers to the ability of SMAs to revert back to their original shape upon heating (typically to a temperature between 60 and 110 °C, although can be extended between − 20 and 200 °C) [148], while superelasticity refers to the ability to endure large amounts of inelastic deformations followed by recovery of the original shape [148]. SMAs possess these unique properties due to the reversible phase transitions between the two crystal phases in an alloy. In the case of Nitinol and similar alloys, these phases are austenite and martensite [147]. The stronger austenite phase is stable at high temperature and has a body-centered cubic crystal structure and can transition to martensite, a weaker phase when cooled down. Martensite, which is stable at low temperature, has an asymmetric parallelogram structure. The martensite phase has 24 geometry variations which the alloy takes advantage of when deformation occurs in order to withstand the stress. Nevertheless, and as expected in any material, applied stress beyond the yield point will result in a plastic (permanent) deformation [147].
SMAs have been used in combination with hollow fibers in order to provide a physical followed by a chemical healing mechanism to fix large cracks [149]. Kuang et al. produced SMA-reinforced concrete beams, with wires made of SMA. Samples were synthesized with different numbers of SMA wires per volume of concrete, and in some cases, epoxy adhesive-filled hollow brittle fibers were added as a healing agent. It was found that in all the SMA-reinforced beams the deformation and the width of the cracks increased during loading; however, after unloading, the deflection of the beams reversed and the crack sealed to near completion. As expected, samples containing epoxy adhesive-filled hollow fibers in addition to SMA wires performed better in repairing the concrete samples as compared to those that only contained SMA wires. The combination of these two healing mechanisms is significantly beneficial in cementitious structures. However, it can also significantly add to concrete cost. The shape-memory effect and superelasticity properties of SMAs allow for SMA-modified concrete to recover from extremely large deformation making it ideal to withstand earthquakes. Thus, this healing methodology could be applied in structures subjected to temperatures in the range of SMA’s transition temperatures and include nuclear reactor structures, pavement, bridges, and commercial and residential infrastructure.
Self-healing using superabsorbent polymers
The use of SAP to promote autogenous and autonomous healing is widely reported in the literature [20, 92,93,94,95,96,97,98,99, 150,151,152,153,154]. Hydrogels, or SAP, possess the capability to absorb a significant amount of water from the environment (up to 500 times their own weight). These materials can absorb these large quantities of fluid and retain the liquid within their structure without dissolving. SAP particles and fibers swell due to the osmotic pressure difference between the hydrogel and the external solution. This property has promoted the use of SAP in the hygiene and food industry, and also in building applications. Indeed, SAP particles have been used in concrete to decrease the autogenous shrinkage by aiding to internal curing [155, 156]. Lee et al. [94] and Snoeck et al. [152] investigated the incorporation of SAP in concrete in order to obtain autogenous and autonomous healing properties.
When water enters a crack, SAP particles swell until they contact the crack walls and block the crack as it was demonstrated by a decrease in water permeability through a crack. This is another example of autonomous healing or self-healing. Furthermore, the absorbed water can also contribute to autogenous healing of the cement and concrete materials where hydration reactions of unreacted cement precursors take place to further seal the crack (Fig. 10) [20, 95,96,97, 151, 152]. This was demonstrated by Snoeck et al. [152] where it was observed that the water absorbed by SAP can be transferred to the cementitious matrix for further hydration and the precipitation of calcium carbonate. Snoeck et al. [20] went even one step further and combined SAP with reinforcing microfibers made of poly(vinyl alcohol) (PVA) to maintain cracks narrow enough (30–50 μm) to be completely healed. They showed that at 7 days, the main healing mechanism is the sewing of a crack by further hydration. Between 28 days of aging and up to 8 years of aging, calcium carbonate crystallization continued to be the main healing mechanism. It was, however, found that after that time, the healing and regaining of mechanical properties decreased, and the healing of aged samples decreased to a third relative to specimens 7 days old. Recovery of mechanical strength was dependent on relative humidity with high relative humidity values generating less strength recovery (about 60%). However, wet/dry cycles generated new cracks instead or reopening of healed cracks showing the potential of these materials for autogenous healing without generally affecting the mechanical strength. SAP that swell in the presence of hydrocarbons have also been investigated as additives in wellbore cement for fossil fuels recovery [157,158,159]. Zichen et al. [157] synthesized an oil swellable polymer (OSP) with oil absorptivity as high as 700%. The OSP dispersion was introduced into the Portland cement paste. It was shown that the addition of OSP decreases the total porosity of cured cement due to the formation of homogeneously distributed polymeric materials in the cement matrix. The self-healing ability of OSP-modified cement was demonstrated by a decrease in oil permeability through a crack due to the expansion of the OSP. Yang et al. [159] combined a number of approaches including microfibers, microencapsulated oil/silica gel core/shell particles. The oil phase was methyl methacrylate monomer and triethylborane as the healing agent; the catalyst was either separately encapsulated as an oil core or surrounded by the passive silica gel capsule. The microcapsules were dispersed in fresh cement mortar along with carbon microfibers. Once cured, self-healing in the cement matrix was triggered by crack propagation through the microcapsules, which then released the healing agent and the catalyst (both described as the oil phase) into the microcracks. Polymerization of the healing agent was initiated by contact with the catalyst, filling the cracks. The self-healing ability was partial with the highest percentage of decrease in the permeability coefficient being 50.2% and 66.8% at ages of 3 days and 30 days, respectively [159].
Autogenous healing mechanisms showing partial (a) and further hydration (b) partial (c) and full calcium carbonate crystallization (d) and the combination of both in a number of cracks (e) and close-up (f). The scale bars amount to 200 μm (a–d, f) and 2 mm (e). Reproduced with permission [20].
Self-healing cement and concrete using OSP or SAP can be applied in subsurface wellbores in unconventional (tight) oil recovery as well as geothermal energy recovery. A main disadvantage of this technology, however, is the fact that fractures need to be (1) connected to an oil or water source (such as a puncture in the casing) and (2) conductive enough for the fluids to access the swellable polymers. Furthermore, this self-healing method should better be described as self-filling since there is no chemical bonding between the swollen polymer and the fracture walls and, as a result, the structural integrity of the cement is still compromised.
Self-healing polymeric cementitious composites
Studies have shown that polymer-modified cements have desirable properties such as increased fracture toughness, low permeability, improved ductility, and chemical resistance [107, 160]. There are an impressive number of publications on self-healing polymers based on a variety of healing mechanisms. These include reversible formation and breakage of hydrogen bonds [161], rearrangement of covalent bonds [162], Diels–Alder reaction [163], reversible formation and breakage of coordination bonds and reversible supramolecular ionic interactions [164]. Although microcapsules and hollow fiber networks are effective vehicles for hosting polymer precursors and other healing additives (including bacteria) in cement and concrete, introducing self-healing polymers without the need of a carrier would simplify the preparation of self-healing cement materials with an associated reduction in manufacturing costs. To this end, several groups have proposed mixing polymers or self-healing polymers with cement in the absence of a carrier [92, 94, 165,166,167,168,169,170,171]. A good number of these polymer-based self-healing cements have been developed for the oil and gas industry but can be extended to other applications. The self-repairing ability was reported to be based on various mechanisms: (1) swelling of polymer (superabsorbent polymers) in the presence of fluids such as water [92, 94, 170] or water reach in H2S and HCl [171]; (2) heat-activation by transitioning from solid to liquid or through different crystalline and amorphous phases [166, 172]; and (3) expansion and contraction of shape-memory polymers [169]. Polymeric healing represents a new pathway for self-healing cement and concrete, and the use of superabsorbent polymers is leading this trend. However, one of the main drawbacks in the modification of cement and concrete materials with superabsorbent polymers is the fact that flow of water or other fluids is required for the polymer to swell and close the fracture and, as importantly, that there is no chemical bond between the filling polymer and the crack walls. For subsurface geothermal and oil/gas wellbore applications or applications in infrastructure, this is not favorable, since corrosion of casing (wellbores) and corrosion of reinforcement materials (such as steel rebar used in infrastructure) bring about additional and, sometimes, more serious problems. Nevertheless, the majority of these polymer-based cements cannot be applied in high-temperature environments due to the thermal degradation of the polymers. In this regard, our group developed the first polymer-modified self-healing cement suitable for high-temperature geothermal wellbore applications [82]. The material consists of thermally stable self-healing thermoset epoxides homogeneously distributed in class-H cement. The resulting polymer–cement composites showed multiple self-healing capability, improved cohesive, rheological and mechanical properties (higher ductility) suitable for geothermal applications.
In the above example, thermoset epoxies with disulfide bonds in the monomer backbone and multifunctional thiol cross-linkers that self-heal through reversible interactions were utilized. The likely intrinsic healing mechanism is proceeding via a substitution reaction of free thiolate group with a disulfide to form a new disulfide group and free thiol (Fig. 11) [82, 173,174,175]. Later, atomistic simulations and spectroscopic studies by our group showed that three kinds of bonding interactions are responsible for the self-healing mechanism of cement modified with the above-described thermoset epoxides. The healing mechanism is explained in detail in "First-principles-based approaches" section. The amount of polymer added as well as the water-to-cement ratio also plays a role in the performance of the material. Other epoxy polymer candidates with intrinsic healing temperatures between 60 and 100 °C have been reported that could also be, in principle, incorporated in cement [173,174,175]. Nevertheless, the polymer-based cement showed high thermal stability (up to 350 °C) and significant permeability reduction (up to 87%) after healing mechanically generated fractures as large as 0.5 mm. The self-healing capability was later reported to be the result of reversible and dynamic chemical interactions between the polymer and cement matrix, which include covalent bonds, hydrogen bonding, and van de Waals interactions [83]. It was also demonstrated that these polymer-modified cements have the ability to self-heal over multiple cycles in the same location resembling “molecular Velcro” in contrast to microencapsulation and hollow fiber-modified cements. Because of their great thermal stability and multi-self-healing capability, these materials represent a superior alternative for subsurface applications as well as in concrete-based structures.
a Chemical structures of reactant monomers and mechanisms of polymer curing and self-healing. Reproduced with permission [82]. Copyright 2017, American Chemical Society. b X-ray microtomograph showing the polymer described in a homogeneously distributed in the cement matrix. Please note a fracture (~ 0.4 mm) has been healed by the polymer. c Cylinder monoliths with a longitudinal fracture mechanically created. Left and center—two different self-healing polymer–cement formulations showing the fracture sealed; right—conventional cement
Modeling of SHCM at (sub)nanoscale
Parallel to experimental approaches to develop SHCM, modeling methods provide invaluable insights regarding the self-healing processes and resulting mechanical properties. Modeling methods can be invaluable tools to guide the design of novel cementitious materials and predict their properties, and in this section, we will present a brief review of modeling work related to cementitious materials. A recent review by Jefferson et al. [176] discusses models addressing various self-healing aspects of cementitious materials at microscale and beyond, such as mechanics or transport. Needless to say, mechanical behavior of a material strongly depends on its local molecular structure and local bonding properties. While at microscale modeling, self-healing cements are considered isotropic materials, at nanoscales, their isotropy no longer exists. In the following sections, we will summarize some of the recent progress using molecular simulation based on numerical studies of (sub-)nanoscales in this emerging and rapidly expanding field.
Force field-based approaches
The majority of reported findings on cementitious materials at nanoscales are obtained from classical force field-based simulations (FF). Force field methods provide fast, computationally tractable approaches. There are force fields especially designed or widely used for this class or similar classes of materials such as CementFF [177], ClayFF [178], IFF [179], CSH-FF [180], or ReaxFF [181]. Although cement has been studied for decades and there have been numerous models of the nanostructure scale [182], it was not until recently that a computation-driven molecular model of calcium–silicate–hydrate (C–S–H), the main component of the hydrated Portland cement, was proposed, based on a “bottom-up” atomistic simulation approach [183]. In this approach, Pellenq et al. began with a monoclinic periodic cell of dry tobermorite, Ca6Si6O16, which contains two CaO layers and eight silicate chains. Guided by rock mass rating (RMR) [184, 185], they remove SiO2 groups in silica tetrahedra, creating a defective structure which subsequently is relaxed at 0 K using the core–shell model interatomic potentials developed by Pellenq et al. [183]. They then carried out Grand Canonical Monte Carlo (GCMC) simulations of water adsorption in the defected cell at 300 K, followed by relaxation at 0 K of the hydrated structure. Here, despite the fact that the authors did not consider the presence of any OH group in their molecular model of (CaO)1.65(SiO2)(H20)1.75, the chemical composition was consistent with experiments regarding chemical composition, structural and mechanical properties. Nevertheless, this model also has other issues such as unrealistic CaO bond lengths and mass density, or the unlikely presence of monomeric silicate units. In a recent study, Kovačević et al. [186] have proposed three different models for C–S–H to help resolve some of these structural issues. These models were very similar to the approach by Pellenq et al. [183], that is, changing the known structure of tobermorite to make it consistent with the correct chemical composition of C–S–H. Specifically, Kovačević et al. [181] considered different ways of removing the silicate units: (1) removal of only bridging silicate tetrahedrons by a random process; (2) removal of silica chain dimers; and (3) randomly removing silicate units. This was followed by further removal of Ca and addition of H atoms to balance the charges. ReaxFF force field-based molecular dynamics simulations were used to identify the most stable structure for C–S–H. From these simulations, the most likely structure has a composition of Ca256Si192O576·224 H2O which provided a Ca/Si ratio of 1.67, close to the experimental composition. These initial molecular models form the basis of a basic CSH model that can be used to build more complex models for self-healing cementitious materials, see "First-principles-based approaches" section.
One of the most fundamental aspects of atomistic modeling of self-healing cements is the understanding of the interfacial properties of C–S–H and additives such as reinforcing materials including carbon nanotubes (CNTs), carbon nanofibers, etc. Such an additive is expected to influence the mechanics and chemistry of C–S–H. As an example, due to exceptional mechanical and thermal properties of CNTs [187], proposed composites of cement and CNTs are in principle viable. It has been shown that cement-based materials can be engineered at nanoscales by addition of CNTs. By incorporating treated multiwalled CNTs into cement, Li et al. [188] found that both compressive and flexural strengths of the cement were greatly increased by 19% and 25%, respectively. Some nanoscale-level understandings of such a composite system (see scheme in Fig. 12) have been recently achieved. Eftekhari and Mohammadi [189] adopted a computer model in which a CNT was inserted to a C–S–H bulk along the normal of the silicate layers, in Fig. 12. The insertion procedure involved an initial creation of a void cylindrical hole running through the C–S–H box by gradually indenting the center of the box with a diameter of 12.5 Å, which was done by means of a built-in function in the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) code [190]. This was followed by the introduction of CNTs. The authors considered two CNT structures, the armchair (6,6) and the zigzag (10,0) [191], which have equal diameters (8.13 Å) and length (40 Å) but possess different mechanical properties. Of the two CNTs, the zigzag structures offered a higher compressive strength (by 10%), while the armchair structure had a higher tensile (by 21%) and shear (by 24%) strengths. Their classical molecular dynamics simulations, based on a standard force field (but excluding dihedral terms) for C–S–H and the Tersoff potentials [192] for CNTs, predict that the z-direction tensile strength of C–S–H + armchair CNT and of C–S–H + zigzag CTN is 4.2 and 3.5 times higher than that of C–S–H, respectively. They did not see any improvement regarding the compressive and shear strengths of C–S–H by CTNs. The authors also pointed out that when a crack in the C–S–H interlayer appears, the CNT bridges two sides of the crack (given the inherently higher strength of CTNs vs C–S–H) and bears the loading until the whole system ruptures. Using a similar computer model to that of Eftekhari and Mohammadi, Lushnikova and Zaoui [193, 194] used classical molecular dynamics simulations. These simulations were based on mixed Morse–Buckingham three-body and spring potentials for C–S–H and the Tersoff potentials for CNTs. The authors considered various CNTs and 11 Å-tobermorite [195] structures of different C–S–H compositions and found that for each C–S–H composition, there is an optimal CNT structure to maximize the CNT/C–S–H adhesion and the improvement in a mechanical property. Building upon the approach by Eftekhari and Mohammadi, in order to have the lowest-energy CNT/C–S–H distance, Lushnikova and Zaoui varied the CNT hole radius, and they found the optimal distance to be 2 Å in most cases. At the Ca/Si ratio of 0.83, it is predicted that the energetically favorable CNT structure for CNTs and tobermorite adhesion is the zigzag CNT(3,0) [193]. With the zigzag CNT(3,0) structure incorporated, all the mechanical parameters were increased; for example, the Young’s modulus increased from 78.4 to 107.7 GPa. They also found that in the CNT diameter range of 3.3–4.1 Å, the composite can change from being brittle to being ductile. At the Ca/Si ratio of 1.0, however, they found that the armchair CNT(2,2) structure incorporated in C–S–H had the best adhesion energy [194]. Meanwhile, CNT(2,2) increased the shear modulus (17.3%), Young’s modulus (13.8%), and plane stress modulus (10.8%). A different trend was apparent in the study by Eftekhari and Mohammadi. Lushnikova and Zaoui also showed that the best concentration of CNT(2,2) that allows for the increase in bulk, shear, and Young’s moduli in tobermorite is 5.2% weight.
Schematic insertion of a CNT into C–S–H. Reproduced with permission [189].
Besides using CNTs to improve mechanical properties of C–S–H, polymers, such as in the form of fibers, were also used as reinforcing agents in cement-based materials as it was discussed earlier [196]. Understanding the interfacial bonding between the polymer and C–S–H then becomes a key issue. By atomistic modeling based on the COMPASS force field [197], Shalchy and Rahbar have shown that the adhesion energies between polymers (nylon-6, poly(vinyl)alcohol (PVA), and polypropylene) and C–S–H gel increased with the Ca/Si ratio and that the electrostatic contribution was the dominant component of the adhesion energies [198]. This indicates a correlation between the adhesion energy and the content of Ca2+ cations. The adhesion energy of nylon-6 is higher than that of PVA, which is higher than that of polypropylene, due to different polarities of each polymer as a result of the presence of different functional groups. Interestingly, despite many polymer hydrogen and C–S–H oxygen atoms forming hydrogen bonds at the interfaces, their contribution to the adhesion energy is very small (nylon-6) or negligible (polypropylene). This is somehow contradictory to the study by Sakhavand et al. [199] based on the CSH-FF potential [200] showing that hydrogen bonding is a major component for the interaction and load transfer between PVA and silica chains of C–S–H. Clearly, the role of functional groups in polymers is crucial. In another study, Zhou et al. investigated structural, dynamical and thermodynamic of the interfaces of C–S–H and poly(acrylic acid) (PAA), PVA, or poly(ethylene glycol) (PEG) [201]. Using the ClayFF [178] force field molecular dynamics simulations, they found that PAA bound most strongly to the C–S–H surface, while PEG showed the weakest interactions with C–S–H. In a water environment, the average residence time of functional groups in the interfacial area of PAA is about four and ten times more than that of PVA and PEG, respectively, showing that the binding affinity of the COOH group to C–S–H was much higher than that of the OH and C–O–C groups. That said, the vertical movement of PAA in the interfacial area was still restricted. Because of the coexistence of OH-based intramolecular and intermolecular hydrogen bonds of PVA, this polymer can move away from the interfacial area to the bulk solution where it is solvated, or back to the interfacial area to interact with C–S–H. Additionally, due to the weak interaction with C–S–H or with another polymer, PEG was found to move relatively freely in the pore in nanopores between C–S–H sheets.
First-principles-based approaches
Thus far, the use of first-principles methods to design self-healing cements and predict their properties has been rather limited. Understandably, given the complexity of these systems, a large number of atoms and electrons in a simulation box are unavoidable and molecular dynamics simulations based on first-principles methods can be challenging. Consequently, to our knowledge, very little has been done in the field at this level of theory. To gain a fundamental understanding on how the chemistry and mechanical properties of self-healing cements change under certain conditions, it is necessary to apply quantum mechanics-based molecular dynamics methods. Among first-principles methods, density functional theory (DFT) [202, 203] has been most widely used in cementitious materials research. To date, almost all the first-principles studies of cementitious materials, employing DFT calculations, are based on static optimizations that offer very limited, if any, understanding of dynamics-related processes such as aggregation or free energy-activated processes. These studies mostly focus on the understanding of fundamental properties (bond lengths, electronic states, elastic constants, etc.) of largely known bulk compounds such as 9,11,14 Å tobermorite mineral [204, 205] and jennite [204]. In a more relevant study, Sakhavand et al. [199] used DFT calculations to estimate the O–H stretching frequency of hydroxyl groups in PVA in contact with a silicon tetrahedron, further verifying results from classical molecular dynamics simulations.
A very recent study by Nguyen et al. [83] is an exception. In this study, by means of ab initio molecular dynamics simulations, the authors validate a hypothesis of the self-healing process/mechanism of polymer–cement composites previously observed experimentally. As shown in Fig. 13, the authors hypothesize that initially a polymer is adsorbed on a natural surface of C–S–H, and when a crack in C–S–H occurs, the polymer will diffuse to the crack, filling the hole. For this study, the authors adopted the mostly like C–S–H composition proposed by Kovačević et al. to build highly disordered C–S–H surface unit cells of about 450 atoms. Two different surfaces, one representing a natural surface of C–S–H crystallites and one representing a cracked surface of C–S–H crystallites, were considered. A single polymer strand (116 atoms) containing different functional groups including hydroxyl and carbonyl was used to represent the polymer used in the synthesis of these materials [82]. By tracking down the dynamics of the functional groups of the polymer at the interfaces, three kinds of bonding were identified: hydrogen bonding (between polymer OH and CH, and C–S–H O atoms), ionic bonds (between polymer O and S, and C–S–H Ca), and van der Waals interactions. The strong hydrogen bonding between the OH groups and the crack surface led to a proton transfer process from the polymer to C–S–H. This proton transfer process was associated with a vibrational frequency of ~ 1270 cm−1, which was further validated with subsequent sum frequency generation spectroscopy experiments. In addition, the soft H-bonds can easily break and reform around areas of cracks, keeping the overall number of H-bonds constant. Overall, this study emphasizes the power of ab initio molecular dynamics to be able to treat both dynamical behavior and chemical changes on equal footing. By computing the potential of mean force, which provides information about the free energy change of a system against a set of reaction coordinates, it was also found that the ionic bonds between the polymer and C–S–H at a crack’s surface were stronger than at the natural surface. By analyzing the elasticity of the polymer on the surface, they found that it became more rigid on the cracked surface than on the natural surface. This further validates that when a crack occurs, the polymer will diffuse to the crack and heal it.
Schematic self-healing process of a polymer–cement composite: polymer diffuses from a natural surface to a crack surface of cement. Reproduced with permission [83].
Given the roughness/disorder at surface cracks, the charge states and coordination number of atoms involved in the interfacial bonding change during the course of time. Building a working force field for such a dynamic interface is a formidable task. The rapid development of numerical approaches and computer hardware allows for large-scale ab initio molecular dynamics simulations that can address at least in part such problems. On the other hand, it has been shown that covalent linkages between polymers and C–S–H promote the dispersion of polymers and enhance the interfacial energy [206, 207], which are major components in composite fracture toughness. Applications of ab initio techniques in self-healing cements research are viable and will become a more available tool in a not very far future.
Conclusions and outlook
Stimulated by the incredibly wide range of applications, cements and concrete materials with superior performance represent an especially important research and development area with the main focus being materials with autonomous healing capability. Additive materials such as fly ash are used to improve mechanical properties, as well as to promote autogenous healing by the presence of unreacted (anhydrate) cement reagents in the so-called geopolymers [23, 130, 208].
ECCs exploit the ability of fibers to control (reduce) crack width, which assists self-healing processes due to the proximity of the two fractured faces [8, 67]. Similarly, shape-memory alloys can physically pull fractures together to aid self-healing. Among self-healing methods bacteria added to the cement matrix provide an attractive approach to bring about self-healing via carbonation reactions [74, 77]. Polymers have also been extensively used to generate self-healing to cement and concrete (via, for example, cross-linking reactions). To this end, microcapsules are typically employed to carry/transport polymer precursors or bacteria to protect them or simply prevent contact with the matrix until a crack is developed. When a crack is formed, the capsules are broken and healing takes place. Likewise, hollow fibers are used to carry and release healing agents along the entire strand and promote healing upon rupture from a crack. Polymer–cement composites incorporate polymers directly into the matrix, without the need for encapsulation, to enable self-healing via the ability of the polymer to migrate to cracks and form reversible bonds with the cement matrix resembling dynamic molecular Velcro.
All of these methodologies have proven to bring about self-healing capability to cementitious materials though each of them has its advantages and disadvantages. For example, geopolymers ultimately rely on unhydrated cementitious material and the contact with water or CO2 (carbonation of dissolved calcium hydroxide) to heal. ECCs rely on similar mechanisms; however, they are extremely ductile resulting in the formation of small and controlled cracks upon failure. SMA physically closes cracks; however, for any actual chemical-based self-healing to occur, hydration of unreacted cementitious reagents, polymer reactions, or bacteria-triggered carbonation is also required. Microencapsulation and hollow fiber networks are the main vehicles for the transport of bacteria or polymer precursors. They need to be homogenously distributed in the cement matrix and with a number density large enough to release the healing agent in the presence of a crack in the material. Nevertheless, once the healing agent at a specific location is used up, it cannot be replenished. Bacterial cement composites along with polymeric cement composites both provide a mechanism for self-healing throughout the entire material independently of the crack location [68, 76, 78, 134]. The main drawback in bacteria-based cements is that bacteria cannot be used in extreme temperature environments and healing rates are significantly slow (weeks to months) for some applications. As described earlier, often bacteria require microcapsules to protect them during cement and concrete mixing and deployment. In addition, bacteria not only have specific conditions in which they can thrive, but they have a limited lifetime and can run out of the necessary reagents (e.g., feed) to survive.
Thermally stable and chemically robust polymer–cement composites present a number of advantages over other self-healing approaches since, in principle, they can self-repair through the lifetime of the structure independently of the number repair cycles or crack location [82, 83] (as compared to other self-healing cement technologies where the number of healing events is limited by the continuous consumption of healing additive) [14, 93]. Polymer–cement composites also offer the possibility of fast (less than 24 h) healing capability due to the presence of reversible and dynamic polymer–cement bonding. These composites increase cement and concrete ductility, a key property to reduce/minimize fracture propagation. Nevertheless, further studies are required to determine the ability of polymer–cement composites to bring about self-healing capability throughout the lifetime of the cement and concrete structures.
To this end, molecular simulations can play a significant role and are already showing promise for a closer look at the atomic-level interactions between cementitious materials and other add-on compounds such as CNTs or polymers [83, 187, 188]. The biggest challenge is to achieve a sufficient approximation of such complex and dynamic systems that cementitious materials represent so new self-healing formulations can be designed toward the preparation of cement-based materials with long-term self-healing capability. We anticipate that modeling and simulations can be utilized to provide useful insights that can be directly validated by experiments and design principles facilitating the advancement of these sophisticated materials for superior performance in a wide range of applications.
One last consideration is cost. Cement is “dirt cheap,” only 10–15 cents a pound, and the incorporation of additives to trigger autonomous healing increases its cost. For example, our group estimated the cost of cement to be about twice the cost of OPC, and although ongoing efforts to reduce polymer concentrations while maintaining self-healing ability are promising, a self-healing cement will always be more expensive than conventional cement. Nonetheless, at the time of constructing new concrete-based infrastructure, the construction industry should evaluate lifetime cost associated with inspection, maintenance, and repair. For example, annual maintenance cost for bridges, tunnels, and other essential infrastructure in the USA alone represents a trillion dollar market [209]. In a 2019 report by Arno Schroten et al. [210], it was stated that maintenance of transport infrastructure alone in Europe reached €38 billion with an additional €69 billion investment in new roads as of 2016. Considering the safety concerns and above-described enormous cost associated with structural repairing, rebuilding and downtime, self-healing cementitious materials need to become a viable alternative to conventional cements, despite their higher cost. Furthermore, the above-described cost considerations do not even account for concrete waste disposal and the significantly high carbon footprint that the cement industry generates, with one pound of CO2 emitted per every pound of cement produced.
The capability of multiple healing cycles at any location offered by advanced cement materials suggests that it is just a matter of time for these cements to be part of next-generation building infrastructure significantly reducing the need for maintenance and replacement of entire concrete structures [82, 83]. An understanding of the properties of these materials and their limitations, independently of the self-healing strategy, will dictate their application and lifetime. Perhaps the continued convergence of different self-healing methods could result in next-generation cement and concrete materials.
References
Van Breugel K (2007) Is there a market for self-healing cement-based materials. In: Proceedings of the first international conference on self-healing materials, Noordwijk. Holland, pp 1–9
Worrell E et al (2001) Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ 26(1):303–329
Limited IR (2020) Concrete and cement market by product, end-user, and geography—forecast and analysis 2020–2024, p 120
Cailleux E, Pollet V (2009) Investigations on the development of self-healing properties in protective coatings for concrete and repair mortars. In: Proceedings of the 2nd international conference on self-healing materials, Chicago, IL, USA
Frank T (2017) Civil engineers say fixing infrastructure will take $4.6 trillion. CNN Money
Yunovich M, Thompson NG (2003) Corrosion of highway bridges: economic impact and control methodologies. Concr Int 25(1):52–57
Hilloulin B et al (2015) Design of polymeric capsules for self-healing concrete. Cem Concr Compos 55:298–307
Lepech MD, Li VC (2009) Water permeability of engineered cementitious composites. Cem Concr Compos 31(10):744–753
Claisse PA (2014) 1—The transport properties of concrete and the equations that describe them. In: Claisse PA (ed) Transport properties of concrete. Woodhead Publishing, Oxford, pp 1–16
Hoseini M, Bindiganavile V, Banthia N (2009) The effect of mechanical stress on permeability of concrete: a review. Cem Concr Compos 31(4):213–220
Jacobsen S, Marchand J, Gerard B (1998) Concrete cracks I: durability and self-healing—a review. Proc Consecutive 98:217–231
Muhammad NZ et al (2016) Tests and methods of evaluating the self-healing efficiency of concrete: a review. Constr Build Mater 112:1123–1132
Vijay K, Murmu M, Deo SV (2017) Bacteria based self-healing concrete—a review. Constr Build Mater 152:1008–1014
Snoeck D, De Belie N (2015) From straw in bricks to modern use of microfibers in cementitious composites for improved autogenous healing—a review. Constr Build Mater 95:774–787
Dong B et al (2018) Chemical self-healing system with novel microcapsules for corrosion inhibition of rebar in concrete. Cem Concr Compos 85:83–91
Wang C-K, Salmon CG (1979) Reinforced concrete design
Cabrera J (1996) Deterioration of concrete due to reinforcement steel corrosion. Cem Concr Compos 18(1):47–59
Guzmán S, Gálvez JC, Sancho JM (2011) Cover cracking of reinforced concrete due to rebar corrosion induced by chloride penetration. Cem Concr Res 41(8):893–902
Shi X et al (2012) Durability of steel reinforced concrete in chloride environments: an overview. Constr Build Mater 30:125–138
Snoeck D, De Belie N (2019) Autogenous healing in strain-hardening cementitious materials with and without superabsorbent polymers: an 8-year study. Front Mater 6(48):1–12
Van Tittelboom K, De Belie N (2013) Self-healing in cementitious materials—a review. Materials 6(6):2182–2217
Li W et al (2018) Recent advances in intrinsic self-healing cementitious materials. Adv Mater 30(17):1705679–1705687
Noushini A et al (2016) Compressive stress-strain model for low-calcium fly ash-based geopolymer and heat-cured Portland cement concrete. Cem Concr Compos 73:136–146
Wilinska I, Pacewska B (2014) Calorimetric and thermal analysis studies on the influence of waste aluminosilicate catalyst on the hydration of fly ash-cement paste. J Therm Anal Calorim 116(2):689–697
Darsanasiri AGND et al (2018) Ternary alkali aluminosilicate cement based on rice husk ash, slag and coal fly ash. J Build Eng 19:36–41
Singh LP et al (2019) Durability studies of nano-engineered fly ash concrete. Constr Build Mater 194:205–215
Pereira EL, de Oliveira AL, Fineza AG (2017) Optimization of mechanical properties in concrete reinforced with fibers from solid urban wastes (PET bottles) for the production of ecological concrete. Constr Build Mater 149:837–848
Marthong C, Sarma DK (2016) Influence of PET fiber geometry on the mechanical properties of concrete: an experimental investigation. Eur J Environ Civ Eng 20(7):771–784
Bui NK, Satomi T, Takahashi H (2018) Recycling woven plastic sack waste and PET bottle waste as fiber in recycled aggregate concrete: an experimental study. Waste Manag 78:79–93
Al-Hadithi AI, Noaman AT, Mosleh WK (2019) Mechanical properties and impact behavior of PET fiber reinforced self-compacting concrete (SCC). Compos Struct 224:111021
Soydan AM et al (2018) Air-cured fiber-cement composite mixtures with different types of cellulose fibers. Adv Mater Sci Eng 2018:3841514
Raabe J et al (2018) Effect of nano-silica deposition on cellulose fibers on the initial hydration of the Portland cement. BioResources 13(2):3525–3544
Palomar I, Barluenga G (2018) A multiscale model for pervious lime-cement mortar with perlite and cellulose fibers. Constr Build Mater 160:136–144
Liu KQ et al (2018) Design of low-density cement optimized by cellulose-based fibre for oil and natural gas wells. Powder Technol 338:506–518
Lee HJ et al (2019) Fire resistance evaluation of fiber-reinforced cement composites using cellulose nanocrystals. Adv Concr Constr 8(4):311–320
Hoyos CG et al (2019) Cellulose nanofibrils extracted from fique fibers as bio-based cement additive. J Clean Prod 235:1540–1548
Garoushi S et al (2020) Incorporation of cellulose fiber in glass ionomer cement. Eur J Oral Sci 128:81–88
Zhang Y et al (2019) Autogenous shrinkage of high-performance cement-based carbon nanofiber composites. Mater Express 9(3):213–225
Yang N, Sun QS (2019) Study on the self-monitoring of bending fatigue cumulative damage for carbon nanofiber polyurethane cement. Appl Sci Basel 9(10):2128–2147
Nishimura T et al (2019) Effects of powdery cellulose nanofiber addition on the properties of glass ionomer cement. Materials 12(19):3077–3084
Kosson M, Brown L, Sanchez F (2020) Early-age performance of 3D printed carbon nanofiber and carbon microfiber cement composites. Transp Res Rec 2674:10–20
Aloulou F, Sabrine A, Sammouda H (2019) Influence and dispersion of nanofiber of wood modified on properties of cement based mortars. J Renew Mater 7(7):631–641
Tang W, Kardani O, Cui H (2015) Robust evaluation of self-healing efficiency in cementitious materials—a review. Constr Build Mater 81:233–247
Gupta S, Dai Pang S, Kua HW (2017) Autonomous healing in concrete by bio-based healing agents—a review. Constr Build Mater 146:419–428
Snoeck D et al (2018) Validation of self-healing properties of construction materials through nondestructive and minimal invasive testing. Adv Mater Interfaces 5(17):1800179
Cohades A et al (2018) Progress in self-healing fiber-reinforced polymer composites. Adv Mater Interfaces 5(17): 1800177
De Belie N et al (2018) A review of self-healing concrete for damage management of structures. Adv Mater Interfaces 5(17):1800074
Ferrara L et al (2018) Experimental characterization of the self-healing capacity of cement based materials and its effects on the material performance: a state of the art report by COST action SARCOS WG2. Constr Build Mater 167:115–142
Jefferson T et al (2018) Research progress on numerical models for self-healing cementitious materials. Adv Mater Interfaces 5(17):1701378
Luhar S, Gourav S (2015) A review paper on self-healing concrete. J Civ Eng Res 5(3):53–58
Wu M, Johannesson B, Geiker M (2012) A review: self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr Build Mater 28(1):571–583
Jakhrani SH et al (2019) Review on the self-healing concrete-approach and evaluation techniques. J Ceram Proc Res 20:1–18
Ravitheja A, Reddy TCS, Sashidhar C (2019) Self-healing concrete with crystalline admixture—a review. J Wuhan Univ Technol Mater Sci Edition 34(5):1143–1154
Wang XF et al (2019) Evaluation of the mechanical performance recovery of self-healing cementitious materials—its methods and future development: a review. Constr Build Mater 212:400–421
Xue CH et al (2019) A review study on encapsulation-based self-healing for cementitious materials. Struct Concr 20(1):198–212
Xu S et al (2019) Investigation of the potential use of calcium alginate capsules for self-healing in porous asphalt concrete. Materials 12(1):168–180
Wu MY et al (2019) Two-component polyurethane healing system: effect of different accelerators and capsules on the healing efficiency of dynamic concrete cracks. Constr Build Mater 227:116700
Tsangouri E et al (2019) Feasibility study on real-scale, self-healing concrete slab by developing a smart capsules network and assessed by a plethora of advanced monitoring techniques. Constr Build Mater 228:116780
Tsangouri E et al (2019) Concrete fracture toughness increase by embedding self-healing capsules using an integrated experimental approach. Constr Build Mater 218:424–433
Savija B et al (2017) Simulation-aided design of tubular polymeric capsules for self-healing concrete. Materials 10(1):10–22
Qureshi TS, Kanellopoulos A, Al-Tabbaa A (2016) Encapsulation of expansive powder minerals within a concentric glass capsule system for self-healing concrete. Constr Build Mater 121:629–643
Lv LY et al (2020) Light induced self-healing in concrete using novel cementitious capsules containing UV curable adhesive. Cem Concr Compos 105:103445
Gruyaert E et al (2016) Capsules with evolving brittleness to resist the preparation of self-healing concrete. Mater Constr 66(323):92-102
Araujo M et al (2018) Poly(methyl methacrylate) capsules as an alternative to the “proof-of-concept” glass capsules used in self-healing concrete. Cem Concr Compos 89:260–271
Oh SR, Choi YW, Kim YJ (2019) Effect of cement powder based self-healing solid capsule on the quality of mortar. Constr Build Mater 214:574–580
Choi YW, Oh SR, Choi BK (2017) A study on the manufacturing properties of crack self-healing capsules using cement powder for addition to cement composites. Adv Mater Sci Eng 2017:5187543
Yang Z et al (2011) A self-healing cementitious composite using oil core/silica gel shell microcapsules. Cem Concr Compos 33(4):506–512
Erşan YÇ et al (2016) Enhanced crack closure performance of microbial mortar through nitrate reduction. Cem Concr Compos 70:159–170
Wang JY et al (2014) Self-healing concrete by use of microencapsulated bacterial spores. Cem Concr Res 56:139–152
Lu S et al (2019) Bacterial self-healing cement based materials: mechanism at nanoscale. AIP Adv 9(10):105312
Rong H et al (2020) Influence of bacterial concentration on crack self-healing of cement-based materials. Constr Build Mater 244:118372
Su YL et al (2019) Influence of bacterial self-healing agent on early age performance of cement-based materials. Constr Build Mater 218:224–234
Bhaskar S et al (2017) Effect of self-healing on strength and durability of zeolite-immobilized bacterial cementitious mortar composites. Cem Concr Compos 82:23–33
Tziviloglou E et al (2016) Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr Build Mater 122:118–125
Ramakrishnan V et al (2005) Improvement of concrete durability by bacterial mineral precipitation. In: Proceedings of 11th international conference on fracture, pp 1–6
Pei R et al (2013) Use of bacterial cell walls to improve the mechanical performance of concrete. Cem Concr Compos 39:122–130
Palin D, Wiktor V, Jonkers H (2016) A bacteria-based bead for possible self-healing marine concrete applications. Smart Mater Struct 25(8):084008
Jonkers HM et al (2010) Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol Eng 36(2):230–235
Huang HL, Ye G (2016) Numerical studies of the effects of water capsules on self-healing efficiency and mechanical properties in cementitious materials. Adv Mater Sci Eng 2016:8271214
Formia A et al (2015) Setup of extruded cementitious hollow tubes as containing/releasing devices in self-healing systems. Materials 8(4):1897–1923
Chen X, Yuan J, Alazhari M (2019) Effect of microbiological growth components for bacteria-based self-healing on the properties of cement mortar. Materials 12(8):1303
Childers MI et al (2017) Polymer-cement composites with self-healing ability for geothermal and fossil energy applications. Chem Mater 29(11):4708–4718
Nguyen M-T et al (2018) Atomic origins of the self-healing function in cement-polymer composites. ACS Appl Mater Interfaces 10(3):3011–3019
Rod KA et al (2019) Insights into the physical and chemical properties of a cement-polymer composite developed for geothermal wellbore applications. Cem Concr Compos 97:279–287
Rod KA et al (2020) Self-repairing polymer-modified cements for high temperature geothermal and fossil energy applications. Geothermics 85:101790
Fernandez CA et al (2019) Into the adhesion and re-adhesion (healing) to formation of novel polymer-cement composites for geothermal applications. In: 15th international congress on the chemistry of cement, Prague, Czech Republic, September 16–20, 2019, p 15
Fernandez CA et al (2018) Advanced polymer-modified cements for subsurface applications. In: Proceedings, 43rd workshop on geothermal reservoir engineering, Stanford University, Stanford, CA, February 12–14, 2018, p 9
Rod KA et al (2020) Polymer-cement composites with adhesion and re-adhesion (healing) to casing capability for geothermal wellbore applications. Cem Concr Compos 107(10):103490
Giergiczny Z (2019) Fly ash and slag. Cem Concr Res 124:105826
Drochytka R et al (2019) Use of secondary crystallization and fly ash in waterproofing materials to increase concrete resistance to aggressive gases and liquids. Adv Civ Eng 2019:7530325
Sugama T, Pyatina T (2019) Self-healing, re-adhering, and carbon-steel corrosion mitigating properties of fly ash-containing calcium aluminum phosphate cement composite at 300 degrees C hydrothermal temperature. Cem Concr Compos 99:1–16
Snoeck D et al (2016) X-ray computed microtomography to study autogenous healing of cementitious materials promoted by superabsorbent polymers. Cem Concr Compos 65:83–93
Snoeck D, De Belie N (2015) Repeated autogenous healing in strain-hardening cementitious composites by using superabsorbent polymers. J Mater Civ Eng 28(1):04015086
Lee H, Wong H, Buenfeld N (2016) Self-sealing of cracks in concrete using superabsorbent polymers. Cem Concr Res 79:194–208
Kua HW et al (2019) Biochar-immobilized bacteria and superabsorbent polymers enable self-healing of fiber-reinforced concrete after multiple damage cycles. Cem Concr Compos 100:35–52
Hu MM et al (2019) Development of Ca2+ -based, ion-responsive superabsorbent hydrogel for cement applications: self-healing and compressive strength. J Colloid Interface Sci 538:397–403
Gwon S, Ahn E, Shin M (2019) Self-healing of modified sulfur composites with calcium sulfoaluminate cement and superabsorbent polymer. Compos Part B Eng 162:469–483
Wang CY et al (2019) Self-healing cement composite: amine- and ammonium-based pH-sensitive superabsorbent polymers. Cem Concr Compos 96:154–162
Mignon A et al (2015) pH-sensitive superabsorbent polymers: a potential candidate material for self-healing concrete. J Mater Sci 50(2):970–979
Sarkar M et al (2014) Autonomous bioremediation of a microbial protein (bioremediase) in Pozzolana cementitious composite. J Mater Sci 49(13):4461–4468
GVR (2017) Self-Healing Materials Market By Product (Concrete, C., Polymers, Asphalt, Fiber-Reinforced Composites, Ceramic, Metal), by technology, by application, by region, and segment forecasts, 2018–2025 grand view research San Francisco
Silva FB et al (2015) Industrial application of biological self-healing concrete: challenges and economical feasibility. J Commer Biotechnol 21(1):31–38
Dam Safety-Fact Sheet #12 Montana Watercourse and Montana Department of Natural Resources and Conservation, C.R p 1–2
Emmons PH, Vaysburd AM (1995) Performance criteria for concrete repair materials. Phase 1. 1995, structural preservation systems Inc Baltimore, MD, p 1–127
Roulin-Moloney A, Berchten A (1987) The repair of concrete structures by injection with epoxide resin. J Adhes 22(2):125–138
Sugama T (2007) Advanced cements for geothermal wells. Brookhaven National Lab (BNL), Upton
Funkhouser GP, Eoff OLS, Norman OLR (2007) Methods and compositions for cementing in well bores. US7238229B2: US7238229B2
Zeldin AN et al (1980) Polymer-cement-geothermal-well-completion materials. Brookhaven National Laboratory/U.S. Department of Energy, Washington
Rockett T (1979) Phosphate-bonded glass cements for geothermal wells. Final report. Department of Materials and Chemical Engineering, Rhode Island University, Kingston, USA
Jackson MD et al (2017) Phillipsite and Al-tobermorite mineral cements produced through low-temperature water-rock reactions in Roman marine concrete. Am Mineral J Earth Planet Mater 102(7):1435–1450
Saslow SA et al (2017) Updated liquid secondary waste grout formulation and preliminary waste form qualification. RPT-SWCS-009, Revision 0, Pacific Northwest National Laboratory, Richland, Washington
Xu H, van Deventer JSJ (2003) The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspars. Colloids Surf A Physicochem Eng Asp 216(1–3):27–44
Rowles M, O’Connor B (2003) Chemical optimization of the compressive strength of aluminosilicate geopolymers synthesized by sodium silicate activation of metakaolinite. J Mater Chem 13(5):1161–1165
De Silva P, Sagoe-Crenstil K, Sirivivatnanon V (2007) Kinetics of geopolymerization: role of Al2O3 and SiO2. Cem Concr Res 37(4):512–518
Pimraksa K et al (2011) Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mater Sci Eng A Struct Mater 528(21):6616–6623
Huang Y, Han MF (2011) The influence of alpha-Al2O3 addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products. J Hazard Mater 193:90–94
Duxson P et al (2005) Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf A Physicochem Eng Asp 269(1–3):47–58
Temuujin J, van Riessen A, MacKenzie KJD (2010) Preparation and characterisation of fly ash based geopolymer mortars. Constr Build Mater 24(10):1906–1910
Ryu GS et al (2013) The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr Build Mater 47:409–418
McLellan BC et al (2011) Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J Clean Prod 19(9–10):1080–1090
Duxson P et al (2007) Geopolymer technology: the current state of the art. J Mater Sci 42(9):2917–2933
Zhang GP, He JA, Gambrell RP (2010) Synthesis, characterization, and mechanical properties of red mud-based geopolymers. Transp Res Rec 2167:1–9
Fletcher RA et al (2005) The composition range of aluminosilicate geopolymers. J Eur Ceram Soc 25(9):1471–1477
Buchwald A et al (2004) Stabilized foam clay material with high performance thermal insulation properties. Cfi-Ceram Forum Int 81(8):E39–E42
Ali MK et al (2015) Self-healing and strength development of geopolymer concrete made with waste byproducts. In: International conference on biological, civil and environmental engineering (BCEE-2015) 3–4 Feb 2015, Bali, p 11
Ahn TH, Kishi T (2010) Crack self-healing behavior of cementitious composites incorporating various mineral admixtures. J Adv Concr Technol 8(2):171–186
Mehta PK (1997) Durability–critical issues for the future. Concr Int 19(7):27–33
Li VC (2003) On engineered cementitious composites (ECC). J Adv Concr Technol 1(3):215–230
Yıldırım G et al (2018) Self-healing performance of aged cementitious composites. Cem Concr Compos 87:172–186
Şahmaran M et al (2008) Self-healing of mechanically-loaded self-consolidating concretes with high volumes of fly ash. Cem Concr Compos 30(10):872–879
Liu H et al (2017) Influence of microcrack self-healing behavior on the permeability of Engineered Cementitious Composites. Cem Concr Compos 82:14–22
Sahmaran M, Yildirim G, Erdem TK (2013) Self-healing capability of cementitious composites incorporating different supplementary cementitious materials. Cem Concr Compos 35(1):89–101
Yang Y, Yang E-H, Li VC (2011) Autogenous healing of engineered cementitious composites at early age. Cem Concr Res 41(2):176–183
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36(2):118–136
Dong B et al (2017) Performance recovery concerning the permeability of concrete by means of a microcapsule based self-healing system. Cem Concr Compos 78:84–96
Perez G et al (2015) Characterisation of cement pastes with innovative self-healing system based in epoxy-amine adhesive. Cem Concr Compos 60:55–64
Van Tittelboom K et al (2011) Self-healing efficiency of cementitious materials containing tubular capsules filled with healing agent. Cem Concr Compos 33(4):497–505
Dong BQ et al (2015) Smart releasing behavior of a chemical self-healing microcapsule in the stimulated concrete pore solution. Cem Concr Compos 56:46–50
Thao TDP (2011) Quasi-brittle self-healing materials: numerical modelling and applications in civil engineering, p 228
Souradeep G, Kua HW (2016) Encapsulation technology and techniques in self-healing concrete. J Mater Civ Eng 28(12):04016165
Dry C (1994) Matrix cracking repair and filling using active and passive modes for smart timed release of chemicals from fibers into cement matrices. Smart Mater Struct 3(2):118–123
Dry C, McMillan W (1996) Three-part methylmethacrylate adhesive system as an internal delivery system for smart responsive concrete. Smart Mater Struct 5(3):297–300
Pang J, Bond I (2005) ‘Bleeding composites’—damage detection and self-repair using a biomimetic approach. Compos A Appl Sci Manuf 36(2):183–188
Pang JW, Bond IP (2005) A hollow fibre reinforced polymer composite encompassing self-healing and enhanced damage visibility. Compos Sci Technol 65(11–12):1791–1799
Jefferson A et al (2010) A new system for crack closure of cementitious materials using shrinkable polymers. Cem Concr Res 40(5):795–801
Sakai Y et al (2003) Experimental study on enhancement of self-restoration of concrete beams using SMA wire. In: Smart structures and materials 2003: smart systems and nondestructive evaluation for civil infrastructures. International Society for Optics and Photonics
Song G, Ma N, Li H-N (2006) Applications of shape memory alloys in civil structures. Eng Struct 28(9):1266–1274
Duerig TW, Melton K, Stöckel D (2013) Engineering aspects of shape memory alloys. Butterworth-Heinemann, Oxford
Kuang Y, Ou J (2008) Self-repairing performance of concrete beams strengthened using super elastic SMA wires in combination with adhesives released from hollow fibers. Smart Mater Struct 17(2):025020
Dejonghe P et al (2011) The behavior of super absorbing polymers as a sealing agent in concrete: absorption kinetics, degradation and water permeability. In: Proceedings of 3rd international conference on self-healing materials, Bath, UK, 2011, p 123
Snoeck D et al (2012) The use of superabsorbent polymers as a crack sealing and crack healing mechanism in cementitious materials. In: Proceedings of 3rd international conference on concrete repair, rehabilitation and retrofitting. Cape Town, South Africa, p 6
Snoeck D et al (2014) Self-healing cementitious materials by the combination of microfibers and superabsorbent polymers. J Intell Mater Syst Struct 25(1):13–24
Snoeck D et al (2012) Visualization of water penetration in cementitious materials with superabsorbent polymers by means of neutron radiography. Cem Concr Res 42(8):1113–1121
Lee HXD, Wong HS, Buenfeld NR (2010) Potential of superabsorbent polymer for self-sealing cracks in concrete. Adv Appl Ceram 109(5):296–302
Jensen OM, Hansen PF (2002) Water-entrained cement-based materials II. Experimental observations. Cem Concr Res 32(6):973–978
Jensen OM, Hansen PF (2001) Water-entrained cement-based materials I Principles and theoretical background. Cem Concr Res 31(4):647–654
Lu ZC et al (2016) Oil swellable polymer modified cement paste: expansion and crack healing upon oil absorption. Constr Build Mater 114:98–108
Zhang R, Mao X, Zhao ZJ (2019) Synthesis of oil-swelling material and evaluation of its self-healing effect in cement paste. Polym Plast Technol Eng 58(6):618–629
Yang Z et al (2009) Feasibility investigation of self-healing cementitious composite using oil core/silica gel shell passive smart microcapsules. In: Proceedings of SPIE—the international society for optical engineering, July 2009, p 13
Morlat R et al (2007) Reinforcement of hydrated Portland cement with high molecular mass water-soluble polymers. J Mater Sci 42(13):4858–4869
Chen Y et al (2012) Multiphase design of autonomic self-healing thermoplastic elastomers. Nat Chem 4(6):467–472
Cash JJ et al (2015) Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 48(7):2098–2106
Yang Y, Urban MW (2013) Self-healing polymeric materials. Chem Soc Rev 42(17):7446–7467
Tournilhac F et al (2010) Self-healing supramolecular networks. Macromol Symp 291–292(1):84–88
Reddy BR, Liang F, Fitzgerald R (2010) Self-healing cements that heal without dependence on fluid contact: a laboratory study. SPE Drill Complet 25(03):309–313
Le Roy-Delage S (Schlumberger Technology Corp), Martin-Al-Khatib L (Schlumberger Technology Corp). Self-repairing cements. 2012, Google Patents: US9683161B2
Le Roy-Delage S (Schlumberger Technology Corp) et al (2013) Self-adaptive cement systems. Google Patents: CA2524514C
Zhao H et al (2015) Blast mitigation effect of the foamed cement-base sacrificial cladding for tunnel structures. Constr Build Mater 94:710–718
Li G (Louisiana State University and Agricultural and Mechanical College), Meng H (Louisiana State University and Agricultural and Mechanical College), Shojaei A (Louisiana State University and Agricultural and Mechanical College) (2016) Self-healing composite of thermoset polymer and programmed super contraction fibers. Google Patents: US9428647B2
Xiaoyong C (Guangzhou Qianke Building Materials Technology Co., Ltd.) (2017) Aqueous epoxy cement self-leveling waterproof mortar and production method thereof. CN 201710745662, p CN 201710745662
Mishra V (Halliburton Energy Services, Inc.), Kadam SS (Halliburton Energy Services, Inc.), Patil RC (Halliburton Energy Services, Inc.) (2018) Self-healing cement comprising polymer capable of swelling in gaseous environment. Google Patents: WO2016053237A1
Reddy BR (Halliburton Energy Services, Inc.) et al (2009) Self-repairing cement compositions and methods of using same. Google Patents: US7530396B1
Canadell J, Goossens H, Klumperman B (2011) Self-healing materials based on disulfide links. Macromolecules 44(8):2536–2541
Lafont U, Van Zeijl H, Van Der Zwaag S (2012) Influence of cross-linkers on the cohesive and adhesive self-healing ability of polysulfide-based thermosets. ACS Appl Mater Interfaces 4(11):6280–6288
Pepels M et al (2013) Self-healing systems based on disulfide–thiol exchange reactions. Polym Chem 4(18):4955–4965
Tony J et al (2018) Research progress on numerical models for self-healing cementitious materials. Adv Mater Interfaces 5:1701378
Freeman CL et al (2007) New forcefields for modeling biomineralization processes. J Phys Chem C 111(32):11943–11951
Cygan RT, Liang JJ, Kalinichev AG (2004) Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J Phys Chem B 108(4):1255–1266
Heinz H et al (2005) Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chem Mater 17(23):5658–5669
Moghaddam SE et al (2017) Morphogenesis of cement hydrate. J Mater Chem A 5(8):3798–3811
van Duin ACT et al (2003) ReaxFF(SiO) reactive force field for silicon and silicon oxide systems. J Phys Chem A 107(19):3803–3811
Papatzani S, Paine K, Calabria-Holley J (2015) A comprehensive review of the models on the nanostructure of calcium silicate hydrates. Constr Build Mater 74:219–234
Pellenq RJM et al (2009) A realistic molecular model of cement hydrates. Proc Natl Acad Sci USA 106(38):16102–16107
Bieniawski ZT (1978) Determining rock mass deformability—experience from case histories. Int J Rock Mech Min Sci 15(5):237–247
Bieniawski ZT (1989) Engineering rock mass classifications : a complete manual for engineers and geologists in mining, civil, and petroleum engineering. Wiley, Hoboken
Kovacevic G et al (2015) Atomistic modeling of crystal structure of Ca1.67SiHx. Cem Concr Res 67:197–203
Popov VN (2004) Carbon nanotubes: properties and application. Mater Sci Eng R Rep 43(3):61–102
Li GY, Wang PM, Zhao XH (2005) Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon 43(6):1239–1245
Eftekhari M, Mohammadi S (2016) Molecular dynamics simulation of the nonlinear behavior of the CNT-reinforced calcium silicate hydrate (C–S–H) composite. Compos Part A Appl Sci Manuf 82:78–87
Plimpton S (1995) Fast parallel algorithms for short-range molecular-dynamics. J Comput Phys 117(1):1–19
Dresselhaus MS, Dresselhaus G, Saito R (1995) Physics of carbon nanotubes. Carbon 33(7):883–891
Tersoff J (1989) Modeling solid-state chemistry: interatomic potentials for multicomponent systems. Phys Rev B Condens Matter 39(8):5566–5568
Lushnikova A, Zaoui A (2017) Improving mechanical properties of C–S–H from inserted carbon nanotubes. J Phys Chem Solids 105:72–80
Lushnikova A, Zaoui A (2018) Influence of single-walled carbon nanotubes structure and density on the ductility of cement paste. Constr Build Mater 172:86–97
Hamid SA (1981) The crystal-structure of the 11-a natural tobermorite Ca2.25 [Si3o7.5(Oh)1.5].1h2o. Zeitschrift Fur Kristallographie 154(3–4):189–198
Zollo RF (1997) Fiber-reinforced concrete: an overview after 30 years of development. Cem Concr Compos 19(2):107–122
Sun H, Ren P, Fried JR (1998) The COMPASS force field: parameterization and validation for phosphazenes. Comput Theor Polym Sci 8(1–2):229–246
Shalchy F, Rahbar N (2015) Nanostructural characteristics and interfacial properties of polymer fibers in cement matrix. ACS Appl Mater Interfaces 7(31):17278–17286
Sakhavand N, Muthuramalingam P, Shahsavari R (2013) Toughness governs the rupture of the interfacial H-bond assemblies at a critical length scale in hybrid materials. Langmuir 29(25):8154–8163
Shahsavari R, Pellenq RJM, Ulm FJ (2011) Empirical force fields for complex hydrated calcio-silicate layered materials. Phys Chem Chem Phys 13(3):1002–1011
Zhou Y et al (2017) Interfacial connection mechanisms in calcium-silicate-hydrates/polymer nanocomposites: a molecular dynamics study. ACS Appl Mater Interfaces 9(46):41014–41025
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev B 136(3b):B864–B871
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4a):1133–1138
Shahsavari R et al (2009) First-principles study of elastic constants and interlayer interactions of complex hydrated oxides: case study of Tobermorite and Jennite. J Am Ceram Soc 92(10):2323–2330
Tunega D, Zaoui A (2011) Understanding of bonding and mechanical characteristics of cementitious mineral tobermorite from first principles. J Comput Chem 32(2):306–314
Franceschini A et al (2007) New covalent bonded polymer–calcium silicate hydrate composites. J Mater Chem 17(9):913–922
Reinhardt H et al (2013) Recovery against environmental action. In: Self-healing phenomena in cement-based materials. Springer, Berlin, pp 65–117
Termkhajornkit P et al (2009) Self-healing ability of fly ash–cement systems. Cem Concr Compos 31(3):195–203
Stephens N (2019) Volcker alliance reveals alarming hidden costs of states’ deferred infrastructure maintenance The Volcker Allience, p 2
Schroten A et al (2019) Overview of transport infrastructure expenditures and costs. European Commission, p 35
Acknowledgements
Funding for this research was provided by the Department of Energy’s Geothermal Technology Office. Pacific Northwest National Laboratory Computational resources were provided by PNNL Institutional Computing (PIC) and the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility supported by the Office of Science of the U.S. DOE. under Contract no. DE-AC02-05CH11231. PNNL is operated by Battelle for the U.S. DOE under contract DE-AC06-76RLO 1830.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: M. Grant Norton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernandez, C.A., Correa, M., Nguyen, MT. et al. Progress and challenges in self-healing cementitious materials. J Mater Sci 56, 201–230 (2021). https://doi.org/10.1007/s10853-020-05164-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05164-7