Abstract
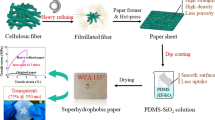
Herein, an eco-friendly and straightforward method was provided for the fabrication of superhydrophobic paper from native wood cellulose fibers via in situ hydrolysis of tetraethyl titanate(IV) without any chemical pretreatment. By simply adjusting the amount of acetic acid (HAc), the surface micro/nanomorphology could be well controlled. After papermaking and hexadecyltrimethoxysilane modification, superhydrophobic paper can be easily achieved with static water contact angle of 152.3° (± 1.3°). The paper also possessed good self-cleaning property against contamination and durability toward mechanical damages of finger wiping over 50 cycles as well as excellent oil/water separation, which expands its utility in various paper-based technologies. The whole procedure possesses the advantages of friendly raw material, mild reaction conditions and with no toxic modifier, which hold potential application in cellulose-based superhydrophobic material in large scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to environmental pollution and resources shortage, bio-based materials made from cellulose fibers, starch, lignocellulosic, chitosan and their bio-derivatives have attracted great attention. Among these, cellulose, as the most widely distributed and abundant polysaccharide in nature, has many excellent properties, including biodegradability, recyclability, excellent mechanical strength as well as flexibility, portability, lightweight and processability [1, 2]. Hence, cellulose fibers are widely used in the manufacture of paper-based materials for printing, packaging [3], stimuli-responsive chromic paper devices [4], supercapacitors and antibacterial [5,6,7]. However, the inherent hydrophilicity of cellulose largely limited paper-based products application in moisture circumstances. Therefore, it is important to improve the hydrophobic properties of cellulose fiber-based paper for practical applications.
Inspired by lotus leaf, lowing surface energy on rough surfaces or fabricating micro/nanoroughness on low-energy surfaces are two key strategies for constructing superhydrophobic surfaces [8,9,10,11,12,13,14,15,16,17,18]. Methods have been reported for the construction of superhydrophobic cellulose fabrics or superhydrophobic paper [19,20,21,22]. Avijit et al. [23] presented an eco-friendly and facile methodology for the fabrication of waterproof paper using fluoroalkyl-functionalized CNFs which were first chemically functionalized with 1H,1H,2H,2H-perfluorooctyltriethoxysilane and 3-(2-aminoethylamino)-propyltrimethoxysilane via a wet chemical process. Satapathy et al. [24] reported a facile strategy to construct superhydrophobic paper via solution casting technique of LLDPE and SiO2 + LLDPE coatings. Liu et al. [25] presented a novel strategy to construct durable superhydrophobic fabrics by pre-applying long alkyl chain silane on the surface of the fabrics and then argon vacuum plasma treatment. However, the complex equipment, complicated synthetic procedures or expensive raw materials greatly limited the application of superhydrophobic materials in large scale. The development of a facile, straightforward and economical method to prepare superhydrophobic paper, especially without changing the original structural properties of cellulose fibers, is in great demand.
Herein, we report a novel and green strategy for the fabrication of superhydrophobic paper from native wood cellulose fibers prepared via in situ hydrolysis of IV without any chemical preprocessing. The surface micro/nanohierarchical rough surface can be well controlled by adjusting the amount of acetic acid (HAc). After papermaking process and subsequent modified with HDTMS, superhydrophobic paper can be well achieved. The structure, chemical composition and surface morphology of the cellulose fibers before and after titanium dioxide functionalization were characterized by FT-IR, XPS, SEM and TGA, respectively. Besides, the wetting stability against abrasion, self-cleaning and oil/water separation properties of the obtained superhydrophobic paper was also studied to evaluate the actual practical performance of the resultant superhydrophobic paper.
Experimental
Materials
Bleached hardwood pulp (moisture content 80 wt%, 75 SR°) was kindly provided by Guangzhou Chenhui Paper Co., Ltd., China. Tetraethyl titanate (IV, 98 wt%), acetic acid (HAc, 99%,) and hexadecyltrimethoxysilane (HDTMS, 99%) were purchased from Sigma-Aldrich. Anhydrous ethanol was purchased from Guangzhou Chemical Reagent Co. Deionized water was obtained from a Millipore Milli-Q water purification system. All the chemicals were used as received without any further purification.
Synthesis of titanium dioxide-functionalized cellulose fibers
The wood cellulose pulp (1.0 g) was suspended in 50 mL ethanol with a mechanical stirrer until it was completely dispersed. Then, IV (500 mg) and a certain amount of HAc were added to the above solution and conducted for 3 h at 50 °C. The titanium dioxide (TiO2)-functionalized cellulose fibers were obtained by centrifugation, ethanol washing and centrifugation repeatedly.
Fabrication of functionalized fiber-based superhydrophobic paper
The functionalized fiber-based superhydrophobic paper was fabricated through a simple vacuum filtration process [26]. Typically, 15 mL aqueous suspension containing 50 mg TiO2-functionalized cellulose fibers was poured onto the filter paper in a sand core funnel with a diameter of 45 mm. After suction filtration and subsequent immersed into petri dish with 30 mL ethanol solution containing HDTMS (1 wt%) for 15 min, superhydrophobic paper was obtained.
Characterization
The structure and chemical composition of cellulose fibers before and after TiO2 functionalization were characterized by FT-IR (Spectrum 2000, PerkinElmer) and XPS (Escalab 250, Thermo Electron) with Al Ka radiation (20 eV) as the exciting source. The morphology of the surfaces of cellulose fibers before and after surface modification were carried out by FE-SEM (Quanta 400F, FEI) at 15 kV. Thermogravimetric analysis (TGA) was measured with TA Instruments (Q600). The samples were heated at a heating rate of 20 °C/min from 40 to 600 °C in nitrogen atmosphere. The static water contact angles (WCAs) were measured at least five different positions on a contact angle system (Dataphysics OCA20) with liquid droplets of 5 μL.
Results and discussion
As is well known, cellulose fibers have a significant number of hydroxyl groups on their surface. Titanate coupling agent that contains four inorganic reactive groups on titanium will bond well to the hydroxyl groups on cellulose fibers. So, the cellulose fibers can act as the template to lead the in situ hydrolysis of IV. This is the basis for modifying cellulose fibers with IV without any chemical pretreatment. Specifically, methoxy groups of the IV can be hydrolyzed under acidic environment to give titanitol groups. The reactive titanitol groups formed can be absorbed rapidly through condensation with the hydroxyl groups present on cellulose fiber surface forming Ti–O–Ti bond linkages. This reaction results in the chemically bonded TiO2 nanoparticles to the cellulose fiber surface, which was beneficial for the construction of micro/nanoroughness structure. The reaction which occurs between cellulose fibers and IV is depicted in Scheme 1.
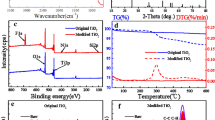
The presence of the TiO2 nanoparticles on the surface of cellulose fibers was confirmed by FT-IR spectra. Figure 1 shows the FT-IR spectra of pristine cellulose fibers and TiO2-functionalized cellulose fibers. Comparing with the spectra of pristine cellulose fibers in Fig. 1a, the wide absorption band at 400–750 cm−1 was the typical absorption peak of Ti–O stretching and Ti–O–Ti bridging stretching modes in TiO2. Besides, the peak in 2854 cm−1 (ν CH2) of the as-synthesized TiO2-functionalized fibers can be attributed to the characteristic frequencies of Ti(OH)x(OC2H5)4−x due to the surface in situ hydrolysis of IV on cellulose fibers [27]. These results proved that TiO2 nanoparticles were successfully grafted onto the surface of the cellulose fibers.
To further prove the above results, XPS were used to measure the surface chemical composition of pristine cellulose fibers and titanium dioxide-functionalized cellulose fibers. As depicted in Fig. 2a, the spectra of pristine cellulose fibers revealed only carbon (284.6 eV) and oxygen (532.6 eV) in cellulose fibers, which was similar to other literature reports [28, 29]. For the titanium dioxide-functionalized cellulose fibers, new peaks with binding energies of 456.36 eV (Ti 2p) and 462.48 eV (Ti 2p1) were appeared in high resolution of Ti 2p region in Fig. 2b, and the relative atomic concentration of carbon increased from 54.12 to 67.08%, and the relative atomic concentration of oxygen decreased from 45.88 to 23.88%, which strongly confirmed that the titanium dioxide particles were covered on the surface of cellulose fibers [30, 31].
XPS survey spectra of pristine cellulose fibers and TiO2 functionalized cellulose fibers (a), high-resolution XPS spectra of Ti 2p (b), C 1 s of pristine cellulose fibers and TiO2 functionalized cellulose fibers (c, d), O1s of pristine cellulose fibers and TiO2 functionalized cellulose fibers (e, f)
High-resolution C1s and O1s XPS spectra for pristine cellulose fiber and TiO2-functionalized cellulose fibers were also performed. As shown in Fig. 2c, the C1s peak can be deconvoluted into three peaks located at 284.8 eV, 286.8 eV and 288.1 eV, which are typical signals of carbon atoms of C–C, C–O and –C=O, respectively [29]. After surface in situ hydrolysis of IV, the C1s peak of fiber@TiO2 composites has been deconvoluted into three peaks located at 284.4 eV, 285.9 eV and 288.2 eV as shown in Fig. 2d. The signal of carbon atoms of C–C at 284.4 eV obviously increased from 5.3 to 19.01 due to the hydrolysis of IV to form the Ti(OH)x(OC2H5)4−x on the surface of cellulose fibers, where x was related to the amount of acetic acid added to the reaction solution [32, 33]. Figure 2e depicts the spectra of O1s region, taken on pristine cellulose fibers. The O1s region can be resolved into three peaks: 531.9 eV (C=O), 533.0 eV (OH) and 534.0 eV (C–O). After modification with TiO2 (Fig. 2f), the high-resolution XPS spectra of O1s region can be divided into five small peaks. The main contribution is attributed to Ti–O bond of TiO2 (529.37 eV) and Ti–O bond of Ti2O3 (530.7 eV) [34]. The other three peaks can be counted by the –OH groups (532.8 eV), the C–O bonds (533.9 eV) and C=O bonds (531.8 eV), respectively. The above analyses strongly proved TiO2 particles were successfully attached on the surface of cellulose fibers.
Since the bleached hardwood pulp used in this study has a moisture content of up to 80 wt%, IV with a relatively low electronegativity can rapidly react with polar solvents, especially water, making the resulting titanium dioxide agglomerate [35,36,37]. So, in order to effectively adjust the hydrolysis of IV, different amounts of HAc were added into the reaction suspension. Surface morphologies of pristine cellulose fibers and TiO2-functionalized cellulose fibers with different amounts of HAc (50 mg, 100 mg, 250 mg and 500 mg) were studied with SEM, as depicted in Fig. 3.
It can be seen that the pristine cellulose pulp was composed of cross-linked micro-nanofibers (Fig. 3a). Higher magnification showed that the surface of pristine cellulose fibers was smooth with natural grooves and veins in Fig. 3b [38]. Figures 3c and 4f show SEM images of TiO2-functionalized cellulose fibers obtained under varied HAc concentrations. When the HAc amount was 50 mg, the cellulose fibers were densely and uniformly covered by TiO2 nanoparticles, which roughen the fibers surface. Besides, heterogeneous TiO2 accumulations were also observed in the region among adjacent fibers which was due to the fast hydrolysis rate of IV (Fig. 3c). When the HAc amount increased to 100 mg (Fig. 3d), the irregularly shaped TiO2 accumulations in the region among adjacent fibers reduced greatly. With the content of HAc increased from 250 to 500 mg, the SEM images (Fig. 3e, f) showed that a homogeneous and complete coverage of TiO2 nanoparticles (20–30 nm) on the fiber surface was presented, forming micro/nanohierarchical rough surface structure. These results indicated that the surface morphology of cellulose fibers functionalized with TiO2 nanoparticles can be well controlled by adjusting the content of HAc, which was crucial to generating a superhydrophobic surface [39, 40].
Moreover, surface elemental mapping analysis was based on X-ray energy-dispersive spectroscopy (EDS) of the TiO2-functionalized cellulose fibers after HDTMS hydrophobic treatment. As shown in Fig. 4, each kind of elements was very complete and uniformly attached on the cellulose fibers, which also certified that cellulose fibers were successfully coated with TiO2 nanoparticles and subsequent HDTMS modification.
Thermogravimetric analysis (TGA) was used to quantitate the thermal properties and the amount of titanium dioxide nanoparticles deposited on cellulose fiber surfaces. Figure 5 shows the TGA results of pristine cellulose fibers and TiO2-functionalized cellulose fibers catalyzed with different HAc (50 mg, 100 mg, 250 mg and 500 mg). It is clearly seen that the residue of raw cellulose fibers was 8.08 wt% due to the present of inorganic ash content [41]. After in situ hydrolysis of IV with 50 mg HAc, the residue content grows to 53.22 wt%. With increasing the amount of HAc from 100 to 500 mg, the final residue weight ratio of the fibers decreased from 52.05 wt%, 41.23 wt% to 31.29 wt%, which was in accordance with SEM analyses. So, 100 mg ~ 250 mg of HAc should be appropriate, since the modified cellulose fibers showed controllable surface roughness.
As is well known, regular pure paper is inherent hydrophilic, and water can completely wet on the paper surface. After surface modification with TiO2 constructing micro/nanohierarchical structure and subsequent modified with HDTMS, superhydrophobic paper (catalyzed with 100 mg HAc) can be easily obtained. Water droplets can stand on the paper surface and maintain their spherical shapes with water contact angle of 152.3° ± 1.3° (Fig. 6a1, a2). Besides, the obtained superhydrophobic paper still maintained excellent mechanical flexibility after repeated folding experiments (Fig. 6a3), which is of vital importance in practice. Figure 6b1–b4 shows the SEM images of the as-prepared superhydrophobic paper. As can be seen, a favorable wrinkling surface morphology that formed over the original cellulose fiber could be gained for the obtained paper sample. It was found to contribute to the promotion of surface roughness for the paper and, thus, an elevated WCA (as high as 152°) because of the high surface roughness of the TiO2-functionalized fiber even after strong sorption vacuum filtration steps.
Optical picture showing static contact angle of water on superhydrophobic paper surface (a1); shapes of water droplet (5 µL) on as-prepared paper surface prepared with 100 mg HAc (a2); physical appearance and flexibility of superhydrophobic paper after refolding tests (a3); SEM image of the as-prepared superhydrophobic paper with different magnification times (b1–b3)
Resistance to staining by water-based fluids is an important feature of superhydrophobic paper. Figure 7a presents raw paper and superhydrophobic paper being immersed in methylene blue dyed water. Figure 7a2 shows raw paper was wetted and stained by the dyed water. Figure 7a4 shows the superhydrophobic paper after immersion in the dyed water showing no wetting or staining. The self-cleaning and antifouling performances are of vital importance for the applications of the final superhydrophobic paper. Hence, to observe self-cleaning properties of the superhydrophobic paper, a layer of carbon black was acted as contaminants spread on the surface, which was more difficult to be washed away than common contamination [42, 43]. As shown in Fig. 7b1–b4 and Movie S1, the carbon powders were easily got washed away with rolling water droplets leaving a thoroughly clean paper surface. This demonstrated that the obtained paper also possessed excellent self-cleaning and antifouling performances.
Images of raw paper (a1, a2) and superhydrophobic paper (a3, a4) before and after immersion in methylene blue dyed water; self-cleaning property of the superhydrophobic paper (b1–b4); images demonstrating the method of the finger-wipe test of the obtained paper (c1–c4); images demonstrating the sandpaper-abrasion test of the obtained paper (d1, d2)
For superhydrophobic interfaces, the superhydrophobicity can be easily destroyed by external friction due to its fragile micro/nanohierarchical surface structure, which was the biggest limitation in practical industrial application. The resistance to mechanical friction of the obtained paper was also investigated, as shown in Fig. 7c, d. Figure 7c1–c4 and Movie S2 demonstrate the finger-wipe test of superhydrophobic paper. It is clearly shown that, after 50 times finger wipes, the water droplets fell off superhydrophobic paper surface quickly, showing excellent durability against finger wiping. Figure 7d1, d2 shows the sandpaper-abrasion test of superhydrophobic paper under the weight of 50 g. Though after rubbed against sandpaper, the fiber debris on the paper surface was worn away, it still remained good superhydrophobic properties, which can further prove that the obtained paper had excellent friction resistance (Movie S3).
Another application of superhydrophobic surfaces is in the separation of oil and water mixtures. The modified paper was superhydrophobic and superoleophilic, which was suitable for the separation of oil and water mixtures. Figure 8a and Movie S4 show the oil/water separation behavior of the obtained paper. When the oil–water mixture (60 mL, chloroform/water, 1/1, v/v) was poured into the separation column, the chloroform was rapidly permeated through the paper and dropped into the beaker below, while the water was intercepted and retained above the funnel. Moreover, the paper after oil–water separation still maintained excellent superhydrophobic property, which implied that the paper showed durable superhydrophobicity even under oil immersion (Fig. 8b, Movie S5).
Conclusion
In summary, a simple and eco-friendly strategy to develop durable superhydrophobic paper based on TiO2-functionalized cellulose fiber as building block was demonstrated. The surface morphology of the TiO2-functionalized cellulose fiber could be well controllable by adjusting the amount of HAc used. After papermaking and HDTMS modification, the obtained paper showed excellent superhydrophobicity with WCA of 152.3° ± 1.3°. It also showed excellent self-cleaning, antifouling and oil/water separation ability. Moreover, the paper also presented durable water-resistant property against finger-wiping and sandpaper-abrasion tests for over 50 times. The whole process was with eco-friendly and low-cost raw materials, simple operational procedure and mild reaction conditions, which provide new opportunities to develop novel superhydrophobic cellulose-based functional materials.
References
Ghasemi S, Tajvidi M, Gardner DJ, Bousfield DW, Shaler SM (2018) Effect of wettability and surface free energy of collection substrates on the structure and morphology of dry-spun cellulose nanofibril filaments. Cellulose 25:6305–6317
Nechyporchuk O, Yu J, Nierstrasz VA, Bordes R (2017) Cellulose nanofibril-based coatings of woven cotton fabrics for improved inkjet printing with a potential in e-textile manufacturing. ACS Sustain Chem Eng 5:4793–4801
Tarrés Q, Oliver-Ortega H, Ferreira PJ, Pèlach MÀ, Mutjé P, Delgado-Aguilar M (2018) Towards a new generation of functional fiber-based packaging: cellulose nanofibers for improved barrier, mechanical and surface properties. Cellulose 25:683–695
Hirotaka K, Masaya N, Akira I (2017) Ionic liquid mediated dispersion and support of functional molecules on cellulose fibers for stimuli-responsive chromic paper devices. ACS Appl Mater Interfaces 9:40914–40920
Ngo YH, Li D, Simon GP, Garnier G (2013) Formation of polyelectrolyte-gold nanoparticle necklaces on paper. J Colloid Interface Sci 405:71–77
Wan C, Jiao Y, Li J (2017) A cellulose fibers-supported hierarchical forest-like cuprous oxide/copper array architecture as a flexible and free-standing electrode for symmetric supercapacitors. J Mater Chem A 5:17267–17278
Duan C, Meng J, Wang X, Meng X, Sun X, Xu Y, Zhao W, Ni Y (2018) Synthesis of novel cellulose-based antibacterial composites of Ag nanoparticles@metal-organic frameworks@carboxymethylated fibers. Carbohydr Polym 193:82–88
Kong X, Zhang J, Xuan Q, Lu J, Feng J (2018) Superhydrophobic coating for antifouling of chinese paintings. Langmuir 34:8294–8301
Zhang JC, Chen FZ, Lu Y et al (2020) Superhydrophilic-superhydrophobic patterned surfaces on glass substrate for water harvesting. J Mater Sci 55:498–508. https://doi.org/10.1007/s10853-019-04046-x
Su X, Li H, Lai X, Zhang L, Liang T, Feng Y, Zeng X (2017) Polydimethylsiloxane-based superhydrophobic surfaces on steel substrate: fabrication, reversibly extreme wettability and oil–water separation. ACS Appl Mater Interfaces 9:3131–3141
Gao A, Yan Y, Li T, Liu F (2019) Biomimetic urchin-like surface based on poly (lactic acid) membrane for robust anti-wetting and anti-bacteria properties. Mater Lett 237:240–244
Guo DY, Hou K, Xu SP, Lin YG, Li L, Wen XF, Pi PH (2018) Superhydrophobic-superoleophilic stainless steel meshes by spray-coating of a POSS hybrid acrylic polymer for oil–water separation. J Mater Sci 53:6403–6413. https://doi.org/10.1007/s10853-017-1542-3
Yin K, Du H, Dong X, Wang C, Duan J, He J (2017) A simple way to achieve bioinspired hybrid wettability surface with micro/nanopatterns for efficient fog collection. Nanoscale 9:14620–14626
Yin K, Yang S, Dong X, Chu D, Duan J, He J (2018) Robust laser-structured asymmetrical PTFE mesh for underwater directional transportation and continuous collection of gas bubbles. Appl Phys Lett 112:243701–243705
Yu M, Lin B, Chen S, Deng Q, Liu G, Wang Q (2018) Biomimetic fabrication of superhydrophobic loofah sponge: robust for highly efficient oil–water separation in harsh environments. RSC Adv 8:24297–24304
Seeharaj P, Pasupong P, Detsri E, Damrongsak P (2018) Superhydrophobilization of SiO2 surface with two alkylsilanes for an application in oil/water separation. J Mater Sci 53:4828–4839. https://doi.org/10.1007/s10853-017-1925-5
Cheng Q, Ye D, Chang C, Zhang L (2017) Facile fabrication of superhydrophilic membranes consisted of fibrous tunicate cellulose nanocrystals for highly efficient oil/water separation. J Membr Sci 525:1–8
Wang Y, Li X, Hu H, Liu G, Rabnawaz M (2014) Hydrophilically patterned superhydrophobic cotton fabrics and their use in ink printing. J Mater Chem A 2:8094–8102
Pegah K, Alistair WTK, Gabriel JP, Leena-Sisko J, Mauri AK, Robin HAR (2018) Superhydrophobic paper from nanostructured fluorinated cellulose esters. ACS Appl Mater Interfaces 10:11280–11288
Peng L, Meng Y, Li H (2018) Facile fabrication of superhydrophobic paper with improved physical strength by a novel layer-by-layer assembly of polyelectrolytes and lignosulfonates-amine. Cellulose 23:2073–2085
Yue X, Zhang T, Yang D, Qiu F, Li Z (2018) Janus ZnO-cellulose/MnO2 hybrid membranes with asymmetric wettability for highly-efficient emulsion separations. Cellulose 25:5951–5965
Yuan Z, Wen Y (2018) Enhancement of hydrophobicity of nanofibrillated cellulose through grafting of alkyl ketene dimer. Cellulose 25:6863–6871
Avijit B, Mohd AG, Swathy JR, Kam CT, Sarit KD, Robin HAR, Thalappil P (2017) Organic solvent-free fabrication of durable and multifunctional superhydrophobic paper from waterborne fluorinated cellulose nanofiber building blocks. ACS Nano 11:11091–11099
Satapathy M, Varshney P, Nanda D, Panda A (2017) Fabrication of superhydrophobic and superoleophilic polymer composite coatings on cellulosic filter paper for oil–water separation. Cellulose 24:4405–4418
Liu S, Zhou H, Wang H et al (2017) Argon plasma treatment of fluorine-free silane coatings: a facile, environment-friendly method to prepare durable, superhydrophobic fabrics. Adv Mater Interfaces 4:1700027–1700035
Yang R, Zhu Y, Chen F, Dong L, Xiong Z (2017) Luminescent, fire-resistant, and water-proof ultralong hydroxyapatite nanowire-based paper for multimode anticounterfeiting applications. ACS Appl Mater Interfaces 9:25455–25464
Chen X, Kuo D, Lu D (2016) N-doped mesoporous TiO2 nanoparticles synthesized by using biological renewable nanocrystalline cellulose as template for the degradation of pollutants under visible and sun light. Chem Eng J 295:192–200
Miao X, Qu D, Yang D, Nie B, Zhao Y, Fan H, Sun Z (2018) Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv Mater 30:1704740–1704747
Wang Q, Chen G, Yu Z, Ouyang X, Tian J, Yu M (2018) Photoluminescent composites of lanthanide-based nanocrystal-functionalized cellulose fibers for anticounterfeiting applications. ACS Sustain Chem Eng 6:13960–13967
Erdem B, Hunsicker RA, Simmons GW, Sudol ED, Dimonie VL, El-Aasser MS (2001) XPS and FTIR surface characterization of TiO2 particles used in polymer encapsulation. Langmuir 17:2664–2669
Li W, Li L, Wu X et al (2018) High infrared blocking cellulose film based on amorphous to anatase transition of TiO2 via atomic layer deposition. ACS Appl Mater Inter 10:21056–21060
Chen B, Qiu J, Sakai E, Kanazawa N, Liang R, Feng H (2016) Robust and superhydrophobic surface modification by a “Paint + Adhesive” method: applications in self-cleaning after oil contamination and oil–water separation. ACS Appl Mater Interfaces 8:17659–17667
Yu J, Su Y, Cheng B, Zhou M (2006) Effects of pH on the microstructures and photocatalytic activity of mesoporous nanocrystalline titania powders prepared via hydrothermal method. J Mol Catal A Chem 258:104–112
Yu J, Zhao X, Zhao Q (2000) Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid Films 379:7–14
Li X, Chen X, Liu S, Chen X, Wang H, Liu Z (2007) Preparation and characterization of nanosize TiO2 photocatalyst obtained from acid catalyzed hydrolysis method. Chin J Appl Chem 24:1279–1283
Lu Y, Sun Q, Liu T, Yang D, Liu Y, Li J (2013) Fabrication, characterization and photocatalytic properties of millimeter-long TiO2 fiber with nanostructures using cellulose fiber as a template. J Alloys Compd 577:569–574
Zhang Q, Gao L, Guo J (2000) Preparation and spectral characterization of quantum-size titanium dioxide in the rutile phase. Chin J Inorg Mater 15:929–934
Wang Q, Yu M, Chen G, Chen Q, Tian J (2017) Facile fabrication of superhydrophobic/superoleophilic cotton for highly efficient oil/water separation. BioResources 12:643–654
Wang Q, Yu M, Chen G, Chen Q, Tian J (2017) Robust fabrication of fluorine-free superhydrophobic steel mesh for efficient oil/water separation. J Mater Sci 52:2549–2559. https://doi.org/10.1007/s10853-016-0548-6
Yu M, Wang Q, Zhang M, Deng Q, Chen D (2017) Facile fabrication of raspberry-like composite microspheres for the construction of superhydrophobic films and applications in highly efficient oil–water separation. RSC Adv 7:39471–39479
Hayaka F, Tsuguyuki S, Tadahisa I, Yoshiaki K, Akira I (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromol 10:162–165
Huang Z, Gurney R, Wang T, Liu D (2018) Environmentally durable superhydrophobic surfaces with robust photocatalytic self-cleaning and self-healing properties prepared via versatile film deposition methods. J Colloid Interface Sci 527:107–116
Wang Q, Chen G, Tian J, Yu Z, Deng Q, Yu M (2018) Facile fabrication of fluorine-free, transparent and self-cleaning superhydrophobic coatings based on biopolymer castor oil. Mater Lett 230:84–87
Funding
This study was funded by GDAS’ Project of Science and Technology Development (2020GDASYL-20200102012), the Special Project of Innovation Capacity Development of Guangdong Academy of Sciences (2018GDASCX-0105), High-Level Talent Start-Up Research Project of Foshan University (gg040945) and the Key Project of Department of Education of Guangdong Province (2016GCZX008), which are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 5999 kb)
Supplementary file2 (AVI 6902 kb)
Supplementary file3 (MP4 23605 kb)
Supplementary file4 (AVI 15855 kb)
Supplementary file5 (AVI 6118 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Xie, D., Chen, J. et al. Superhydrophobic paper fabricated via nanostructured titanium dioxide-functionalized wood cellulose fibers. J Mater Sci 55, 7084–7094 (2020). https://doi.org/10.1007/s10853-020-04489-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04489-7