Abstract
The development of hierarchical micro-/nanostructures has received great attention in the field of electrochemical energy storage and conversion. In this report, we demonstrate a template-free synthesis of LiV3O8 hollow microspheres from vanadium ethylene glycolate intermediate, which in turn was obtained by a facile solvothermal route. Evaluation of morphological features with FE-SEM and HR-TEM shows hollow spheres of size 1.8 ± 0.1 µm diameters with wall thickness and void size of 200 ± 20 nm and 1.5 ± 0.1 µm, respectively. Powder X-ray diffraction confirms that both the intermediate and LiV3O8 hollow spheres are highly crystalline without any impurity phase. Tested as a positive electrode in lithium-ion batteries, the LiV3O8 hollow spheres constructed electrode shows a discharge capacity of 304 mAh g−1 at 0.1 C rate and 246 mAh g−1 at 1 C rate during the first discharge. More importantly, LiV3O8 electrode delivers stable cycle-life performance with a specific capacity of 156 mAh g−1 after 300 (dis)charges at 1 C rate. The present hollow LiV3O8 structures would be expected to offer additional active sites, void space to counter volume expansion, and thin-shell structures to provide shortest path for Li+ ion/electron transport, thereby enhancing the cycling stability and rate performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vanadium-based layered intercalation compounds have appealing redox properties as cathode in lithium-ion batteries (LIBs), because of their high theoretical capacity due to accommodation of more than one Li per formula unit [1,2,3]. Among the vanadium oxides, lithium trivanadate LiV3O8 has garnered great interest as positive electrode material because of its layered structure which could intercalate more than 3 Li per formula unit [4,5,6]. In the past decades, LiV3O8 has been extensively studied because of its capability to achieve high specific capacity, safety, and power capability. The crystal structure of LiV3O8 mainly consists of VO6 octahedral- and VO5 distorted trigonal bipyramidal-structural units, where the V3O8− layered structures are linked together by inter-layered lithium ions [7]. This unique structure leads to an overall accommodation of up to 3 Li per formula unit, thereby delivering high specific capacity in LIBs [8]. Despite its high specific capacity, huge volume change of the electrode materials during (de)lithiation processes causes capacity fading even at low current rates. Furthermore, the slow intrinsic Li+ diffusion kinetic between the LiV3O8 layers as well as within the particles could impede the rate performance. In order to circumvent these issues, particle size reduction strategy was explored in the past to enhance the life-cycling as well as rate performances [9,10,11,12,13]. In addition, morphologically different nanostructures such as nanorods, nanobelts, nanowires, and nanosheets were also scrutinized to provide facile Li-diffusion pathway for ions/electrons transport and improve the electrode/electrolyte interface vis-à-vis overall electrochemical performance [14,15,16,17,18].
In the past decades, intensive research efforts have been made to fabricate hollow micro-/nanostructures with 10 nm–10 µm range, emphasizing their applications in the field of energy conversion and storage [19, 20]. In particular, hollow spheres are believed to mitigate the problem of capacity decay and rate performance by providing significantly reduced paths for de(insertion). As a result of significantly mitigated electrode pulverization and polarization, better performance is anticipated for hollow sphere constructed electrodes [19, 20]. The microstructure of these hollow spheres would be expected to offer extra active sites, void space to counter volume expansion, thin-shell structures to provide shortest path for Li+ ions and electrons movement, thereby enhancing the cycling stability and rate performance. However, fabrication of V-based hollow micro-/nanospheres was scarcely reported. In this context, V2O5 hollow micro-/nanostructures were fabricated by using several soft template approaches [21,22,23,24]. We have reported V2O5 hollow nanospheres with ~ 28 nm using poly(styrene-b-2-vinyl-methylpyridiniumiodide-b-ethylene oxide)-based micelles with core–shell-corona architecture as soft template, and it showed high-rate capability as well as cycling stability as a positive electrode in LIBs [25]. Later on, Liu et al. reported V2O5 yolk-shell structure using a template-free method using N,N-dimethylformamide as solvent [26]. Afterward, Zhou et al. [27] synthesized LiV3O8 hollow spheres by solid-phase oxidation and lithiation using V2O3 as precursors. In this context, further advancement of template-free, simple method for fabrication of LiV3O8 with hierarchical structures would be highly desirable, but quite challenging.
Recently, David Lou group have used solvothermal method for synthesis of V2O5 hollow spheres starting from vanadium intermediate compound and explained the formation mechanism of hollow spheres by Oswald repining [22,23,24]. Herein, we demonstrate nanorods-assembled LiV3O8 hollow microspheres by solvothermal method using ethylene glycol as a solvent. The in situ formed vanadium ethylene glycolate hollow sphere intermediate serves as a template for further synthesis of LiV3O8 hollow microspheres (LiV3O8-HS) on treatment with LiNO3. The crystal structure and morphology of synthesized LiV3O8-HS were analyzed by XRD, FE-SEM, and HR-TEM techniques. The LiV3O8-HS was further investigated as positive electrode material for LIBs, which shows high lithium storage capacity of 304 mAh g−1 at 0.1 C rate.
Experimental section
Synthesis of LiV3O8 hollow spheres
In a typical synthesis, an aqueous vanadium oxalate solution that acts as a vanadium source was prepared by dissolving vanadium oxide and oxalic acid in 1:3 ratio under continuous stirring at 80 °C for 12 h [23]. Then, 3 mL of vanadium oxalate solution thus obtained was added to a 30 mL of ethylene glycol under stirring and the content was stirred for another 30 min. The solution was then transferred to a 50 mL Teflon-lined stainless steel autoclave and subjected to solvothermal treatment at 200 °C for 12 h. After completion of the reaction, the precipitate was collected by centrifugation, washed with ethanol and water, and dried in an oven at 80 °C for 12 h. Further lithiation of vanadium ethylene glycolate (VEG) intermediate product was carried out by dispersing the VEG precipitate in ethanolic LiNO3. Then, the mixture was stirred for 30 min at 60 °C till the solvent gets evaporated. Finally, the resultant solid powder was annealed at 500 °C for 6 h with heating rate of 3 °C/min to get LiV3O8 hollow spheres (LiV3O8-HS) as depicted in Scheme 1.
Characterization of LiV3O8 hollow spheres
The phase purity and crystallinity of vanadium ethylene glycolate (VEG) obtained by solvothermal method and LiV3O8-HS by subsequent annealing step were confirmed by powder X-ray diffraction technique using Bruker (D8 Advance, Da Vinci) analytical instruments with Cu-kα radiation source (1.54 Å). The XRD measurements were conducted with 2θ angle ranging from 10° to 80° at a scan speed of 3° min−1 with 0.04 step size. FT-IR spectroscopy (IRTracer-100) was employed to identify functional groups. The TGA analysis was performed using STA 2500 Regulus-Netzsch instrument at oxygen atmosphere with temperature range from 25 to 600 °C (10˚/min). The morphological evolution of VEG and LiV3O8-HS was observed with field-emission scanning electron microscopy (FE-SEM) operating at 10 kV using ZESS electron microscopy. High-resolution transmission electron microscopy (HR-TEM) technique was used to confirm the fine structures using a JEOL JEM-2010 instrument operated at 200 kV. The N2 adsorption/desorption isotherms and specific surface area of the synthesized LiV3O8-HS were analyzed using Quntachrome instrument. X-ray photoelectron spectroscopy (XPS) was recorded to identify the oxidation states of the LiV3O8 using a 1032 instrument with Al-alpha as the X-ray source.
Electrochemical characterization of LiV3O8 hollow spheres
Electrochemical properties of as-synthesized LiV3O8-HS were evaluated using CR-2032 coin-type cells, which were assembled inside an argon-filled glove box, maintained at less than 0.5 ppm of oxygen and moisture level. The working electrode was made by intimately mixing the active material, SP carbon, and PVDF binder in the ratio 80:15:5 with material loading of 4.5 mg/cm2. The electrode slurry was prepared using N-methyl pyrrolidine (NMP) as a solvent. Then, the slurry was uniformly coated on Al-foil using doctor blade coater and dried in a vacuum oven maintained at 120 °C for 12 h. The coated electrode was cut into circular disk with 15 mm diameter and was used as working electrode in coin cells. Glass microfiber (Whatman 47 mm) choked with few drops of electrolyte was used as separator, and Li metal was used as reference electrode. The cell components were stacked and sealed inside the glove box. The electrolyte used was 1.0 M LiPF6 dissolved in ethylene carbonate and dimethylene carbonate in the ratio 1:1 (EC/DMC). Electrochemical techniques like cyclic voltammetry, galvanostatic charge/discharges, and electrochemical impedance techniques were employed using Biologic instrument BCS 810 series battery cycler at various current densities in the potential window of 2.0–4.0 V. Cyclic voltammetry study was carried out at 0.2 mV s−1 scan rate in the voltage window of 2.0–4.0 V. Galvanostatic impedance was recorded in the frequency range of 100 kHz–10 mHz at 10 mA amplitude.
Results and discussion
Material characterization
Upon treatment of aqueous vanadium oxalate solution with ethylene glycol under solvothermal conditions, ligand exchange between oxalate and glycolate anions readily occurs to provide vanadium ethylene glycolate (VEG) as shown in Scheme 1. The ethylene glycol (EG) serves as ligand as well as reducing agent under solvothermal conditions and has a tendency to coordinate with the central Vn+ ion to form VEG intermediate. The bands appearing at 1598, 1405–1465, and 890 cm−1 in the FT-IR spectrum are assigned to C–H bending vibrations of –CH2 groups, whereas the bands at 992 and 539 cm−1 are attributed to V=O and V–O stretching vibrations, respectively (Fig. 1a) [12, 13]. Furthermore, the VEG composite particles exhibit two-step weight loss behavior in thermo-gravimetric profile (TG, Fig. 2) with increasing temperature from 25 to 600 °C. The weight loss below 150 °C is attributed to adsorbed water (12%), whereas the second weight loss occurring between 150 and 320 °C corresponds to the decomposition of organic compounds and the amount of organics was found to be 23% (Fig. 2). These results clearly indicate that the VEG intermediate contains both organic and inorganic components. Subsequent treatment of VEG intermediate with LiNO3·xH2O (1:3) by solid-state mixing and calcination at 500 °C led to the formation of desired LiV3O8 hollow spheres (Scheme 1). However, further increasing temperature from 400 to 600 °C in the TG analysis (Fig. 2), no significant weight loss was observed, indicating the complete removal of template to produce hollow spheres (LiV3O8-HS). After calcinations (Fig. 1b), the retention of adsorption peaks at 950, 745 and 550 cm−1 corresponding to V=O stretching vibration, symmetric and asymmetric stretching vibrations of V–O–V linkage confirms the presence of vanadium compound [12].
The crystalline nature and purity of vanadium ethylene glycolate intermediate as well as LiV3O8 hollow spheres were examined by XRD (Fig. 3). The diffraction pattern of VEG intermediate (Fig. 3a) matches well with the literature report (JCPDS card data no. 49-2797). Furthermore, after treatment with LiNO3 the reflections at 13.98, 15.2, and 28.3° corresponding to (200), (202), and (− 111) planes of LiV3O8 are clearly seen (Fig. 3b, JCPDS No -72-1193). All observed reflections of LiV3O8 can be indexed to a layered monoclinic crystal structure with P21/m space group with lattice parameters of a = 6.6, b = 3.3, and c = 12.1. The diffraction peaks located at 2θ = 14° and 28.3° correspond to the (100) and (− 111) planes which are presumed to be electrochemically active planes of LiV3O8. Furthermore, the low intensity of (100) reflection compared to (− 111) peak suggests that the present material would be expected to favor better electrochemical performance by providing a short Li-ion diffusion pathway [13].
To get further insights into the morphology and detailed surface structure of VEG intermediate and LiV3O8 particles, field-emission scanning electron microscope (FE-SEM) observation was made as shown in Fig. 4a, b. The low- and high-magnified FE-SEM images of VEG intermediate clearly show the spherical morphology, and the estimated particle size was found to be 1.8 ± 0.1 µm. The formation mechanism of hollow spheres was reported to be driven by Ostwald ripening, where the smaller particles get dissolved in the growth medium and further re-deposited on the larger particle’s surface due to difference in surface-free energy between the interior and exterior particles. Therefore, the inner particles of the sphere having higher surface energy try to move to the exterior surface in order to reduce their surface energy, thereby forming hollow structures [23]. Indeed, the formation of hollow structured VEG intermediate under solvothermal conditions is clearly evidenced in Fig. 4b. Interestingly, subsequent conversion of VEG to LiV3O8-HS upon treatment with LiNO3 at high-temperature, the spherical morphology is well retained and the size of the hollow spheres was approximately 1.1 ± 0.1 µm (Fig. 4c, d). Furthermore, the particle surface is constructed by self-assembly of small nanorods with size of about 40–60 nm (Fig. 4c, d) and the hollow particles also exhibited some degree of aggregation presumably due to high-temperature calcinations. In addition, comparison of particle size difference between VEG and LiV3O8-HS suggests that the size of the latter shrunk significantly to about 60% after calcinations and this kind of shrinkage during the calcinations has also been reported for other metal oxides [20]. Thus, the nanorods constructed hierarchical LiV3O8-HS with high inter-particles contact could provide a better Li-ion diffusivity and improved electrical conductivity.
The presence of void space in the VEG intermediate as well as LiV3O8-HS was further visualized with high-resolution transmission electron microscope (HR-TEM) as shown in Fig. 5. For VEG intermediate (Fig. 5a, b), the light contrast in the electron micrograph confirms the void space and the dark contrast indicates the shell domain of the hollow spheres. High-magnified image of VEG (Fig. 5b) suggests that the surface is covered by needle-like rods and the particle sizes are found to be about 1.8 ± 0.1 nm corroborating the FE-SEM results. Figure 5c, d shows void space in the LiV3O8-HS, and the surface of LiV3O8-HS nanoparticles is rather agglomerated probably due to re-crystallization that is happening during high-temperature sintering. Next, textural properties such as porosity, pore size, and BET surface area information of LiV3O8-HS were obtained from N2 sorption isotherms as shown Fig. 6a, b. The N2 adsorption/desorption isotherms show a histogram which is not characteristics of Type IV isotherms (Fig. 6a), and the Brunner–Emmet–Teller (BET) surface area was found to be 31.9 m2/g. The pore volume estimated was found to be 0.07 mL/g from nitrogen desorption curves at p/p0 = 0.99. The LiV3O8-HS material has both micro- as well as mesopores in the range of about 2–7 nm in diameter (Fig. 6b). Apparently, the presence of modest surface area, and hierarchical pore size features would be expected to enhance the electrochemical behavior of hollow sphere constructed LiV3O8 electrode. The XPS spectrum confirms the existence of different elements and its valence in the LiV3O8-HS (Fig. 7). The survey spectrum (Fig. 7a) shows the presence of vanadium (V) and oxygen (O) in the LiV3O8-HS. The vanadium peak (Fig. 7b) has been deconvuluted into two peaks, where the high intense peak at 516.3 eV and low intense peak at 523.8 eV corresponding to V 2p3/2 and V 2p1/2 confirm the + 5 oxidation state of vanadium. Furthermore, the peak position at 530 eV is associated with O 1s (Fig. 7c) of LiV3O8-HS.
Electrochemical studies
To evaluate the electrochemical performance of LiV3O8 hollow sphere constructed as positive electrode in lithium-ion batteries, we used cyclic voltammetry (CV) and galvanostatic (dis)charge (GCPL) techniques. The CV traces were acquired at a scan rate of 0.2 mV/s in the potential window of 2.0–4.0 V (vs. Li/Li+) as displayed in Fig. 8a, and for clarity, only 1st and 5th cycles are provided. The Li (de)insertion processes are very obvious from CV curves. During the cathodic run, three major reduction peaks at 2.8 V, 2.54 V, and 3.50 V (weak) are noted. The peaks at 2.78 and 3.50 V (weak) are noted due to Li+ ion occupancy in the empty tetrahedral sites during single-phase reaction and two-phase transformation from Li3V3O8 to Li4V3O8, respectively [28, 29]. The minor peak at 2.3 V in the cathodic sweep is related to the slower insertion kinetic step where the single-phase transition corresponding to the Li4V3O8 takes place. The corresponding anodic sweeps exhibit peaks at 2.5 and 2.8 V due to the Li-ion extraction from the tetrahedral and octahedral sites of host LiV3O8 structure. The CV curves of 1st and 5th cycles of LiV3O8-HS electrode match very well with negligible changes in the current produced, and these results indeed indicate good redox properties as well as high reversibility upon (de)intercalation.
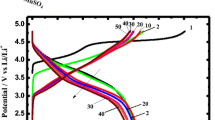
To get more insight into the Li-intercalation properties, the CV features are further correlated with galvanostatic (dis)charge behavior of LiV3O8-HS performed at 0.1 C in the potential range 2.0–4.0 V. For clarity, only selected cycles such as 1st, 10th, and 50th cycles are shown in Fig. 8b. The profiles showed plateaus at 3.5 (weak), 2.8, 2.6, and 2.3 V during the discharge, whereas peaks at 2.3, 2.7, and 3.7 V (weak) during charging were noted, which are in good agreement with the CV results discussed above. The LiV3O8-HS delivered an initial discharge capacity of 304 mAh g−1 at 0.1 C rate and delivered 235 mAh g−1 after 50th cycles, and the decrease in capacity is mainly ascribed to electrolyte decomposition to form interface film (SEI) similar to other electrodes and poor electrical conductivity of LiV3O8-HS. The observed capacity of LiV3O8-HS has also been compared with previously reported nanostructured materials as shown in Table S1.
To understand further, dQ/dV analysis of LiV3O8-HS was considered since it is associated with capacity fading of an electrode (Fig. 8c). It is seen from Fig. 8c that three peaks were appeared at 2.80, 2.71, and 2.57 V in the cathodic as well as anodic sweeps (2.63, 2.65, and 2.84 V). The dQ/dV plots can be used to assess structural stability and reversibility of electrode vis-à-vis capacity fading. For the initial 10 cycles, the dQ/dV curves overlap with each other, indicating a good structural stability and reversibility of LiV3O8-HS constructed electrode. However, after 50 cycles, the peak potentials were shifting gradually owing to polarization of electrode and electrolyte decomposition [30]. The appearance of new electrochemical peak at lower potential ~ 2.2 V at cathodic process and 3.3 V for anodic sweep is indicative of capacity fading of LiV3O8-HS [31]. Rate capability of as-synthesized LiV3O8-HS has also been evaluated by applying different currents, and the obtained capacity values are displayed in Fig. 8d. The LiV3O8-HS delivered discharge capacity of 304 mAh g−1 at 0.1 C, which upon further increasing the current densities to 0.5 C, 1 C, 3 C and 5 C delivered 253, 236, 192, and 168 mAh g−1, respectively. Finally, when the cell was subjected to lower current of 0.1 C again, the electrode regained its original capacity, thus demonstrating its good reversibility.
The cycling and structural stability of LiV3O8-HS have been further evaluated by cycling at 1 C rate up to 300 cycles, which are displayed in Fig. 9. The LiV3O8-HS electrode delivered a maximum capacity of 246 mAh g−1 at 1 C rate and maintained the capacity value of 156 mAh g−1 even after 300 cycles. There is no much capacity fading owing to hollow spherical morphology of LiV3O8, which helps to control the pulverization of particles during cycling process. The void space present in the hollow spheres would also be expected to accommodate the mechanical strain during repeated (dis)charges in addition to providing short-diffusion pathway for Li+ ion diffusion through thin-shell domain.
To understand conductivity behavior of LiV3O8 hollow spheres, electrochemical impedance spectroscopy (EIS) was acquired. The EIS analysis was performed in the frequency ranges of 100 kHz–10 mHz for both fresh and cycled cells (50 cycles at 0.1 C rate), which are shown in Fig. 10a. The characteristic high-frequency semicircle and low-frequency Warburg region (Zw) correspond to charge transfer resistance (Rct) and lithium-ion diffusion within the electrode materials, respectively. Furthermore, the charge transfer resistance of fresh electrode has comparatively lower Rct value than the cycled electrode owing to the decrease in the electrical conductivity vis-à-vis capacity fading upon prolonged cycling. Additionally, the Li+ diffusion coefficient (DLi+) was also calculated from Eqs. (1) and (2):
where DLi is the Li-ion diffusion coefficient, R is the gas constant, T is the absolute temperature, A is the surface area of the electrode, n is the number of electron transferred, F is the Faraday constant, C is the concentration of Li+, and σ is the Warburg factor which can be related to Z. Figure 10b displays linear fitted equation plots between real part of Warburg impedance against ω−1/2 curve for fresh and cycled LiV3O8-HS electrodes. The calculated Li-ion diffusion coefficients were 5.53 × 10−9 cm2 s−1 for fresh and 5.52 × 10−8 cm2 s−1 cycled electrodes. The fresh electrode showed a higher Li-ion diffusion coefficient value compared to cycled electrode (for 50 cycles), indicating capacity fading upon cycling.
The EIS spectrum of LiV3O8-HS at different states of charge, i.e., before cycling and after first discharge/charge, was evaluated which is shown in Fig. S2. The impedance spectrum at initial state indicates two semicircles at high-frequency and high-to-medium frequency region corresponding to charge transfer resistance at electrode surface and solid/electrolyte interface (SEI). After first discharge, the EIS spectrum shows the increased charge transfer resistance and the Warburg line is inclined toward the real axis, indicating surface passivation of active materials and current collector [6]. However, after charging to 4 V, the charge transfer resistance decreased and Warburg line was inclined toward imaginary axis owing to surface activation process. Furthermore, the decreased capacity of LiV3O8-HS with prolonged cycling is ascribed to the decreased electrical conductivity.
Conclusions
The LiV3O8 hollow spheres have been synthesized successfully by annealing the vanadium ethylene glycolate synthesized under solvothermal conditions as precursor in presence of Li-source. The XRD, FE-SEM, and HR-TEM results of LiV3O8-HS confirmed the monoclinic crystal structure having hollow spherical morphology with average particle size of about 1.8 ± 0.1 µm. Tested as positive electrode materials for lithium-ion batteries, the LiV3O8-HS showed very good electrochemical performance with specific discharge capacity of 304 mAh g−1 at 0.1 C and 246 mAh g−1 at 1 C rate during the first cycle. The enhanced electrochemical performance is attributed to the thin-shell structure, fast lithium-ion diffusivity, and accommodation of strain by the hollow void space during repeated charge and discharges.
References
Braithwaite JS, Catlow CRA, Gale JD, Harding JH (1999) Lithium intercalation into vanadium pentoxide: a theoretical study. Chem Mater 11:1990–1998. https://doi.org/10.1021/cm980735r
Chae OB, Kim J, Park I, Jeong H, Ku JH, Ryu JH, Kang K, Oh SM (2014) Reversible lithium storage at highly populated vacant sites in an amorphous vanadium pentoxide electrode. Chem Mater 26:5874–5881. https://doi.org/10.1021/cm502268u
Liu M, Su B, Tang Y, Jiang X, Yu A (2017) Recent advances in nanostructured vanadium oxides and composites for energy conversion. Adv Energy Mater 7:1700885. https://doi.org/10.1002/aenm.201700885
Qiao YQ, Tu JP, Wang XL, Zhang J, Yu YX, Gu CD (2011) Self-assembled synthesis of hierarchical waferlike porous Li–V–O composites as cathode materials for lithium ion batteries. J Phys Chem C 115:25508–25518. https://doi.org/10.1021/jp2080176
West K, Zachau-Christiansen B, Skaarup S, Said Y, Barker J, Olsen II, Pynenburg R, Koksbang R (1996) Comparison of LiV3O8 cathode materials prepared by different methods. J Electrochem Soc 143:820. https://doi.org/10.1149/1.1836543
Sarkar S, Banda H, Mitra S (2013) High capacity lithium-ion battery cathode using LiV3O8 nanorods. Electrochim Acta 99:242–252. https://doi.org/10.1016/j.electacta.2013.03.083
Wadsley AD (1957) Crystal chemistry of non-stoichiometric pentavalent vanadium oxides: crystal structure of Li1+xV3O8. Acta Crystallogr 10:261–267. https://doi.org/10.1107/S0365110X57000821
Pan A, Liu J, Zhang J-G, Cao G, Xu W, Nie Z, Jie X, Choi D, Arey BW, Wang C, Liang S (2011) Template free synthesis of LiV 3 O 8 nanorods as a cathode material for high-rate secondary lithium batteries. J Mater Chem 21:1153–1161. https://doi.org/10.1039/C0JM02810J
Xu HY, Wang H, Song ZQ, Wang YW, Yan H, Yoshimura M (2004) Novel chemical method for synthesis of LiV3O8 nanorods as cathode materials for lithium ion batteries. Electrochim Acta 49:349–353. https://doi.org/10.1016/j.electacta.2003.08.017
Chen Z, Xu F, Cao S, Li Z, Yang H, Ai X, Cao Y (2017) High rate, long lifespan LiV3 O8 nanorods as a cathode material for lithium-ion batteries. Small 13:1603148. https://doi.org/10.1002/smll.201603148
Liu H, Wang Y, Wang K, Wang K, Zhou H (2009) Synthesis and electrochemical properties of single-crystalline LiV3O8 nanorods as cathode materials for rechargeable lithium batteries. J Power Sources 192:668–673. https://doi.org/10.1016/j.jpowsour.2009.03.066
Huang S, Wang XL, Lu Y, Jian XM, Zhao XY, Tang H, Cai JB, Gu CD, Tu JP (2014) Facile synthesis of cookies-shaped LiV3O8 cathode materials with good cycling performance for lithium-ion batteries. J Alloys Compd 584:41–46. https://doi.org/10.1016/j.jallcom.2013.09.034
Wang H, Ren Y, Wang Y, Wang W, Liu S (2012) Synthesis of LiV3O8 nanosheets as a high-rate cathode material for rechargeable lithium batteries. CrystEngComm 14:2831. https://doi.org/10.1039/c2ce06326c
Heli H, Yadegari H, Jabbari A (2011) Investigation of the Lithium Intercalation Behavior of Nanosheets of LiV3 O8 in an Aqueous Solution. J Phys Chem C 115:10889–10897. https://doi.org/10.1021/jp201382n
Tan H, Rui X, Sun W, Yan Q, Lim TM (2015) Vanadium-based nanostructure materials for secondary lithium battery applications. Nanoscale 7:14595–14607. https://doi.org/10.1039/c5nr04126k
Ren W, Zheng Z, Luo Y, Chen W, Niu C, Zhao K, Yan M, Zhang L, Meng J, Mai L (2015) An electrospun hierarchical LiV 3 O 8 nanowire-in-network for high-rate and long-life lithium batteries. J Mater Chem A 3:19850–19856. https://doi.org/10.1039/c5ta04643b
Nair VS, Cheah YL, Madhavi S (2014) Symmetric aqueous rechargeable lithium battery using Na 1.16 V 3 O 8 nanobelts electrodes for safe high volume energy storage applications. J Electrochem Soc 161:A256–A263. https://doi.org/10.1149/2.025403jes
Mo R, Du Y, Zhang N, Rooney D, Sun K (2013) In situ synthesis of LiV3O8 nanorods on graphene as high rate-performance cathode materials for rechargeable lithium batteries. Chem Commun 49:9143–9145. https://doi.org/10.1039/c3cc43975e
Lou XW, Archer LA, Yang Z (2008) Hollow micro-/nanostructures: synthesis and applications. Adv Mater 20:3987–4019. https://doi.org/10.1002/adma.200800854
Sasidharan M, Nakashima K (2014) Core–shell–corona polymeric micelles as a versatile template for synthesis of inorganic hollow nanospheres. Acc Chem Res 47:157–167. https://doi.org/10.1021/ar4001026
Cao A-M, Hu J-S, Liang H-P, Wan L-J (2005) Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries. Angew Chemie Int Ed 44:4391–4395. https://doi.org/10.1002/anie.200500946
Bin WuH, Chen JS, Hng HH, Lou XW (2012) Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 4:2526–2542. https://doi.org/10.1039/c2nr11966h
Pan A, Zhu T, Bin WuH, Lou XW (2013) Template-free synthesis of hierarchical vanadium-glycolate hollow microspheres and their conversion to V2O5 with improved lithium storage capability. Chem A Eur J 19:494–500. https://doi.org/10.1002/chem.201203596
Pan A, Bin WuH, Yu L, Lou XWD (2013) Template-free synthesis of VO2 hollow microspheres with various interiors and their conversion into V2O5 for lithium-ion batteries. Angew Chem Int Ed 52:2226–2230. https://doi.org/10.1002/anie.201209535
Sasidharan M, Gunawardhana N, Yoshio M, Nakashima K (2012) V2O5 hollow nanospheres: a lithium intercalation host with good rate capability and capacity retention. J Electrochem Soc 159:A618–A621. https://doi.org/10.1149/2.082205jes
Liu J, Zhou Y, Wang J, Pan Y, Xue D (2011) Template-free solvothermal synthesis of yolk-shell V 2O 5 microspheres as cathode materials for Li-ion batteries. Chem Commun 47:10380–10382. https://doi.org/10.1039/c1cc13779d
Liu J, Liu W, Wan Y, Ji S, Wang J, Zhou Y (2012) Facile synthesis of layered LiV3O8 hollow nanospheres as superior cathode materials for high-rate Li-ion batteries. RSC Adv 2:10470. https://doi.org/10.1039/c2ra20969a
Thamodaran P, Kesavan T, Vivekanantha M, Senthilkumar B, Barpanda P, Sasidharan M (2019) Operando structural and electrochemical investigation of Li1.5V3O8 nanorods in li-ion batteries. ACS Appl Energy Mater 2:852–859. https://doi.org/10.1021/acsaem.8b01915
Bonino F, Panero S, Pasquali M, Pistoia G (1995) Rechargeable lithium batteries based on Li1 + xV3O8 thin films. J Power Sources 56:193–196. https://doi.org/10.1016/0378-7753(95)80033-D
Tanguy F, Gaubicher J, Guyomard D (2010) Capacity fading on cycling nano size grains of Li1.1V3O8, electrochemical investigation. Electrochim Acta 55:3979–3986. https://doi.org/10.1016/j.electacta.2009.12.038
Acknowledgements
The authors acknowledge the Ministry of New and Renewable Energy (MNRE), Govt. of India, for financial assistance (No. 31/03/2014-15/PVSE-R&D). The authors thank SRM SCIF and Nanotechnology Research Centre, SRMIST, for providing facility for FE-SEM and HR-TEM analysis. T. P. thanks Council of Scientific and Industrial Research (CSIR) for providing senior research fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Partheeban, T., Sasidharan, M. Template-free synthesis of LiV3O8 hollow microspheres as positive electrode for Li-ion batteries. J Mater Sci 55, 2155–2165 (2020). https://doi.org/10.1007/s10853-019-04086-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04086-3