Abstract
Bi2S3, with earth-abundant compositions and a low thermal conductivity, is regarded as a candidate thermoelectric material. In this work, Bi2S3 samples that were doped with x % mol InCl3 were successfully fabricated via a mechanical alloying and spark plasma sintering process. InCl3, as an n-type donor dopant, was added to the Bi2S3 system to improve its electrical transport properties and optimize its thermal conductivity. Upon doping, the electrical conductivity of Bi2S3 doped with 1 mol% InCl3 reaches up to 62 Scm−1, and the Seebeck coefficient maintains a relatively large value of −244 μV K−1 at 673 K, which results in a maximum power factor of 363 μW m−1 K−2. Furthermore, due to a simultaneously reduced thermal conductivity at high temperature, a ZT peak of 0.57 is obtained at 673 K along the parallel to the press direction for the sample doped with 1.0 mol% InCl3, which is almost four times higher than that of pristine Bi2S3 (0.14 at 673 K). The elastic properties and Debye temperature of Bi2S3 are also calculated to analyze the origin of the intrinsically low thermal conductivity and are compared to those of other thermoelectric materials with a low thermal conductivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Given the great increasing demand for clean renewable energy, thermoelectric materials are considered to be an effective method to alleviate the energy crisis [1]. Thermoelectric (TE) materials offer a recipe to provide a green, clean and renewable source of energy by converting waste heat directly into electrical energy [2, 3]. The conversion efficiency of TE materials can be estimated by the figure of merit (ZT), which is defined as ZT = (α2σ/κ)T, where σ is the electric conductivity, α is the Seebeck coefficient, κ is the thermal conductivity and T is the temperature in kelvin. Notably, a high power factor (α2σ) and a low value of κ are required for efficient TE materials. However, the laws of physics affect this situation of ours synchronously. The Wiedemann–Franz law indicates there is a strong coupling between σ and κ, and the Pisarenko relation reveals that α is inversely proportional to the concentration of carriers (n); therefore, it is difficult to optimize these parameters independently. To gain a relative high ZT value, several methods were attempted, such as doping to improve the carrier concentration (n) and carrier mobility (μ) simultaneously [4, 5], nano-structuring engineering and compositing to reduce the lattice thermal conductivity (κL) [6,7,8], and band engineering to modify the Seebeck coefficient (S) and effective mass (m*) [9, 10].
Aside from a high performance, low toxicity and cost also require crucial consideration. It is known that Bi2Te3-based [11] and PbTe-based [12] thermoelectric materials have been investigated for decades owing to the excellent TE properties during a certain temperature range. Because Pb is toxic and Te is lacking in earth’s crust, the rich abundance and environmental benignity of sulfur-based composites have attracted attention as promising alternative TEs [13,14,15,16,17]. Bismuth sulfide (Bi2S3), which has intrinsically low thermal conductivity, has been investigated as an underlying TE material for a long time owing to its potential TE properties. However, similar to most TE materials of low thermal conductivity, such as SnSe [18,19,20], BiCuSeO [5, 21, 22] and Bi2O2Se [23, 24], high electrical resistivity restricts their widespread application. It has been widely demonstrated that more electrons will be generated when halogen group elements (Cl, Br and I) are doped at the S site [13, 25, 26]. Furthermore, plenty of thermoelectric materials obtain a giant leap in performance due to the introduction of elemental indium (In) [27,28,29]. Herein, indium chloride (InCl3) is used as an n-type dopant to optimize the electrical transport properties of Bi2S3.
Mechanical alloying (MA) has been utilized to prepare nanoscale materials due to its unique advantages, such as the production of uniform compositions and short fabrication times. Additionally, spark plasma sintering (SPS) is a suitable hot-sintering process for preparing nanoscale bulk materials due to its heating and cooling processes being achieved in a very short time, making it possible to inhibit the excessive growth of grains during the heat preservation process. In this work, the effect of InCl3 doping on the electrical transport and thermal transport properties of Bi2S3 has been investigated. The elastic properties of Bi2S3 have been also calculated to compare with low thermal conductivity materials to further analyze the origin of low thermal conductivity. The results reveal that the combination of low thermal conductivity and an effectively enhanced power factor, a ZT peak of 0.57, is obtained for Bi2S3 doped with 1.0% mol InCl3 at 673 K.
Experimental section
Synthesis
The Bi2S3 powders nominally doped with x mol% InCl3 (x = 0, 0.25, 0.5, 1.0 and 1.5) were prepared by adding the appropriate proportion of high-purity Bi (99.9%), S (99.9%) and InCl3 (99.99%) starting materials to mechanical alloy in a planetary ball mill (QM-3SP2, Nanjing University, China) at 425 r for 12 h. Then, a stainless steel vessel and balls were used, and the ratio of ball to powder weight was maintained at 20:1, as before [30]. The as-prepared Bi2S3 samples were densified in a Ф15-mm graphite mold under an axial pressure of 40 MPa using a spark plasma sintering (SPS) system (Sumitomo SPS632 lx, Japan) under vacuum at 673 K for 5 min. The detailed sintering process is shown below: Firstly, the pressure rose to 40 MPa during 1 min; next, the temperature was increased from room temperature to 623 K in 4 min, then increased to 673 K in 1 min and held at this temperature for 5 min and subsequently decreased to room temperature.
Characterization
X-ray diffraction (XRD) patterns were recorded on a MiniFlex 600 using Cu Kα1 radiation (40 kV, 15 mA, λ = 1.541 Å, step size = 0.02°, 5°min−1 from 20° to 70°). Field emission scanning electron microscopy (FE-SEM, Zeiss; Sigma 300, Germany) was used to characterize the fractured surface morphologies of all samples. The electronic absorption spectra of pristine and doped Bi2S3 samples were characterized on a Shimadzu UV-3600 plus UV–Vis–NIR spectrophotometer using an integrating sphere accessory. The transverse and longitudinal acoustic velocities were measured using an UMS Advanced Ultrasonic Modulus measurement system (Techlab, France). The bulk sample was cut and polished into disk shape of Φ6 mm × 1 mm for the measurement of the sound speed. Then, the transverse phonon velocity and the longitudinal phonon velocity were measured via transverse probe (V157-RM, 5 MHz) and longitudinal probe (V116-RM, 20 MHz) under the voltage of 50 V and 250 V, respectively.
Thermoelectric performance characterization
The obtained bulk samples were cut and ground into disk shapes of Φ6 mm × 1.5 mm and bars of 2 mm × 2 mm × 10 mm for the measurements of the thermal diffusivity coefficient and electrical properties, respectively. The measurements of the thermal and electrical transport behavior in this work were done along the parallel to the press direction of the SPS. The electrical resistivity and Seebeck coefficient of the bulk samples were measured using a Seebeck coefficient/electrical resistance measuring system (ZEM-3, Ulvac-Riko, Japan) from 323 to 673 K under the protection of a helium atmosphere. The thermal conductivity (κ) was calculated by the correlation κ = DCpρ, where the thermal diffusivity coefficient (D) was measured by the laser flash method (LFA457, Netzsch, Germany), the specific heat (Cp) was measured by thermal analysis (STA449, Netzsch, Germany), and the sample density (ρ) was determined according to the Archimedes method, i.e., \( \rho = \frac{{M_{\text{O}} }}{{M_{2} - M_{1} }}\rho_{\text{h}} , \) where MO is the quality of the dry sample in air, M2 is the quality of the sample in air after fully absorbing water, M1 is the quality of the sample in water after fully absorbing water and ρh is the density of water. Additionally, the porosity was obtained via subtracting the relative density of the actual sample from the relative density of the fully dense bulk sample, namely P = ρ0/ρ0 − ρ/ρ0= 1 − ρ/ρ0, where ρ is the actual density, ρ0 is the theoretical density.
Results and discussion
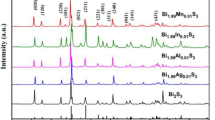
Figure 1a reveals typical powder X-ray diffraction (XRD) patterns of the Bi2S3 + x mol% InCl3 (x = 0, 0.25, 0.5, 1.0, 1.5) samples. All main peaks are well matched with orthorhombic Bi2S3 (PDF#75-1306) in the Pbnm space group. After the spark plasma sintering (SPS) process, all diffraction peaks become narrower and sharp, indicating the random growth of grains (see Fig. 1b). Furthermore, a second phase appears as x > 1.0 and can be indexed as In6S7 (PDF#72-0011). As shown in Fig. 1c, the 2-theta angle of 28.5° for the pristine Bi2S3 bulk sample is slightly lower than the 28.6° that is given by the standard card, and this difference is due to the volatilization of S during the SPS process. The enlarged patterns of the 2-theta range from 28° to 29° for Bi2S3 samples reveal a shift after InCl3 doping. The main peak for the (2 3 0) plane moves to higher angles when x < 1.0 and then shifts back, which implies a lattice deformation of Bi2S3. Considering that the ionic radius of Cl (0.181 nm) is quite close to that of S (0.184 nm), the rightward shift in the diffraction peaks is attributed more to the substitution of In (0.08 nm) at the Bi (0.103 nm) site. When x ≥ 1.0, the expansion of the host lattice is likely due to a portion of In3+ entering the interstitials of the Bi2S3 lattice to increase the lattice parameter. The lattice parameter variations of all InCl3-doped Bi2S3 samples are obtained by refining the bulk sample XRD data. The lattice parameters are found to change along all three axes. The volume of the unit cell first decreases and then increases with the InCl3 doping content, as plotted in Fig. 1d, which is a good match with the shift direction of XRD and with the details we discussed above.
Figure 1e shows the electronic absorption spectra of pristine and doped Bi2S3 powders, and the insert presents the obtained band gap values. It is widely known that Bi2S3 is a direct band gap semiconductor, and the optical band gap can be estimated according to the Kubelka–Munk theory [31, 32]: F(R)*(hν)= (hν − Eg)1/2, where R, F(R), h, ν and Eg are the reflectance, the proportional to the extinction coefficient, Planck’s constant, the frequency of light and the band gap, respectively. After a series of transformations, the pristine Bi2S3 has a band gap value of 1.328 eV, which is quite close to the theoretical value reported before [33]. Upon InCl3 doping, the electronic absorption spectra of Bi2S3 + x mol% InCl3 reveal an initial shift in the absorption edge to a lower energy when x = 0.25 and then moves to higher energies as x > 0.25. The band gap values of all samples are 1.328 eV, 1.309 eV, 1.317 eV, 1.324 eV and 1.332 eV. From the shift in the XRD diffraction and the variation trend of the lattice parameters and band gap values as a function of InCl3 doping content, it is not hard to conclude that the InCl3 is successfully introduced into the Bi2S3 lattice.
The fractographic results for pristine Bi2S3 and all Bi2S3 bulk samples doped with x mol% (x = 0.25, 0.5, 1.0 and 1.5) InCl3 fractured in the direction parallel to the SPS press direction are presented in Fig. 2. The orthorhombic layered structure of pristine Bi2S3 with an average grain size of 400 nm is shown in Fig. 2a. The grain size decreases with increasing x up to 0.5 and then increases when x is greater than 1.0, which is perhaps ascribed to the activation of lattice deformation by introducing InCl3 [34]. Upon InCl3 doping, the grain size variation in the Bi2S3 system agrees well with the changes in lattice parameters. Additionally, compared to those of the pristine Bi2S3 bulk sample, the number of pores increases after doping, which leads to low density and relative density. The densities of pristine Bi2S3 and Bi2S3 doped with x mol% InCl3 (x = 0.25, 0.5, 1.0 and 1.5) are 6.579, 6.372, 6.389, 6.242 and 6.418, respectively, and the relative densities of all samples are greater than 90%, as shown in Fig. 2f. The increased pores also play a significant role in reducing the lattice thermal conductivity, and the details will be discussed later.
The electrical transport properties as a function of the measured temperature for all samples (Bi2S3 + x % mol InCl3, x = 0, 0.25, 0.5, 1.0 and 1.5) are shown in Fig. 3. The electrical conductivities of pristine and InCl3-doped Bi2S3 samples increase with temperature, which indicates characteristic semiconductor behavior. Additionally, even the electrical conductivity value for pristine Bi2S3 increases from 0.14 Scm−1 at 323 K to 5 Scm−1 at 673 K, which remains a long way from that of high-performance thermoelectric materials. The extremely low electrical conductivity originates from the intrinsically low carrier concentration. With the addition of InCl3, the electrical conductivities of all samples show an upward tendency over the measured temperature range. The maximum value of 62 Scm−1 is obtained for the Bi2S3 + 1.0 mol% InCl3 bulk sample at 673 K. Then, the electrical conductivity begins to decrease as the dopant content rises above 1.0 mol%, which is ascribed to carrier scattering caused by defects induced via doping. After InCl3 is added to the host matrix, there are two kinds of defect reactions corresponding to substitutional and interstitial equations, which are expressed as follows:
When x < 1.0, InCl3 substitutes the position of Bi2S3 to form a substitutional solid solution, resulting in the decrease in the lattice parameters. With x ≥ 1.0, a portion of the substitute InCl3 enters the interstitial sites to form an interstitial solid solution, which leads to the increase in parameter. The increased lattice parameter is derived from the expansion of the unit cell volume. The electron concentration is increased when Cl− substitutes S2− and further increased after In3+ enters the host lattice, as revealed in Eqs. (1) and (2). Owing to the high electrical conductivity appearing at high temperature, here, we assume that the electrical transport benefits from thermal activation and the carrier concentration, mobility and electrical conductivity are indicated as [8, 35, 36]
where A, B and C are parameters related to the material and D is a specific constant. An approximately linear relationship between ln(σT−2/3) and 1000T−1 exists for all samples at high temperature. Here, the results in Fig. 3b indicate that thermal activation has a commanding influence on the electrical conductivity with increasing temperature. The thermal activation temperature (Ta) for all InCl3-doped samples appears at 529 K, which is in good agreement with the temperature marking the rising point of electrical conductivity. Different from the doped samples, the undoped sample shows a weak thermal activation effect above 413 K.
Figure 3c shows the Seebeck coefficient dependence on the measured temperature for pristine and InCl3-doped Bi2S3 samples. A negative Seebeck coefficient indicates an n-type electrical transport behavior with electrons contributing more to the whole transport process. The absolute value of the Seebeck coefficient of pristine Bi2S3 increases for a temperature range of 323–423 K and then gently decreases above 423 K. The maximum Seebeck coefficient value of − 517 μV K−1 is obtained at 423 K. With increasing InCl3 doping content, the absolute value of the Seebeck coefficient of Bi2S3 decreases remarkably from − 500 μV K−1 for pristine Bi2S3 to − 130 μV K−1 for Bi2S3 doped with 1.0 mol% InCl3 at 323 K, which is strongly correlated with the increase in carrier concentration. Upon InCl3 doping, the absolute Seebeck coefficient of Bi2S3 increases monotonically with the temperature in the measured range. According to the Mott expression [37], \( S = (\pi^{2} k_{\text{B}}^{2} T/3e)\left\{ {{\text{d}}n\left( E \right)/n{\text{d}}E + {\text{d}}\mu \left( E \right)/\mu {\text{d}}E)} \right._{{E = E_{\text{F}} }} \), the carrier concentration simultaneously affects the absolute value of the Seebeck coefficient along with the carrier mobility. Therefore, when the dopant content surpasses 1.0% mol, the increase in the Seebeck coefficient is probably due to a reduced carrier mobility, suggesting that the electron mobility influences the Seebeck coefficient to a greater extent than carrier concentration.
The power factor is significantly enhanced upon InCl3 doping, particularly for 1.0% mol InCl3 doping, which is improved by approximately five times than that of pristine Bi2S3. The maximum value of the power factor is obtained at 673 K as approximately 363 μW m−1 K−2 for Bi2S3 doped with 1.0 mol% InCl3 and is benefited by the remarkably improved electrical conductivity and the relatively large Seebeck coefficient. The power factors of the Bi2S3 samples show an upward tendency similar to that of the electrical conductivity upon InCl3 doping, which indicates that the electrical conductivity contributes more to the power factor when the Seebeck coefficient is large.
Figure 4 shows the thermal transport properties as a function of temperature for the pristine Bi2S3- and InCl3-doped samples. The pristine Bi2S3 exhibits a relatively low total thermal conductivity (κtot), which may be ascribed to the interatomic bonding strength (the weak ionic and van der Waals forces) and to the lattice vibration anharmonicity. As revealed in Fig. 4a, κtot increases with the InCl3 doping content, and κtot presents similar values when x ≥ 0.5. Additionally, Bi2S3 doped with 1.5 mol% InCl3 possesses the maximum κtot, with values ranging from 0.61 W m−1 K−1 at 323 K to 0.42 W m−1 K−1 at 673 K. The total thermal conductivity consists of two typical parts, the electrical thermal conductivity (κe) and the lattice thermal conductivity (κL), as follows: κtot= κe+ κL. The value of κe can be obtained by κe= LσT, where L, σ and T are the Lorenz number (L = 2.45 × 10−8 V2 K−2), electrical conductivity and temperature in kelvin, respectively. The value of κe is directly proportional to the electrical conductivity and herein exhibits an upward tendency similar to that of the electrical conductivity. The maximum κe of 0.1 W m−1 K−1 is achieved by the Bi2S3 sample doped with 1.0 mol% at 673 K (Fig. 4b). After subtracting κe, κL shows a relatively large value at low temperature and decreases with increasing temperature until a low κL of 0.32 W m−1 K−1 is finally obtained at 673 K for x = 1.0. A low κL has been proved to derive from the interatomic bonding type (ionic bond and covalent bond) and the lattice vibrations, apart from the abundance of pores in the host matrix. The effect of pores on reducing the lattice thermal conductivity is evaluated by the following formula [38]:
where κL,f is the lattice thermal conductivity of the full dense bulk material and P is the porosity, as listed in Table 1 (P = 1 − ρ/ρ0, ρ/ρ0 is the relative density). The κL,f for the Bi2S3 + x mol% InCl3 samples (x = 0, 0.25, 0.5, 1.0 and 1.5) at room temperature are 0.541, 0.585, 0.651, 0.663 and 0.661 W m−1 K−1, which suggests that the pores play an important role in scattering phonons to further reduce the lattice thermal conductivity. The rapidly dropped lattice thermal conductivity for Bi2S3 doped with 1.0, 1.5 mol% InCl3 may be stem from the increased porosity and the generated secondary phase.
Furthermore, the ultrasonic pulse reflection method is used to evaluate the elastic properties and determine the origin of the low lattice thermal conductivity. On the basis of formulas (7) and (8) [39,40,41], a small Young’s modulus (E) and Debye temperature (θD) and a large Grüneisen constant (γ) are necessary for a low lattice thermal conductivity.
where ρ, M, n, Ma, a3, υ, vt, vl, va, h, kB, N, V, νp and G are the sample density, the atomic weight of the molecule, the atomic number of the molecule, the mean atomic weight of the constituent atoms, the average volume occupied by one atom, the lattice numbers of the primitive unit cell, the transverse phonon velocity, the longitudinal phonon velocity, the average phonon velocity, Planck’s constant, the Boltzmann constant, the number of atoms in a unit cell, the unit cell volume, the Poisson ratio and the shear modulus, respectively. Here, the transverse phonon velocity (vt) and the longitudinal phonon velocity (vl) can be obtained via the ultrasonic pulse reflection method, while va, νp, G, E, θD and γ can be calculated using Eqs. (10), (11), (12), (13) and (14) [39, 41,42,43]. The results are listed in Table 2.
The elastic properties of some typical materials with low lattice thermal conductivities are listed in Table 2 for comparison with those of Bi2S3. In this work, the average phonon velocity of Bi2S3 (1781 ms−1) is lower than that of BiCuSeO (2107 ms−1) and Bi2Se3 (2083 ms−1), which results in the small Young’s modulus of Bi2S3. It is worth noting that the covalent nature of a material decreases with the E value. Therefore, a small Young’s modulus will weaken the covalent nature of a material to reduce the lattice thermal conductivity. Furthermore, the E of Bi2S3 is significantly lower than that of BiCuSeO (76.5 GPa) and Bi2Se3 (70.3 GPa) and is comparable to materials with ultralow lattice thermal conductivity, such as BiSbSe3 (34.9 GPa) and K2Bi8Se13 (37.1 GPa). Additionally, the Poisson ratio (νp) reflects the interatomic bonding type in a material; a small νp (0.1) value reveals a covalent bonding nature, whereas a typical νp value of 0.25 is obtained for ionic materials [44]. As shown in Table 2, the νp of Bi2S3 and all thermoelectric materials with low lattice thermal conductivities are larger than 0.25, indicating that there is strong ionic bonding between the atoms in the materials (the weak bonding interaction). The Grüneisen constant (γ) has a strong link with the lattice vibration anharmonicity. As far as we know, the ultralow thermal conductivity of SnSe, with a large γ of ~ 3.13, stems from the lattice vibration anharmonicity in the crystal owing to the lone pair electrons of Sn. The Grüneisen constant for Bi2S3 is larger than that for BiCuSeO (1.5) and is comparable to that for Bi2Se3 (1.65) and K2Bi8Se13 (1.77). The Debye temperature (θD) is also one of the most significant parameters to reflect the low thermal conductivity, other than the elastic properties (phonon velocity, Young’s modulus, shear modulus and Grüneisen constant) that we discussed above. The Debye temperature of Bi2S3 is 182 K, which is lower than that of BiCuSeO (243 K) and Bi2Se3 (205 K), as listed in Table 2. Herein, the weak bonding interaction and the lattice vibration anharmonicity in the material’s crystal lead to the intrinsically low thermal conductivity of Bi2S3.
The dimensionless figure of merit (ZT) as a function of measured temperature for Bi2S3 + x mol% InCl3 (x = 0, 0.25, 0.5, 1.0 and 1.5) is shown in Fig. 4d. The ZT values for all Bi2S3 samples increase with the temperature, and by combing the significantly enhanced power factor and the intrinsically low thermal conductivity, finally, a maximum ZT value of 0.57 is obtained at 673 K, which is almost four times higher than that of pristine Bi2S3 (0.14 at 673 K). This is also a relatively high value in Bi2S3 system. The thermoelectric properties of Bi2S3 could be further enhanced by composting with high electrical conductivity materials and co-doping. This work showed the Bi2S3-based materials have the possibility to be a candidate of high-performance thermoelectric.
Conclusion
Bi2S3-based materials with high performance were prepared via InCl3 doping by ball milling and spark plasma sintering. InCl3 is an n-type dopant that can efficiently increase the electrical conductivity of Bi2S3, especially for the Bi2S3 + 1.0 mol% InCl3 bulk sample, with a value up to 62 Scm−1 at 673 K. Finally, a maximum ZT value of 0.57 is obtained at 673 K along the parallel to the press direction for the sample doped with 1.0 mol% InCl3 because of the remarkably enhanced power factor (363 μW m−1 K−2 at 673 K) and the intrinsically low thermal conductivity (0.43 W m−1 K−1 at 673 K). The improved thermoelectric performance of Bi2S3 is ascribed to the enhanced electrical transport properties via the introduction of InCl3 elements.
References
Hayder A-M, Gao M (2017) Effective use of thermal energy at both hot and cold side of thermoelectric module for developing efficient thermoelectric water distillation system. Energy Convers Manag 133:14–19
Bell LE (2008) Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 321:1457–1461
Zhou C, Dun C, Wang K, Zhang X, Shi Z, Liu G, Hewitt CA, Qiao G, Carroll DL (2016) General method of synthesis ultrathin ternary metal chalcogenide nanowires for potential thermoelectric applications. Nano Energy 30:709–716
Kang H, Li J, Liu Y, Guo E, Chen Z, Liu D, Fan G, Zhang Y, Jiang X, Wang T (2018) Optimizing the thermoelectric transport properties of BiCuSeO via doping with the rare-earth variable-valence element Yb. J Mater Chem C 6:8479–8487
Pei YL, Wu H, Wu D, Zheng F, He J (2014) High thermoelectric performance realized in a BiCuSeO system by improving carrier mobility through 3D modulation doping. J Am Chem Soc 136:13902–13908
Ge Z-H, Qin P, He D, Chong X, Feng D, Ji Y-H, Feng J, He J (2017) Highly enhanced thermoelectric properties of Bi/Bi2S3 nanocomposites. ACS Appl Mater Interfaces 9:4828–4834
Yang L, Chen ZG, Hong M, Han G, Zou J (2015) Enhanced thermoelectric performance of nanostructured Bi2Te3 through significant phonon scattering. ACS Appl Mater Interfaces 7:23694–23699
Zhu Y, Carrete J, Meng Q-L, Huang Z, Mingo N, Jiang P, Bao X (2018) Independently tuning the power factor and thermal conductivity of SnSe via Ag2S addition and nanostructuring. J Mater Chem A 6:7959–7966
Pei Y, Wang H, Snyder GJ (2012) Band engineering of thermoelectric materials. Adv Mater 24:6125–6135
Zhu H, Sun W, Armiento R, Lazić P, Ceder G (2014) Band structure engineering through orbital interaction for enhanced thermoelectric power factor. Appl Phys Lett 104:082107
Zhao L-D, Zhang B-P, Li J-F, Zhou M, Liu W-S, Liu J (2008) Thermoelectric and mechanical properties of nano-SiC-dispersed Bi2Te3 fabricated by mechanical alloying and spark plasma sintering. J Alloys Compd 455:259–264
Wu HJ, Zhao LD, Zheng FS, Wu D, Pei YL, Tong X, Kanatzidis MG, He JQ (2014) Broad temperature plateau for thermoelectric figure of merit ZT > 2 in phase-separated PbTe0.7S0.3. Nat Commun 5:4515
Biswas K, Zhao L-D, Kanatzidis MG (2012) Tellurium-free thermoelectric: the anisotropic n-type semiconductor Bi2S3. Adv Energy Mater 2:634–638
Chang C, Xiao Y, Zhang X, Pei Y, Li F, Ma S, Yuan B, Liu Y, Gong S, Zhao L-D (2016) High performance thermoelectrics from earth-abundant materials: enhanced figure of merit in PbS through nanostructuring grain size. J Alloys Compd 664:411–416
Cheng X, Wang L, Wang X, Chen G (2018) Flexible films of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)/SnS nanobelt thermoelectric composites. Compos Sci Technol 155:247–251
Xie H, Su X, Zheng G, Zhu T, Yin K, Yan Y, Uher C, Kanatzidis MG, Tang X (2017) The role of Zn in chalcopyrite CuFeS2: enhanced thermoelectric properties of Cu1−xZnxFeS2 with in situ nanoprecipitates. Adv Energy Mater 7:1601299
Zhang R-z, Chen K, Du B, Reece MJ (2017) Screening for Cu–S based thermoelectric materials using crystal structure features. J Mater Chem A 5:5013–5019
Ge ZH, Song D, Chong X, Zheng F, Jin L, Qian X, Zheng L, Dunin-Borkowski RE, Qin P, Feng J, Zhao LD (2017) Boosting the thermoelectric performance of (Na, K)-codoped polycrystalline SnSe by synergistic tailoring of the band structure and atomic-scale defect phonon scattering. J Am Chem Soc 139:9714–9720
Li F, Wang W, Ge ZH, Zheng Z, Luo J, Fan P, Li B (2018) Enhanced thermoelectric properties of polycrystalline SnSe via LaCl(3) doping. Materials 11:203
Zhao LD, Lo SH, Zhang Y, Sun H, Tan G, Uher C, Wolverton C, Dravid VP, Kanatzidis MG (2014) Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 508:373
Li J, Sui J, Pei Y, Meng X, Berardan D, Dragoe N, Cai W, Zhao L-D (2014) The roles of Na doping in BiCuSeO oxyselenides as a thermoelectric material. J Mater Chem A 2:4903–4906
Zhao L-D, He J, Berardan D, Lin Y, Li J-F, Nan C-W, Dragoe N (2014) BiCuSeO oxyselenides: new promising thermoelectric materials. Energy Environ Sci 7:2900–2924
Liu R, Lan J-l, Tan X, Liu Y-c, Ren G-k, Liu C, Zhou Z-f, Nan C-w, Lin Y-h (2018) Carrier concentration optimization for thermoelectric performance enhancement in n-type Bi2O2Se. J Eur Ceram Soc 38:2472–2746
Tan X, Liu Y, Hu K, Ren G, Li Y, Liu R, Lin Y-H, Lan J-L, Nan C-W (2017) Synergistically optimizing electrical and thermal transport properties of Bi2O2Se ceramics by Te-substitution. J Am Ceram Soc 101:326–333
Liu Z, Pei Y, Geng H, Zhou J, Meng X, Cai W, Liu W, Sui J (2015) Enhanced thermoelectric performance of Bi2S3 by synergistical action of bromine substitution and copper nanoparticles. Nano Energy 13:554–562
Yang J, Liu G, Yan J, Zhang X, Shi Z, Qiao G (2017) Enhanced the thermoelectric properties of n -type Bi2S3 polycrystalline by iodine doping. J Alloys Compd 728:351
Meng Q-L, Kong S, Huang Z, Zhu Y, Liu H-C, Lu X, Jiang P, Bao X (2016) Simultaneous enhancement in the power factor and thermoelectric performance of copper sulfide by In2S3 doping. J Mater Chem A 4:12624–12629
Tsutsui M, Zhang LT, Ito K, Yamaguchi M (2004) Effects of in-doping on the thermoelectric properties of β-Zn4Sb3. Intermetallics 12:809–813
Zhang Q, Liao B, Lan Y, Lukas K, Liu W, Esfarjani K, Opeil C, Broido D, Chen G, Ren Z (2013) High thermoelectric performance by resonant dopant indium in nanostructured SnTe. Proc Natl Acad Sci USA 110:13261–13266
Ge ZH, Zhang BP, Liu Y, Li JF (2012) Nanostructured Bi(2−x)Cu(x)S3 bulk materials with enhanced thermoelectric performance. Phys Chem Chem Phys 14:4475–4481
Guo J, Ge Z, Hu M, Qin P, Feng J (2018) Facile synthesis of NaBiS2 nanoribbons as a promising visible light-driven photocatalyst. Phys Status Solidi-R 12:1800135
López R, Gómez R (2011) Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: a comparative study. J Sol–Gel Sci Technol 61:1–7
Pei J, Zhang L-J, Zhang B-P, Shang P-P, Liu Y-C (2017) Enhancing the thermoelectric performance of CexBi2S3 by optimizing the carrier concentration combined with band engineering. J Mater Chem C 5:12492–12499
Du X, Cai F, Wang X (2014) Enhanced thermoelectric performance of chloride doped bismuth sulfide prepared by mechanical alloying and spark plasma sintering. J Alloys Compd 587:6–9
Morin FJ, Maita JP (1954) Conductivity and Hall effect in the intrinsic range of germanium. Phys Rev 94:1525
Morin FJ, Maita JP (1954) Electrical properties of silicon containing arsenic and boron. Phys Rev 96:28
Heremans JP, Jovovic V, Toberer ES, Saramat A, Kurosaki K, Charoenphakdee A, Yamanaka S, Snyder GJ (2008) Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 321:554–557
Wan CL, Pan W, Xu Q, Qin YX, Wang JD, Qu ZX, Fang MH (2006) Effect of point defects on the thermal transport properties of (LaxGd1−x)2Zr2O7: experiment and theoretical model. Phys Rev B 74:144109
Kurosaki K, Kosuga A, Muta H, Uno M, Yamanaka S (2005) Ag9TlTe5: a high-performance thermoelectric bulk material with extremely low thermal conductivity. Appl Phys Lett 87:061919
Liu X, Wang D, Wu H, Wang J, Zhang Y, Wang G, Pennycook SJ, Zhao L-D (2018) Intrinsically low thermal conductivity in BiSbSe3: a promising thermoelectric material with multiple conduction bands. Adv Funct Mater 29:1806558
Xiao Y, Chang C, Pei Y, Wu D, Peng K, Zhou X, Gong S, He J (2016) Origin of low thermal conductivity in SnSe. Phys Rev B 94:125203
Anderson OL (1963) A simplified method for calculating the Debye temperature from elastic constants. J Phys Chem Solids 24:909–917
Ge C, Hu M, Wu P, Tan Q, Chen Z, Wang Y, Shi J, Feng J (2018) Ultralow thermal conductivity and ultrahigh thermal expansion of single-crystal organic-inorganic hybrid perovskite CH3NH3PbX3 (X = Cl, Br, I). J Phys Chem C 122:15973–15978
Koc H, Ozisik H, Deligoz E, Mamedov AM, Ozbay E (2014) Mechanical, electronic, and optical properties of Bi(2)S(3) and Bi(2)Se(3) compounds: first principle investigations. J Mol Model 20:2180
Gao X, Zhou M, Cheng Y, Ji G (2016) First-principles study of structural, elastic, electronic and thermodynamic properties of topological insulator Bi2Se3 under pressure. Philos Mag 96:208–222
Pei Y, Chang C, Wang Z, Yin M, Wu M, Tan G, Wu H, Chen Y, Zheng L, Gong S, Zhu T, Zhao X, Huang L, He J, Kanatzidis MG, Zhao LD (2016) Multiple converged conduction bands in K2Bi8Se13: a promising thermoelectric material with extremely low thermal conductivity. J Am Chem Soc 138:16364–16371
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11764025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, J., Ge, ZH., Qian, F. et al. Achieving high thermoelectric properties of Bi2S3 via InCl3 doping. J Mater Sci 55, 263–273 (2020). https://doi.org/10.1007/s10853-019-04008-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04008-3