Abstract
A series of semimetal Bi-doped carbon fibers (Bi/CFs) were prepared by electrospinning-calcined polyacrylonitrile technique and characterized by XRD, SEM, EDS, XPS and UV–Vis DRS spectra to probe the morphology, structure and optical properties. Then, the Bi/CFs hybrids as photocatalysts were investigated the photocatalytic performances for degradation of representative environmental contaminants (Congo red dye and antibiotics sulfanilamide) under visible light irradiation (λ > 420 nm). In different compositions, the photocatalytic efficiencies have followed the order as Bi (5%)/CFs > Bi (10%)/CFs > Bi (1%)/CFs > polyacrylonitrile carbon fibers, that was mainly for the surface plasmon resonance effect of semimetal Bi, which made Bi/CFs hybrids had high light-harvesting properties and effective separation abilities of photogenerated carriers. According to the results of optical performance and the scavengers trapping experiments, the feasible photocatalytic mechanism was deduced. This study provided semimetal Bi, a new plasmonic co-catalyst, as a well substitute for Au and Ag noble metal to be a promising potential for environmental contaminants treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, in the wake of rapid development of economy and industry, environmental water contamination issues became one of the intractable problems all over the world [1]. Photocatalytic water purification was a green and effective technology to treatment of water pollutants, which utilized inexhaustible solar energy [2, 3]. Therefore, development of various of photocatalysts became the focus for more and more researchers [4,5,6].
Surface plasmon resonance (SPR) effect of noble metal was deemed to be an effective means for photocatalytic remediation of environmental pollutant [7,8,9,10]. Au, Ag and Pt were widely used as co-catalyst for photocatalysis due to their SPR effect [11,12,13,14]. While in view of the over-expenditure for water treatment, inexpensive and eco-friendly metals were sought to replace of these noble metals. Recently, a typical semimetal material bismuth (Bi) draws attentions because of its non-toxic, green, high carrier motility and low cost [15,16,17]. It was found that semimetal Bi was an attractive plasmonic photocatalyst and can act as the good substitute of the noble metal. For example, Zhu et al. obtained C/Bi/Bi2O3 composite by calcining the precursor to photocatalytic degradation of 2,4-dichlorophenol [18]. Chiu et al. found that Bi/C/α-Bi2O3 prepared by thermal decomposition exhibited superior photocatalytic activity for MO and MG degradation under visible light irradiation [19]. Bi microsphere/g-C3N4 was synthesized and used for photocatalytic H2 evolution and NO removal under visible light irradiation [16, 20]. Nevertheless, as far as we know, doping semimetal Bi with carbon fibers by electrospinning of polyacrylonitrile and its photocatalytic performance have no other report.
Herein, a series of semimetal Bi coupling with carbon fibers derived from electrospinning of polyacrylonitrile were prepared and the photocatalytic activities were investigated by degradation of environmental water contaminants including dye (Congo red) and antibiotics (sulfanilamide) under visible light irradiation. Based on the trapping experiments, the possible photocatalytic mechanism of Bi/CFs was speculated.
Experimental section
Synthesis of Bi/CFs hybrids
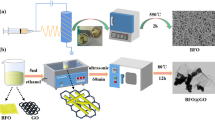
The formation process of Bi/CFs hybrids is demonstrated in Scheme 1 in detail. Typically, the carbon nanofiber webs were woven by electrospinning method. Firstly, the precursor solution containing 16wt% solute was obtained by mixing polyacrylonitrile (PAN, Mw = 90,000) with N–N-dimethylformamide (DMF) solvent at 70 °C. Then, it was separated° into four bottles, each containing 30 mL. After that, BiCl3 of 0.48 g, 0.24 g and 0.048 g were, respectively, added into four bottles under vigorous stirring. The uniform mixed solution was loaded into a syringe equipped with an 18G stainless steel needle. A high voltage (21 kV) was applied to the needle, and the solution was propelled at a speed of 3 mL h−1. Besides, the rotating speed of collecting drum was 300 rpm and the distance between the needle and collecting drum was 20 cm. The prepared nanofiber webs were pre-oxidized in a tubular furnace for 30 min in the air and then carbonized at 1000 °C for 1.5 h in nitrogen atmosphere with a heating rate of 5 °C min−1. Then, the products named Bi (10%)/CFs, Bi (5%)/CFs, Bi (1%)/CFs, respectively, were obtained.
Characterization
Scanning electron microscopy (SEM) (FEI INSPECT-F, USA) was used to detect the morphology of the materials. X-ray diffraction (XRD) patterns with diffractometer D8 (Bruker AXS, Germany) were performed in the range of 2θ = 10°–80°. An ESCAlab 250Xi electron spectrometer from Thermo Scientific (300 W Al Kα radiations) was investigated X-ray photoelectron spectroscopy (XPS). A Metash UV-8000S with BaSO4 as the reflectance standard recorded the UV–visible diffuse reflectance spectra (DRS).
Photocatalytic experiments
The photocatalytic properties of the hybrids were investigated by photodegradation of target contaminant (Congo red (CR) dye or sulfanilamide (SA) antibiotics) under visible light using 300 W Xe lamp (PLS-SXE300, Beijing Perfect Light) with UV-cutoff filter (λ > 420 nm). Typically, 0.5 g L−1 of the sample was added to 50 mL of target contaminant aqueous solution (10 mg L−1) under vigorous stirring. Before irradiation, the suspension was kept in dark for 30 min with continuous stirring to achieve adsorption–desorption equilibrium. In the process of visible light irradiation, 3 mL aliquots were sampled at a certain interval of time and removed the catalysts by centrifugation. Then, the concentration of target contaminant aqueous solution was monitored by UV–Vis spectrophotometer.
Results and discussion
Characterization
Figure 1 shows XRD patterns of the as-prepared samples to characterize the phase and the structure. Figure 1a suggests that two diffraction peaks were at 2θ = 15.68° and 22.61°, which referred to the lateral packing of crystalline regions in PAN molecules. Also, a weak and wide peak located at about 42.3° corresponded to (100) characteristic reflection peak of carbon materials, indicating that the spun nanofibers were completely carbonized with the carbonization temperature over 1000 °C [21, 22]. As displayed in Fig. 1b–d, it can be found that the two peaks located at 10°–25° belong to the characteristic peaks of PAN-C. Then, the diffraction peaks at 27.81°, 37.60°, 39.87°, 48.49° and 64.16° were well matched with the (012), (104), (110), (202) and (122) planes of semimetal Bi (JCPDS No. 44-1246) [16], illustrating that semimetallic Bi was doped on carbon fibers.
Figure 2a displays the SEM image of carbon fibers formed by electrostatic spinning of polyacrylonitrile, which had a diameter in the range of 300–500 nm, and the surface was smooth without other impurity. The smooth surface of PAN-CFs (inset of Fig. 2a) can be attributed to the carbonized procedure and the high calcined temperature to 1000 °C. As displayed in image of Fig. 2a, b–d, mass of Bi spheres in situ grew on the surface of PAN-CFs with different Bi-doped contents from 1 to 10%. From the image of inset of Fig. 2c, the diameter of Bi microsphere was approximately 1–2 μm. It can be found that Bi microspheres were uniformly immobilized on the surface of PAN-CFs. In Fig. 2d, as the Bi-doped content increased to 10%, many Bi microspheres agglomerated severely each other, which will hinder the active sites and affect the photocatalytic activity. The corresponding EDS mapping images of Bi (5%)/CFs sample clearly demonstrated the homogeneous spatial distribution of C, O, Bi elements (Fig. 2e).
The elemental and chemical states of Bi/CFs hybrid were investigated by XPS spectra (Fig. 3). Figure 3a exhibits all of the detected elements of PAN-CFs and Bi (10%)/CFs in survey XPS spectra. It was noted that the existence of N in the survey spectra was probable due to nitrogen contamination species from XPS measurement and the N2 calcination process. From Fig. 3b, c, the peak of C 1 s at binding energy 283.6 eV shifted to 281.4 eV and the peak of O 1 s at 530.8 eV shifted to 528.7 eV. Apparently, blueshift happened after doping Bi, indicating the interaction between semimetallic Bi microspheres and PAN-CFs. As shown in Fig. 3d, two peaks at binding energy of 164.4 eV and 161.3 eV belong to Bi–Bi bond of semimetallic Bi microsphere. In consequence, XPS results further verified the existence of the elements in Bi/CFs hybrids, which was in accordance with EDS.
Figure 4 illustrates the UV–Vis DRS of PAN-CFs and different content Bi/CFs hybrids. Compared with PAN-CFs, the light adsorption capability was conspicuously heightened from 400 to 800 nm after Bi doped. That was mainly because of the charge-transfer transition between Bi microspheres and PAN-CFs. Furthermore, Bi/CFs hybrids demonstrated wide absorption peak over 500–600 nm, which was the obvious characteristic SPR peak of semimetal Bi [20, 23]. The enhanced light adsorption ability was inevitably in favor of prompting photoexciting electron–holes to improve the photocatalytic performance of Bi/CFs hybrids.
Photocatalytic capabilities of Bi/CFs hybrids
The photocatalytic performances of Bi/CFs hybrids were evaluated by degradation CR dye solution and antibiotic SA solution under visible light irradiation in 120 min. As shown in Fig. 5a, the photodegradation of CR dye was almost negligible in 120 min without any catalyst under visible light irradiation (λ > 420 nm). In the same way, the PAN-CFs as the catalyst gave poor photodegraded activity. However, a series of Bi/CFs hybrids, including Bi (1%)/CFs, Bi (5%)/CFs and Bi (10%)/CFs, exhibited satisfied degradation capacities. The superior photocatalytic activity of Bi/CFs hybrids may be attributed to SPR effect of semimetal Bi doped with PAN-CFs and the visible light absorption capability of PAN-CFs [18]. Bi (5%)/CFs illustrated the highest photocatalytic performance (97.2% degradation efficiency) than Bi (1%)/CFs (67.1%) and Bi (10%)/CFs (89.4%). When the content of semimetal Bi increased in the hybrid, excessive Bi will serve as the mediators for recombination of photoinduced carriers, which brought down the photocatalytic activity [24]. Figure 5b displays similar photocatalytic tendency for degradation of antibiotic SA solution.
Figure 6a illustrates the result of four recycle runs for degradation of CR and SA over Bi (5%)/CFs hybrid under the same experimental conditions. It was obvious that, after four runs, insignificant more decrease for degradation efficiency in photocatalytic activity was found. That may be a bit of loss during wash-dry circulation process. Namely, the Bi (5%)/CFs hybrid was stable and prospective for environmental wastewater treatment under visible light irradiation.
Deduced photocatalytic mechanism
To further explore the active species and deduce the photocatalytic mechanism, the trapping experiments were performed. Different scavengers, Na2C2O4 served as hole (h+) scavenger [25], isopropanol (IPA) and methyl alcohol (MeOH) act as ·OH and ·O2− scavenger [26, 27], were used in the photodegradation reaction. As shown in Fig. 6b, with the introduction of IPA, the degradation efficiency of CR/SA decreased a little. However, the degradation efficiency of CR/SA was remarkably hampered as MeOH was introduced into the system. When Na2C2O4 was introduced, a certain repression was also found. That manifested that ·O2− radical was the main active species as well as h+ was subordinate active species for the whole degradation process.
According to the whole experimental results, the schematically deduced photocatalytic mechanism is demonstrated in Scheme 2. A survey of the previous literature showed that semimetal Bi had surface plasmon effect which facilitated the separation of photogenerated electron–holes. Under the visible light irradiation, the hot electrons (e−) can be aroused and emitted from the materials due to the SPR of semimetal Bi. Then, the hot electrons will transfer quickly from SPR Bi materials to the PAN-CFs, leaving positive charges (h+) on Ef (− 0.17 eV) [28]. Ultimately, the e− transferred to the surface of PAN-CFs will accept O2 to form ·O2− radical, and the h+ oxidized H2O molecular to ·OH, which effectively inhibited the recombination of e−/h+ pairs. As we know, the semimetal Bi can perform as electron traps to accelerate the separation of photogenerated e−/h+ pairs. Then, a number of pollutant molecules (CR dye or SA antibiotics) absorbed on the surface of Bi/CFs were oxidized by active species ·O2−, h+ and ·OH to decompose step by step.
Comparing with other photocatalysts
The photocatalyst Bi/CFs hybrid was compared to other photocatalysts for determination of the photocatalytic capability from the viewpoint of preparation methods, type of target pollutants and degradation efficiency (Table 1). Due to the special SPR effect of semimetal Bi, Bi/CFs hybrid processed high light-harvesting properties and effective separation abilities of photogenerated carriers, which result in satisfied photocatalytic performance. It was further evidenced that the photocatalyst Bi/CFs had promising potential applications for eliminating various organic contaminants.
Conclusion
Summarily, a series of Bi/CFs were prepared by electrospinning technique as photocatalyst, which exhibited outstanding photocatalytic performance for degradation of Congo red dye and antibiotics sulfanilamide under visible light (λ > 420 nm), that mainly belongs to the special SPR effect of semimetal Bi. The hybrids Bi/CFs not only had favorable light-harvesting ability, but also possessed higher separation capability of photogenerated carriers. This work provided a new sight for nonmetallic material as the expensive plasmonic catalyst Au or Ag substitute in environmental contamination remediation.
References
Rodriguez-Narvaez OM, Peralta-Hernandez JM, Goonetilleke A, Bandala ER (2017) Chem Eng J 323:361–380
You J, Guo Y, Guo R, Liu X (2019) Chem Eng J 373:624–641
Bolisetty S, Peydayesh M, Mezzenga R (2019) Chem Soc Rev 48:463–487
Wang Z, Li C, Domen K (2019) Chem Soc Rev 48:2109–2125
Yang WT, Liu XL, Li D, Fan LZ, Li YC (2015) Phys Chem Chem Phys 17:14532–14541
Liu XL, Li D, Yang WT, Tang SL, Li XH, Fan LZ (2016) YC Li. J Mater Sci 51:11021–11037
Hou W, Cronin SB (2013) Adv Funct Mater 23:1612–1619
Wu N (2018) Nanoscale 10:2679–2696
Wang M, Ye M, Iocozzia J, Lin C, Lin Z (2016) Adv Sci 3:1600024
Shahzad A, Kim W-S, Yu T (2016) Dalton T 45:9158–9165
Zhang W, Hu Y, Yan C et al (2019) Nanoscale 11:9053–9060
Xu B, Li Y, Gao Y et al (2019) Appl Catal B-Environ 246:140–148
Yang X, Liu S, Li J, Chen J, Rui Z (2020) Chemosphere 249:126096
Fang J, Chen X, Wu Y, Liu H (2020) J Mater Sci 55:5880–5891
Li J, Zhang W, Ran M, Sun Y, Huang H, Dong F (2019) Appl Catal B-Environ 243:313–321
Dong F, Zhao Z, Sun Y, Zhang Y, Yan S, Wu Z (2015) Environ Sci Technol 49:12432–12440
Hua C, Dong X, Wang Y, Zheng N, Ma H, Zhang X (2019) J Mater Sci 54:9397–9413
Hao Q, Wang R, Lu H et al (2017) Appl Catal B-Environ 219:63–72
Ma Y, Han Q, Chiu T-W, Wang X, Zhu J (2020) Catal Today 340:40–48
Wei Z, Liu J, Fang W et al (2019) Chem Eng J 358:944–954
Song C, Wang T, Qiu Y, Qiu J, Cheng H (2009) J Porous Mat 16:197–203
Miao F, Shao C, Li X, Wang K, Liu Y (2016) J Mater Chem A 4:4180–4187
Yu Y, Cao C, Liu H et al (2014) J Mater Chem A 2:1677–1681
Weng S, Chen B, Xie L, Zheng Z, Liu P (2013) J Mater Chem A 1:3068–3075
Wu Y, Li Y, Fang C, Li C (2019) ChemCatChem 11:2297–2303
Tan L, Yu C, Wang M et al (2019) Appl Surf Sci 467–468:286–292
Zeng L, Xiao L, Shi X, Wei M, Cao J, Long Y (2019) J Colloid Interf Sci 534:586–594
Dong F, Li Q, Sun Y, Ho W-K (2014) ACS Catal 4:4341–4350
Fiorenza R, Di Mauro A, Cantarella M et al (2020) Mat Sci Semicon Proc 112:105019–105025
Ray SK, Dhakal D, Lee SW (2020) Mat Sci Semicon Proc 105:104697–104706
Babu B, Koutavarapu R, Shim J, Yoo K (2020) Sep Purif Technol 240:116652–116667
Acknowledgements
This project was supported by the Doctoral Start-up Foundation of Liaoning Provincial Natural Science Foundation of China (2019-BS-129, 2019-BS-128), the Doctoral Start-up Foundation of Liaoning Institute of Science and Technology (Nos. 1810B07, 1810B09) and the Scientific Foundation Project of Education Department of Liaoning Province (L2019lkyjc-02). The authors also thank their colleagues who participated in this work.
Author information
Authors and Affiliations
Contributions
DFZ and JGL conceived and designed the study. ZYX, TL and CWA performed the experiments. DFZ and HZ wrote the paper. DFZ, ZYX, HZ, TL, CWA and JGL reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, D., Xu, Z., Zhao, H. et al. Semimetal Bi/carbon fibers derived from electrospinning polyacrylonitrile and its visible light photocatalytic performance. J Mater Sci 55, 10765–10772 (2020). https://doi.org/10.1007/s10853-020-04696-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04696-2