Abstract
Monolayer (ML) Tl2O, as a newly discovered metal-shrouded two-dimensional semiconductor with an appropriate bandgap and high carrier mobility, is a promising candidate as the channel materials for the next-generation field-effect transistors (FETs). Using ab initio electronic structure calculation and quantum transport simulation, the contact properties of ML Tl2O–metal interfaces based on FET are comprehensively investigated with Au, Sc, Tl, Ni, graphene, Ti2C, Ti2CF2, and Ti2C(OH)2 electrodes. ML Tl2O undergoes metallization with Au, Sc, Tl, Ni, and Ti2C, while it forms van der Waals-type contact with graphene, Ti2CF2, and Ti2C(OH)2. An n-type lateral Schottky contact is formed with Ni, Au, Sc, and Ti2C electrodes with the electron Schottky barrier height (SBH) of 0.25, 0.27, 0.27, and 0.36 eV, respectively, while a p-type lateral Schottky contact is formed with graphene electrode with the hole SBH of 0.10 eV. Surprisingly, a desired n-type Ohmic contact arises with Tl and Ti2C(OH)2 electrodes and a desired p-type Ohmic contact arises with Ti2CF2 electrode. The study not only provides a deep understanding of the interfacial properties of the ML Tl2O FETs but also reveals a versatile approach to realize both n- and p-type Ohmic contact for the ML Tl2O FETs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last decades, two-dimensional (2D) semiconductors have been drawing extensive attention for their intriguing physical properties and promising applications in nanoelectronic [1,2,3]. Due to improved carrier transport associated with the absence of dangling bond at the interface and the reduced scattering at smooth surface and remarkable gate electrostatics associated with atomically thin thickness, 2D semiconductors exhibit great potentials to be the channel materials beyond traditional semiconductors for the next-generation nanoelectronic devices [4,5,6]. A successful 2D semiconductor successor of traditional semiconductor not only requires an advisable bandgap greater than 0.4 eV [6] but also needs to meet two other criteria: ambient stability and high carrier mobility. 2D semiconductors, such as 2D MoS2 and WSe2, black phosphorene, and 2D InSe, are intensively researched for the electronics with the proper band gaps [7, 8]. Unfortunately, these 2D semiconductors cannot meet the other two criteria mentioned above. For example, 2D MoS2 has relatively low carrier mobility that causes the low on-currents in experiments, which is less than 250 μA μm−1 [9,10,11]. Although black phosphorene and 2D InSe possess high carrier mobility, their insufficient ambient stability results in the rapid degradation of device performance when exposed to the air [12,13,14,15]. Consequently, looking for a proper 2D semiconductor that meets all these criteria is critical.
Monolayer (ML) Tl2O, the first metal-shrouded 2D semiconductor, has been recently predicted with a direct bandgap about 1.0 eV [16]. The calculated carrier mobility of ML Tl2O is comparable to that of ML phosphorene and 2D InSe, which is up to 4.3 × 103 cm2 V−1 s−1. What is more, it is dynamically and thermally stable and is easy to be mechanically exfoliated from the bulk Tl2O with a low exfoliation energy (0.43 J m−2) [16, 17]. These characteristics indicate that ML Tl2O is a promising candidate for the future high-performance and low-power nanoelectronic devices applications. In an actual transistor of 2D semiconductor, forming low-resistance contacts at the interfaces is just as important as the semiconducting characters itself [18,19,20]. Contacting with metal electrodes is inevitable in 2D semiconductors, and Schottky barrier and Fermi level pinning (FLP) effects always appear at the 2D semiconductors–metal interfaces in their transistors [21,22,23]. The interfacial properties of ML Tl2O transistors are still exclusive, and forming Ohmic contacts of ML Tl2O–metal interface is expected. 2D metals, such as graphene and MXene, are new and preferable choice for the metal electrodes, which can form low-resistance van der Waals (vdW)-stacked junctions with 2D semiconductors [24,25,26,27,28]. Particularly, the work function (WF) of MXene can be tuned by the functional group at the surfaces, indicating that the carrier polarity of 2D semiconductor field-effect transistor (FET) can be tuned by choosing the surficial functional group (Table 1).
In this article, based on the FET configuration, the interfacial properties between ML Tl2O and eight metals (traditional bulk metals: Au, Sc, Tl, and Ni; 2D metals: graphene, Ti2C, Ti2CF2, and Ti2C(OH)2) are systematically investigated by utilizing ab initio electronic structure calculations and quantum transport simulations. ML Tl2O encounters a metallization contacting with Au, Sc, Tl, Ni, and Ti2C electrodes, while it forms vdW-type contacts with graphene, Ti2CF2, and Ti2C(OH)2 electrodes with the band structure of ML Tl2O kept. In the ML Tl2O FETs, an n-type lateral Schottky contact is formed when Sc, Ni, Ti2C, and Au are electrodes with the electron Schottky barrier height (SBH) of 0.27, 0.25, 0.35, and 0.27 eV, respectively, while a p-type lateral Schottky contact is formed when graphene is the electrode with the hole SBH of 0.10 eV. The desired n-type Ohmic contact is formed with Ti2C(OH)2 and Tl electrodes, and the desired p-type Ohmic contact is formed with Ti2CF2 electrode. Because of the metal-induced gap states (MIGS) and the interaction between the channel ML Tl2O and the electrodes, strong FLP is discovered in the ML Tl2O FETs with the pinning factor of 0.15.
Methodology
Interface and device models
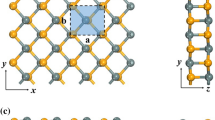
Au, Ni, Sc, Tl, graphene, Ti2C(OH)2, Ti2CF2, and Ti2C are taken into consideration as electrodes, which cover a wide range of WF. For bulk metal Au, Ni, Sc, and Tl, five layers of metal atoms are constructed to model the metal surface, while for two-dimensional metal graphene and Ti2C without and with F/OH termination, ML structure is constructed. ML Tl2O is absorbed on one side of the metal surfaces as shown in Fig. 1c. The lattice constant of ML Tl2O is fixed with the optimized lattice constant a = b = 3.59 Å, and the lattice constants of metal surface are changed to adapt it correspondingly. The 1 × 1 unit cell of ML Tl2O matches 1 × 1 unit cell of Sc and Tl surface, and the 2 × 2 unit cells of ML Tl2O match 3 × 3 unit cell of Au (111), Ni (111), and graphene surface, and the \( \surd 5 \) × \( \surd 5 \) unit cells of ML Tl2O match the 2 × 2 unit cells of Ti2C, Ti2CF2, and Ti2C(OH)2 surfaces. The mismatches of lattice constant with Ti2C(OH)2, Ti2CF2, Ti2C, graphene, Ni, Tl, Au, and Sc surfaces are 0.56%, 1.10%, 1.51%, 1.79%, 2.59%, 2.62%, 3.89%, and 3.96%, respectively. ML Tl2O mainly couples with the top three layers of metal atoms for Ni, Tl, Au, and Sc [29], so the bottom two layers of the metal atom freeze in the geometry optimizations.
a Top and b side views of the free-standing ML Tl2O. The rhombus indicates the unit cell of ML Tl2O. c Atomic configuration of ML Tl2O–metal interfaces. d Schematic diagram of a ML Tl2O FET at the upper-right portion. The enlarged picture of the electrode region at the lower-left portion. Black rows represent the pathways that the carrier transfers from the electrode to the channel ML Tl2O. Blue dashed lines indicate two interfaces at ML Tl2O FET, i.e., vertical interface A and lateral interface B. ΦV and ΦL represent the corresponding vertical and lateral SBH, respectively
We build a two-probe ML Tl2O FET to study its interfacial properties as shown in Fig. 1d. Pristine ML Tl2O is used in the channel region with the channel length of 5–6 nm along the transport direction, and the optimized ML Tl2O–metal interfaces are used in the left and the right electrode region with semi-infinite length.
Computational methods
The geometry optimizations and the electronic properties of the ML Tl2O–metal interfaces are calculated based on density functional theory (DFT) performed in the Vienna ab initio simulation package (VASP) code [30,31,32]. Ion–electron interactions are described by utilizing projected augmented wave (PAW) approximation [33]. The plane-wave basis is applied with cut-off energy of 500 eV. In the interfacial systems, vdW correction is processed by zero damping DFT-D3 method of Grimme [34]. The convergence threshold for the residual force is not more than 0.01 eV/Å and the energy difference converges to 1 × 10−6 eV for per atom during geometry optimization. The Monkhorst–Pack k-point grid is sampled in the Brillouin zone [35] with a separation of 0.02 Å−1. To satisfy decoupling between the adjacent slabs, the vacuum buffer layer is set at least 15 Å.
DFT combining with nonequilibrium Green’s function (NEGF) method is applied for the ML Tl2O FET calculations, which are carried out in Atomistix ToolKit (ATK) 2019 package [36,37,38]. The transmission coefficient \( T (k_{y} , E) \) is defined by the following equation:
where G(ky, E) (\( G^{ + } \left( {k_{y} , \, E} \right) \)) represent a retarded (advanced) Green function in the central region and ky is the y portion of the reduced wave vector. (The coordinate axis is labeled in Fig. 1d.) The self-energy correction term \( \varGamma_{L/R} = i(\sum_{L/R}^{r} - \sum_{L/R}^{a} ) \) expresses the level broadening that is produced by the interaction between the center region and the electrode, and the electrode self-energy can be regarded as an effective Hamiltonian to describe the interplay between the lead and the device [39]. In the simulations, temperature is set to 300 K. The linear combination of the atomic orbits (LCAO) basis set with the double-ξ polarization (DZP) form is taken into account. The Monkhorst–Pack [35] k-point meshes are sampled in the device with a separation of 0.08 Å−1 in x orientation and 0.007 Å−1 in z orientation. The Periodic, Neumann, and Dirichlet boundary condition are implemented in the x, y, z orientation of the ML Tl2O FETs, respectively.
In the paper, the generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE) method is adopted to describe the exchange and correlation interactions [40, 41]. When contacting with metals, 2D semiconductors are significantly doped with the carrier. Electron–electron interactions are screened in the doped 2D semiconductor, and thus, DFT–GGA comes to be a good approximation for the estimation of the bandgap and Schottky barriers [42, 43]. For instance, the bandgap of the doped ML MoSe2 obtained at the DFT–GGA level of 1.53 eV is in good agreement with that derived from the GW calculation of 1.59 eV [43]. What is more, the SBH in ML/bilayer/trilayer phosphorene FET is well reproduced, i.e., the calculated electron SBHs of 0.34/0.19/0.20 eV at the DFT–GGA level are well kept with those of 0.39/0.23/0.21 eV from the experimental measurements [44,45,46,47].
Results and discussion
Configuration and bonding degree of ML Tl2O–metal interfaces
After optimization, the atomic configurations of the ML Tl2O–metal interfaces are presented in Fig. 2 and figure S1. The geometrical structure of ML Tl2O is well maintained on all the metal surfaces. On Ni (111) and Au (111) surface, both the Tl and O atoms are almost located in the center of the triangle formed by the three layers of metal atoms; on the Tl and Sc (0001) surface, the Tl atoms exactly sit above the metal atoms, and the O atoms sit above the center of the hexagon of metal atoms; on Ti2C surface, the O atoms sit above the topmost Ti atoms and C atoms, and the Tl atoms sit above the center of triangle shown in figure S1(i); on graphene, Ti2CF2, and Ti2C(OH)2 surfaces, the O and Tl atoms are randomly scattered on these metal surfaces.
To illustrate the binding level of these interfaces, we have calculated the binding energy and the interlayer distance. The binding energy Eb between ML Tl2O and metals is defined as follows:
where ET, EM, and ES-M stand for the optimized energy for the free-standing ML Tl2O, the clean crystalline metal, and the corresponding combined system per supercell, respectively, and the N denotes the number of Tl atom in the sublayer near metals per supercell. The equilibrium separation of the interlayer dz is the average distance in the vertical direction to the interfaces between the nearest metal atom layer to ML Tl2O and the neighboring Tl atom layer as indicated in Fig. 1c. According to the binding energy and the equilibrium interlayer distance, the ML Tl2O–metal interfaces are divided into three categories. Weak bonding is formed for the ML Tl2O–Ti2CF2, Ti2C(OH)2, and graphene interfaces with 2.97 < dz < 3.45 Å and 0.24 < Eb < 0.43 eV; medium bonding is formed for the ML Tl2O–Tl interface with dZ = 3.18 Å and Eb = 0.50 eV; strong bonding is formed for the ML Tl2O–Au, Sc, Ti2C, and Ni interfaces with 2.29 < dz < 2.76 Å and 0.63 < Eb < 1.36 eV. In the case of the weak bonding, the small binding energy suggests that vdW-type stacking is formed between ML Tl2O and graphene, Ti2CF2, and Ti2C(OH)2. In the most cases, the binding degree of ML Tl2O with metals is stronger than that of ML transition-metal dichalcogenides (TMDs) with metals, such as the binding energy of ML Tl2O vs. ML MoS2 (Ni: 1.36 > 0.83 eV; Au: 0.63 > 0.307 eV) [48, 49]. This may be because the metal-shrouded ML Tl2O with Tl atoms is more active than the nonmetal-shrouded ML TMDs.
Electronic structure of ML Tl2O–metal interfaces
The band structures of the pristine ML Tl2O and the interfacial systems are shown in Fig. 3. The free-standing ML Tl2O is a semiconductor with a direct bandgap of 1.0 eV located at M point by the plane-wave basis set with the PBE level, which agrees with the previous theoretical results [14]. The bandgap of ML Tl2O is also calculated by the DZP basis set in the ATK package with the same value of 1.0 eV as shown in figure S2. The effective mass m* (\( \frac{1}{{m^{*} }} = \frac{1}{{\hbar^{2} }}\frac{{{\text{d}}^{2} E}}{{{\text{d}}k^{2} }} \)) is calculated by the band structure of ML Tl2O. The band near the conduction band minimum (CBM) is relatively steep, bringing about a really small electron effective mass of 0.18me and 0.62me (me is the mass of an electron) along M → Γ and M → K direction, respectively; the band near the valence band maximum (VBM) along M → Γ direction is also quite steep, resulting in a small hole effective mass of 0.37me. On the contrary, the band near the VBM along M → K direction is flat, resulting in a large hole effective mass of 5.90me. The calculated effective masses are well consistent with the previous results [50]. The small effective masses promise a large carrier mobility comparable to that of black phosphorene [51, 52].
Band structures of the pristine ML Tl2O and the ML Tl2O–graphene, Ti2CF2, Ti2C(OH)2, Ti2C, Tl, Sc, Au, and Ni interfaces. Gray lines represent the band structure of the interfacial systems, and blue lines represent the band structure projected to the ML Tl2O in the interfacial systems. The line width is proportional to the weight. The Fermi level is set at zero energy and denoted by the black dashed lines. The inset graph shows the Brillouin zone
The band structures of the ML Tl2O in the interfacial systems, which reflect the difference of the bonding level, are shown in Fig. 3. In the weak bonding, although band folding exists owing to the supercell calculation, the intrinsic band structure of ML Tl2O is preserved with the formation of the vdW-type interactions. The bandgap is maintained with the value of 0.92, 0.98, and 1.00 eV after contacting with graphene, Ti2CF2, and Ti2C(OH)2, respectively. Electron transfer occurs at these interfaces, which leads to the energy level shift of the ML Tl2O. As for the medium and the strong bonding with Tl, Au, Sc, Ti2C, and Ni electrode, the band structures of the ML Tl2O are destroyed with the bandgap disappearing, resulting in the metallization of the ML Tl2O. It is found that although the ML Tl2O-Tl system has a smaller Eb and a larger dz than the strong bonding, the energy band structure of ML Tl2O is also heavily hybridized resulting from the consistency of the orbital of Tl atoms in the metal substrates and the ML Tl2O.
In order to get a deeper insight into the electronic properties of the ML Tl2O contacted with metals, we further analyze the partial density of states (PDOS) of the ML Tl2O in the interfacial systems, as shown in Fig. 4. In agreement with the band structure, the bandgap of the ML Tl2O is kept in the case of the weak bonding with the value of 0.90, 0.89, and 0.85 eV in contacting with graphene, Ti2C(OH)2, and Ti2CF2 electrodes, respectively. By contrast, a large amount of DOS are extended into the bandgap of the ML Tl2O in the case of the medium and the strong bonding, and thus, electron state appears at the Fermi level, which is a typical character of metallization. The DOS near the CBM is mainly composed of s and p orbitals, while that near the CBM is mainly composed of px + py orbitals in the free-standing ML Tl2O. The orbital component of the ML Tl2O in the case of the weak bonding interfacial systems is similar to that of the free-standing ML Tl2O. In the medium and strong bonding case, the s and p orbitals of the metalized ML Tl2O nearly make the same contributions at the Fermi level. To further visualize the physical picture of the interaction at the interface, the real-space total electron distribution of a slice that crosses both ML Tl2O and metal atoms is plotted in figure S3. There is apparently more electron accumulation at the interfaces for the medium and strong binding (ML Tl2O–Ti2C, Tl, Au, Sc, and Ni interfaces) than that for the weak binding interfaces (ML Tl2O–Ti2C(OH)2, Ti2CF2 and graphene interfaces).
Schottky barriers in ML Tl2O FETs
When electron transfers from the metal electrode to the channel ML Tl2O, it will encounter two kinds of interfaces: One is between metal electrode and the underneath contacted ML Tl2O in the vertical direction, which is called vertical interface (labeled as interface A in Fig. 1d); the other is between the ML Tl2O–metal systems and the channel ML Tl2O in the lateral direction, which is called the lateral interface (labeled as interface B in Fig. 1d). There may be Schottky barrier and tunneling barrier at the two interfaces. For the weak bonding (the vdW-type interactions) with the band structures of ML Tl2O preserved, Schottky barrier and tunneling barrier may appear at the vertical interfaces A. However, for the medium and strong bonding, Schottky barrier and tunneling barrier will disappear at the vertical interfaces A owing to the metallization of the underneath contacted ML Tl2O. The metalized ML Tl2O–metal systems are treated as a new metal system, and therefore, lateral Schottky barrier may exist at the interfaces B. Tunneling barrier is determined by the electrostatic potential barrier going through the Fermi level at the ML Tl2O–metal interfaces. Figure 2 shows the average electrostatic potential profiles normal to the interface between ML Tl2O and metals. Apparently, the electrostatic potential is below the Fermi level at the interfaces, so the tunneling barrier is zero in all the checked systems.
The vertical electron/hole Schottky barrier \( \varPhi_{\text{V}}^{\text{e}} \) / \( \varPhi_{\text{V}}^{\text{h}} \) is defined as the energy difference between the Fermi level of the interfacial systems and the VBM/CBM of the ML Tl2O in electrode. From the band structure analysis, a p-type and an n-type vertical Ohmic contact are formed with Ti2CF2 and Ti2C(OH)2, respectively, because of the Fermi level of the interfacial systems under the VBM or above the CBM of the ML Tl2O. When contacting with graphene, a p-type vertical Schottky contact is established, and the hole vertical SBH is 0.10 eV. Due to the metallization of ML Tl2O under the metal electrodes, vertical Ohmic contact is formed at the ML Tl2O-Tl, Au, Sc, Ti2C, and Ni interfaces.
The commonly used method to examine the lateral SBH is work function approximation (WFA), in which the lateral electron/hole SBH \( \varPhi_{\text{L}}^{\text{e, WFA}} \)/\( \varPhi_{\text{L}}^{\text{h, WFA}} \) is determined by the energy difference between the CBM/VBM of the channel materials and the Fermi level of the interfacial systems. We plot the band alignment of the free-standing ML Tl2O and line-up of the WF of the interfacial systems in Fig. 5. An n-type lateral Ohmic contact is formed with Ti2C(OH)2, Tl, and Sc electrodes, while a p-type lateral Ohmic contact is established with Ti2CF2 and Au electrodes in ML Tl2O FETs. What is more, an n-type Schottky contact is established with Ti2C electrode with the lateral electron SBH \( \varPhi_{\text{L}}^{\text{e, WFA}} \) of 0.37 eV, and a p-type Schottky contact is established with graphene and Ni electrodes with the lateral hole SBHs \( \varPhi_{\text{L}}^{\text{h, WFA}} \) of 0.39 and 0.01 eV, respectively.
Band alignment of the free-standing ML Tl2O and line-up of the work functions of the interfacial systems in terms of separate electronic energy band calculations. Ef denotes the Fermi level of the ML Tl2O. \( E_{\text{C}}^{\text{DFT}} \) and \( E_{\text{V}}^{\text{DFT}} \) are the conduction and valence band edges of the ML Tl2O calculated at DFT level, respectively. The orange dashed and the green solid lines present the work functions of the pristine metals and the corresponding ML Tl2O–metals systems, respectively. The unit of the data in the figure is eV
The electrode and the channel ML Tl2O are treated as the independent parts in the WFA, and thus, the coupling between the two parts is ignored. The more direct and reliable method to calculate the lateral SBH in 2D semiconductor FETs is ab initio quantum transport simulation based on a real two-probe FET model with including the coupling. For example, the calculated SBHs of ML, bilayer, and trilayer black phosphorene with Ni electrode by quantum transport simulations are 0.34, 0.19, 0.20 eV, respectively, consistent well with that of 0.39, 0.23, and 0.21 eV in the experimental measurements [44,45,46]. The SBHs of ML MoS2 with Au and Pd electrodes by quantum transport simulations are in better agreement with those observed in experiments than that by the WFA [22, 49, 53, 54].
The transport properties of the two-probe ML Tl2O FETs are calculated by using the quantum transport simulation. Figure 6 is the local device density of states (LDDOS) of ML Tl2O FETs under zero-bias and zero-gate voltage with graphene, Ti2CF2, Ti2C(OH)2, Ti2C, Ni, Au, Tl, and Sc electrodes, where the density of states is projected into the ML Tl2O. In the electrode regions, the hybridization degree of the electronic band for the ML Tl2O is well consistent with that in the band structure. The bandgap of ML Tl2O is well preserved when contacting with the graphene, Ti2CF2, and Ti2C(OH)2, while the band structure of ML Tl2O hybridizes with that of the metal electrode when contacting with Ti2C, Au, Ni, Sc, and Tl. According to the energy-level distribution in the electrode region, a p-type vertical Schottky contact is established with the SBH of 0.1 eV for graphene, while an n-type and a p-type Ohmic contact are established with Ti2C(OH)2 and Ti2CF2 electrode, respectively. Because of electron transfers from Ti2CF2 electrodes to the channel ML Tl2O, the CBM of the ML Tl2O is obviously bent downward at the interface. The band hybridization of ML Tl2O makes carrier directly go thorough from the metal to the underneath ML Tl2O without vertical Schottky barrier with Ti2C, Au, Ni, Sc, and Tl electrodes.
Zero-bias and zero-gate voltage LDDOS (left panel) and transmission spectra (right panel) of the ML Tl2O FETs with graphene, Ti2CF2, Ti2C(OH)2, Ti2C, Tl, Sc, Au, and Ni electrodes. The Fermi level is represented by the black dash line. The white dash lines represent the VBM and the CBM of the channel ML Tl2O. The MIGS are represented by the red short dash lines
According to the LDDOS of ML Tl2O FET at the lateral interface, it is found that ML Tl2O forms n-type lateral Schottky contact with Ti2C, Sc, Au, and Ni electrodes with the lateral electron SBHs \( \varPhi_{\text{L}}^{\text{e, trans}} \) of 0.36, 0.27, 0.27, and 0.25 eV, respectively. For the Tl electrode, the Fermi level is above the CBM of ML Tl2O at the interfaces indicating that an n-type Ohmic contact is established in ML Tl2O FETs. Transmission gap is defined as the sum of the lateral holes and electrons SBH: \( E_{\text{g}}^{\text{trans}} { = }\varPhi_{\text{L}}^{\text{e,trans}} { + }\;\varPhi_{\text{L}}^{\text{h,trans}} \). The transport gap of ML Tl2O FETs extracted from the LDDOS is 1.15, 1.02, 1.04, 1.02, 1.01, 0.98, 0.98, and 0.95 eV with Ni, graphene, Au, Ti2C(OH)2, Ti2C, Ti2CF2, Sc, and Tl electrodes, respectively. The transport band gaps are similar to the bandgap of the free-standing ML Tl2O.
The transmission spectrum of the ML Tl2O FETs under zero-bias and gate voltage is plotted in Fig. 6 (right side of each figure). When Ti2C, Au, Ni, and Sc are used as electrodes, n-type FETs are established with the electron SBHs of 0.33, 0.29, 0.28, and 0.24 eV, respectively. When graphene is used as electrode, a p-type Schottky FET is established with the hole SBH of 0.14 eV. Obviously, Tl and Ti2C(OH)2 electrodes form the desired n-type Ohmic contacts, and Ti2CF2 electrode forms an expected p-type Ohmic contact in ML Tl2O FETs. The height and polarity of the lateral Schottky barrier calculated by the transmission spectrum are generally consistent with those obtained from the LDDOS.
The desired Ohmic contact is realized with Ti2C(OH)2 and Ti2CF2 electrodes in the ML Tl2O FETs, and the carrier polarity is changed from n-type with OH functional group to p-type with F functional group. The modulation of the contact type with the terminal Ti2C electrodes is beneficial from the significant difference of WF for Ti2C with different functional groups. For example, the WFs of Ti2C(OH)2 and Ti2CF2 are 1.10 and 4.81 eV, respectively. The phenomenon is also reported in the ML MoS2 counterpart [55]. What is more, weak vdW junction is always formed between the ML Tl2O and terminated Ti2C with functional group, and the band structures of the ML Tl2O are preserved, which favors the performance of 2D semiconductor FETs. Thus, MXene is an excellent electrode candidate for the ML Tl2O FETs, and the contact can be tuned by changing the functional group at the surface of MXene.
Discussions
The polarity and height of the lateral Schottky barriers obtained from the quantum transport simulation and the WFA are compared in Fig. 7. The carrier polarity of the ML Tl2O FETs is the same in the two methods for Sc, Tl, Ti2C, graphene, Ti2C(OH)2, and Ti2CF2 electrodes, while it is completely reversed in the two approaches for Au and Ni electrodes. The n-type lateral Schottky contacts in the quantum transport simulations are changed into p-type Ohmic contact and quasi-Ohmic contact in the WFA for Au and Ni electrode, respectively. The SBH of the ML Tl2O FET is similar for Ti2C electrode by using the two approaches; Ohmic contacts are both formed when Ti2C(OH)2, Tl, and Ti2C as electrodes in the two approaches; the SBH of ML Tl2O FET is overestimated by the WFA than the quantum transport simulation for graphene electrode; a false Ohmic contact is examined with Sc electrode by using WFA. The discrepancy of the Schottky barrier of the two approaches is huge for the ML Tl2O–metal contact, and the phenomena are common in the FET of 2D semiconductors [44, 48, 49]. The cause of the discrepancy is that the interaction between the electrode and the channel region is taken into consideration in the quantum transport simulations, but it is neglected in the WFA. The interaction is weak in the case of the weak binding (Ti2C(OH)2, Ti2CF2, and graphene electrodes), and the two methods give similar Schottky barrier. However, the interaction is strong in the strong binding case (ML Tl2O-Ti2C Tl, Sc, Au, and Ni electrodes), and the two approaches give the apparently different Schottky barrier type and height. The quantum transport simulation is more reliable to examine the lateral SBH by using a real two-probe model.
Comparison of the lateral SBHs obtained by the ab initio work function approximation and quantum transport calculation for the ML Tl2O–Ti2C(OH)2, Tl, Sc, Ni, Au, Ti2C, graphene, and Ti2CF2 interfaces. Ef is the Fermi level. \( \Phi _{L}^{e} \) and \( \Phi _{\text{L}}^{h} \) represent the lateral electron and hole SBH, respectively
Strong FLP effect always occurs at 2D semiconductor–metal interfaces, such as for ML MoS2 [22] and black phosphorene [44, 56], and the degree of FLP is decided by the pinning factor S, which is described by the following equation:
where \( \varPhi^{\text{e}} \) denotes the SBH of the electron and Wm is the WF of the pristine metal. The \( \varPhi^{\text{e}} \) as a function of the Wm is plotted in Fig. 8. The value of pinning factor is 0.15 by using the quantum transport calculation, indicating a strong FLP appearing between the ML Tl2O and metals. The strong FLP is from the MIGS, which apparently appears at the lateral interfaces with graphene, Ti2C, Sc, and Au electrodes, as presented in Fig. 6. The other reason for the strong FLP is the interaction between the channel ML Tl2O and the metal electrode considered in the quantum transport simulation. The pinning factor calculated by the WFA is 0.54 without including the coupling, which is apparently larger than that (0.15) calculated by the quantum transport simulation. The FLP effect of the ML Tl2O FET is comparable with that of the ML MoS2 FET with the pinning factor of 0.187 [53].
Conclusions
In conclusion, on the basis of ab initio electronic structure calculation and the quantum transport simulation, the interfacial properties of the ML Tl2O FETs are comprehensively investigated with Au, Sc, Tl, Ni, graphene, Ti2C, Ti2CF2, and Ti2C(OH)2 electrodes. The n-type Schottky FETs are discovered with Ni, Au, Sc, and Ti2C electrodes with the lateral electron SBH of 0.25, 0.27, 0.27, and 0.36 eV, respectively, while the p-type Schottky FET is discovered with graphene electrode with the lateral hole SBH of 0.10 eV. Surprisingly, the desired n-type Ohmic contacts arise with Tl and Ti2C(OH)2 electrodes, and a desired p-type Ohmic contact arises with Ti2CF2 electrode in the ML Tl2O FETs. Strong FLP is observed in the ML Tl2O FETs with a small pinning factor of 0.15, which is mainly from the MIGS and the coupling between the electrode and the channel ML Tl2O. Our result reveals a versatile approach to realize both n- and p-type Ohmic contact in the ML Tl2O FETs.
References
Geng D, Yang HY (2018) Recent advances in growth of novel 2D materials: beyond graphene and transition metal dichalcogenides. Adv Mater 30(45):1800865
Zeng M, Xiao Y, Liu J, Yang K, Fu L (2018) Exploring two-dimensional materials toward the next-generation circuits: from monomer design to assembly control. Chem Rev 118(13):6236–6296
Xu M, Liang T, Shi M, Chen H (2013) Graphene-like two-dimensional materials. Chem Rev 113(5):3766–3798
Desai SB, Madhvapathy SR, Sachid AB, Llinas JP, Wang Q, Ahn GH, Pitner G, Kim MJ, Bokor J, Hu C, Wong HSP, Javey A (2016) MoS2 transistors with 1-nanometer gate lengths. Science 354(6308):99–102
Allain A, Kang J, Banerjee K, Kis A (2015) Electrical contacts to two-dimensional semiconductors. Nat Mater 14:1195–1205
Schwierz F (2010) Graphene transistors. Nat Nanotechnol 5:487–496
Fiori G, Bonaccorso F, Iannaccone G, Palacios T, Neumaier D, Seabaugh A, Banerjee SK, Colombo L (2014) Electronics based on two-dimensional materials. Nat Nanotechnol 9(10):768–779
Chhowalla M, Jena D, Zhang H (2016) Two-dimensional semiconductors for transistors. Nat Rev Mater 1(11):1–15
Xie L, Liao M, Wang S, Yu H, Du L, Tang J, Zhao J, Zhang J, Chen P, Lu X, Wang G, Xie G, Yang R, Shi D, Zhang G (2017) Graphene-contacted ultrashort channel monolayer MoS2 transistors. Adv Mater 29(37):1702522
Ni Z, Ye M, Ma J, Wang Y, Quhe R, Zheng J, Dai L, Yu D, Shi J, Yang J, Watanabe S, Lu J (2016) Performance upper limit of sub-10 nm monolayer MoS2 transistors. Adv Electron Mater 2(9):1600191
Xu K, Chen D, Yang F, Wang Z, Yin L, Wang F, Cheng R, Liu K, Xiong J, Liu Q, He J (2017) Sub-10 nm nanopattern architecture for 2D material field-effect transistors. Nano Lett 17(2):1065–1070
Ho P-H, Chang Y-R, Chu Y-C, Li M-K, Tsai C-A, Wang W-H, Ho C-H, Chen C-W, Chiu P-W (2017) High-mobility InSe transistors: the role of surface oxides. ACS Nano 11(7):7362–7370
Bandurin DA, Tyurnina AV, Yu GL, Mishchenko A, Zólyomi V, Morozov SV, Kumar RK, Gorbachev RV, Kudrynskyi ZR, Pezzini S, Kovalyuk ZD, Zeitler U, Novoselov KS, Patanè A, Eaves L, Grigorieva IV, Fal’ko VI, Geim AK, Cao Y (2017) High electron mobility, quantum Hall effect and anomalous optical response in atomically thin InSe. Nat Nanotechnol 12(3):223–227
Li L, Yu Y, Ye GJ, Ge Q, Ou X, Wu H, Feng D, Chen XH, Zhang Y (2014) Black phosphorus field-effect transistors. Nat Nanotechnol 9:372–377
Du Y, Liu H, Deng Y, Ye PD (2014) Device perspective for black phosphorus field-effect transistors: contact resistance, ambipolar behavior, and scaling. ACS Nano 8(10):10035–10042
Ma Y, Kuc A, Heine T (2017) Single-layer Tl2O: a metal-shrouded 2D semiconductor with high electronic mobility. J Am Chem Soc 139(34):11694–11697
Wang H, Zhou Y, Zeng Z-Y, Cheng Y, Chen Q-F (2019) First-principles study of elastic, thermal and optical properties of a metal-shrouded two-dimensional semiconductor Tl2O. Solid State Commun 293:40–47
Kang J, Liu W, Sarkar D, Jena D, Banerjee K (2014) Computational study of metal contacts to monolayer transition-metal dichalcogenide semiconductors. Phys Rev X 4(3):031005
Iannaccone G, Bonaccorso F, Colombo L, Fiori G (2018) Quantum engineering of transistors based on 2D materials heterostructures. Nat Nanotechnol 13(3):183–191
Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A (2011) Single-layer MoS2 transistors. Nat Nanotechnol 6:147–150
Gong C, Colombo L, Wallace RM, Cho K (2014) The unusual mechanism of partial Fermi level pinning at metal–MoS2 interfaces. Nano Lett 14(4):1714–1720
Kim C, Moon I, Lee D, Choi MS, Ahmed F, Nam S, Cho Y, Shin HJ, Park S, Yoo WJ (2017) Fermi level pinning at electrical metal contacts of monolayer molybdenum dichalcogenides. ACS Nano 11(2):1588–1596
Tung RT (2000) Chemical bonding and Fermi level pinning at metal-semiconductor interfaces. Phys Rev Lett 84(26):6078–6081
Liu Y, Xiao H, Goddard WA (2016) Schottky-barrier-free contacts with two-dimensional semiconductors by surface-engineered MXenes. J Am Chem Soc 138(49):15853–15856
Liu Y, Stradins P, Wei S-H (2016) Van der Waals metal-semiconductor junction: weak Fermi level pinning enables effective tuning of Schottky barrier. Sci Adv 2(4):e1600069
Liu Y, Wu H, Cheng H-C, Yang S, Zhu E, He Q, Ding M, Li D, Guo J, Weiss NO, Huang Y, Duan X (2015) Toward barrier free contact to molybdenum disulfide using graphene electrodes. Nano Lett 15(5):3030–3034
Zhang J, Xie W, Agiorgousis ML, Choe DH, Meunier V, Xu X, Zhao J, Zhang S (2018) Quantum oscillation in carrier transport in two-dimensional junctions. Nanoscale 10(17):7912–7917
Zhang J, Xie W, Zhao J, Zhang S (2016) Band alignment of two-dimensional lateral heterostructures. 2D Mater 4(1):015038
Popov I, Seifert G, Tománek D (2012) Designing electrical contacts to MoS2 monolayers: a computational study. Phys Rev Lett 108(15):156802
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186
Kresse G, Furthmüller J (1996) Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6(1):15–50
Kresse G, Hafner J (1993) Ab Initio molecular dynamics for liquid metals. Phys Rev B 47(1):558–561
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59(3):1758–1775
Klimeš J, Bowler DR, Michaelides A (2009) Chemical accuracy for the van der Waals density functional. J Phys Condens Mat 22(2):022201
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192
Taylor J, Guo H, Wang J (2001) Ab Initio modeling of quantum transport properties of molecular electronic devices. Phys Rev B 63(24):245407
Brandbyge M, Mozos J-L, Ordejón P, Taylor J, Stokbro K (2002) Density-functional method for nonequilibrium electron transport. Phys Rev B 65(16):165401
Atomistix ToolKit, version 2019. QuantumWise a/s: Copenhagen, Denmark
Çakır D, Peeters FM (2014) Dependence of the electronic and transport properties of Metal–MoSe2 interfaces on contact structures. Phys Rev B 89(24):245403
Hu Z, Metiu H (2011) Choice of U for DFT + U calculations for titanium oxides. J Phys Chem C 115(13):5841–5845
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Gao S, Yang L (2017) Renormalization of the quasiparticle band gap in doped two-dimensional materials from many-body calculations. Phys Rev B 96(15):155410
Liang Y, Yang L (2015) Carrier plasmon induced nonlinear band gap renormalization in two-dimensional semiconductors. Phys Rev Lett 114(6):063001
Pan Y, Wang Y, Ye M, Quhe R, Zhong H, Song Z, Peng X, Yu D, Yang J, Shi J, Lu J (2016) Monolayer phosphorene-metal contacts. Chem Mater 28(7):2100–2109
Pan Y, Dan Y, Wang Y, Ye M, Zhang H, Quhe R, Zhang X, Li J, Guo W, Yang L, Lu J (2017) Schottky barriers in bilayer phosphorene transistors. ACS Appl Mater Interface 9(14):12694–12705
Zhang X, Pan Y, Ye M, Quhe R, Wang Y, Guo Y, Zhang H, Dan Y, Song Z, Li J, Yang J, Guo W, Lu J (2018) Three-layer phosphorene-metal interfaces. Nano Res 11(2):707–721
Das S, Zhang W, Demarteau M, Hoffmann A, Dubey M, Roelofs A (2014) Tunable transport gap in phosphorene. Nano Lett 14(10):5733–5739
Pan Y, Li S, Ye M, Quhe R, Song Z, Wang Y, Zheng J, Pan F, Guo W, Yang J, Lu J (2016) Interfacial properties of monolayer MoSe2–Metal contacts. J Phys Chem C 120(24):13063–13070
Zhong H, Quhe R, Wang Y, Ni Z, Ye M, Song Z, Pan Y, Yang J, Yang L, Lei M, Shi J, Lu J (2016) Interfacial properties of monolayer and bilayer MoS2 contacts with metals: beyond the energy band calculations. Sci Rep 6:21786
Huang H, Xing G, Fan X, Singh D (2019) Layered Tl2O: a model thermoelectric material. J Mater Chem C 7:5094–5103
Liu H, Hu K, Yan D, Chen R, Zou Y, Liu H, Wang S (2018) Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv Mater 30(32):1800295
Yang S, Zhang K, Ricciardulli AG, Zhang P, Liao Z, Lohe MR, Zschech E, Blom PWM, Pisula W, Müllen K, Feng X (2018) A delamination strategy for thinly layered defect-free high-mobility black phosphorus flakes. Angew Chem Int Ed 57(17):4677–4681
Pan Y, Gu J, Tang H, Zhang X, Li J, Shi B, Yang J, Zhang H, Yan J, Liu S, Hu H, Wu M, Lu J (2019) Reexamination of the Schottky barrier heights in monolayer MoS2 field-effect transistors. ACS Appl Nano Mater 2(8):4717–4726
Liu Y, Guo J, Zhu E, Liao L, Lee SJ, Ding M, Shakir I, Gambin V, Huang Y, Duan X (2018) Approaching the Schottky-Mott limit in van der Waals metal–semiconductor junctions. Nature 557(7707):696–700
Peng Q, Si C, Zhou J, Sun Z (2019) Modulating the Schottky barriers in MoS2/MXenes heterostructures via surface functionalization and electric field. Appl Surf Sci 480:199–204
Li Y, Lin S, Liu Y, Chai Y, Lau SP (2019) Tunable Schottky barriers in ultrathin black phosphorus field effect transistors via polymer capping. 2D Mater 6(2):024001
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Nos. 51572296, U1662113, 11904409), the Ministry of Science and Technology of China (No. 2016YFA0301300), the Fundamental Research Funds for the Central Universities (Nos. 17CX06029, 19CX05002A), China Postdoctoral Science Foundation (No. 2018M642721), and Shandong Postdoctoral Funded Project (201901012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, Y., Dai, J., Liu, Z. et al. Ohmic contacts of monolayer Tl2O field-effect transistors. J Mater Sci 55, 11439–11450 (2020). https://doi.org/10.1007/s10853-020-04866-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04866-2