Abstract
Iron (Fe) and chromium (Cr) powders at various concentrations of Cr (6, 9, 12 and 14 wt%) were mechanically alloyed via high-energy ball milling for 10 h. The milled Fe–Cr alloy powders were analyzed using scanning electron microscopy (SEM) and X-ray diffraction (XRD) for understanding the impact of varying chromium content on the characteristics of the milled powder. The powder samples were then consolidated via spark plasma sintering (SPS) at 1000 °C for 45 min at 80 MPa pressure. The density of the consolidated samples was measured and found to reach a maximum of 98%. Microstructural characterization of the spark plasma sintered samples was performed using SEM and XRD. Electrochemical corrosion tests were performed on spark plasma sintered samples in 0.5 M H2SO4, 0.05 M NaH2PO4 and 1 M NaOH solutions using potentiodynamic polarization, electrochemical impedance spectroscopy and Mott–Schottky analysis. The corrosion behavior of the spark plasma sintered materials was compared with that of a wrought HT-9 steel (12Cr based ferritic–martensitic steel). The work presented here is a comparative study of microstructural evolution and corrosion behavior between the different spark plasma sintered Fe–Cr model alloys and wrought HT-9 steel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-chromium (9–14 wt%)-containing steels have potential for structural and fuel cladding applications in advanced nuclear reactors including sodium-cooled fast reactors [1,2,3], which require high radiation swelling resistance and resistance to attack from liquid sodium [4]. Oxide dispersion strengthened (ODS) steels based on Fe-(9–14) wt% Cr alloys are also promising for such applications. The processing routes of such steels involve mechanical alloying that incorporates rare earth oxides such as yttrium oxide in the base Fe–Cr matrix. Mechanical alloying is a processing method that involves repeated fracturing, welding and re-fracturing of a mixture of powder particles, generally in a high-energy ball mill, to produce a controlled, extremely fine microstructure, which cannot be easily produced via conventional ingot metallurgy. The milled powder is then consolidated into bulk shapes and heat-treated to obtain the desired characteristics [5]. It has been reported that the morphology, grain size distribution and contamination of the milled powders strongly depend on the process variables in mechanical alloying such as milling time, process control agent (PCA), ball-to-powder weight ratio (BPR) and milling atmosphere [6,7,8,9,10,11,12]. Mechanical alloying is a potential processing technique for producing nanocrystalline materials, amorphous and non-equilibrium phases. During this process, solid solubility significantly increases with increasing energy input. Solid solubility limit is determined by the ratio of intermixing (caused by shear forces) to the decomposition of the solid solution due to thermally activated jumps. If this ratio is very large, a fully random solid solution is achieved. Formation of nanostructures during milling has been established as the most effective mechanism to increase solid solubility due to higher fraction of atoms at the grain boundaries and enhanced (short-circuit) diffusion path.
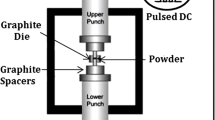
Spark plasma sintering (SPS) is a compaction/sintering process that utilizes a combined action of a pulsed direct electrical current (DC) and pressure to perform fast consolidation of powder. The direct way of heating allows high heating rates, thus increasing densification due to enhanced diffusion [13,14,15]. It is regarded as a rapid sintering method in which the heating power is distributed over the volume of the powder compact and the heat is dissipated exactly at the locations in the microscopic scale, where energy is required for the sintering process, mainly at the contact points of the powder particles. SPS systems offer many advantages over conventional systems using hot pressing (HP), hot isostatic pressing (HIP) or atmospheric furnaces, including ease of operation and accurate control of sintering energy resulting in lower-temperature sintering and less time, and applicability to both electrically conductive and non-conductive materials.
The nature of the passive oxide film formation on as-cast iron has been the subject of investigation since Schonbein and Faraday [16] first developed the theory of protective oxide layer. Recent studies have shown that the passivity is due to the presence of a thin oxide film 1–4 nm thick, which isolates the metal surface from a corrosive aqueous environment. A lot of work has been done on the chemical composition and structure of passive films formed on as-cast ferrous alloys [17,18,19,20,21,22,23]. But the study on the electrochemical and transport properties of these films has not been done in detail. An increase in transpassive dissolution tendency with increasing chromium content in a Fe–Cr alloy was reported by Betova et al [24]. Nonetheless, the reason behind the complex nature of the passive film formed at the interfaces of metal and the electrolyte is yet to be fully understood. This complexity has made it difficult to predict the kinetic and transport parameters of the passive layer.
Fe–Cr alloys have been primarily made by casting followed by thermomechanical processing and heat treatment [25, 26], but only limited work has been reported on the powder metallurgy source Fe–Cr alloys. In the work by Heintze et al. [27], high-energy ball milling was conducted followed by SPS and HIP. However, the ball-milled powders for different milling durations were not fully analyzed and results were not interpreted. Also, corrosion study has not been done on spark plasma-sintered sample Fe–Cr alloy system. Although analyses of corrosion and microstructural behavior have been reported for complex Fe–Cr-X alloy systems previously [28,29,30], a comparative study of the corrosion behavior between spark plasma sintered Fe–Cr-based alloys has not been done yet. Hence, the present work was undertaken to obtain an understanding of the microstructural evolution and corrosion characteristics of the Fe–xCr (x = 6, 9, 12 and 14 wt%) alloys processed via high-energy ball milling and SPS, as a function of composition.
Materials and method
Processing methods and metallographic characterization
Iron powder (99.9+% metal basis) of around 10 µm size and chromium powder (99.95% metal basis) of 50 µm size were weighed in order to make nominal compositions of Fe–xCr powder batches with 6, 9, 12 and 14 wt% Cr, which were poured into stainless steel vials along with stainless steel balls (milling medium) of 8 mm diameter under an argon atmosphere inside a glove box to prevent oxidation/contamination. No surfactant was added to the milling mixture. The ball-to-powder weight ratio was around 10:1 [31]. The filled vials were loaded in a shaker mill (SPEX 8000M) and milled for 10 h. The carbon and nitrogen content of the powders were estimated to be approximately 0.020 wt% and 0.024 wt%, respectively, using combustion analysis.
The milled powders were characterized in a Zeiss Supra 35 VP field-emission gun scanning electron microscope (FEG-SEM) to examine the powder size and morphology. X-ray diffraction (XRD) of ball-milled powders was performed using Siemens 5000D diffractometer with Cu Kα X-ray radiation (wavelength of 0.1541 nm). Using the XRD data, lattice parameters of the milled powders were calculated based on the Nelson–Riley relation [32]. The crystallite size and lattice strain were evaluated using the Williamson–Hall equation [33]:
where βhkl is the full width half maxima (FWHM) of the XRD peak, L is the crystallite size and ε is the lattice strain.
All the 10-h ball-milled powder batches were consolidated via SPS at an applied pressure of 80 MPa for 45 min at 1000 °C using a Dr. Sinter 515 SPS machine. The heating rate used was 100 °C/min, and the cooling rate was 40 °C/min. The samples made were about 12.5 mm in diameter and 7 mm in thickness. Each composition had 3 samples prepared for further investigation. Density of the polished (0.5 µm finish) sintered samples was measured by Archimedes principle. The weight of the samples was taken in air and fully immersed condition in deionized water using an Ohaus precision balance (HRB-200). The density of deionized water (1 g/cm3) and the density of air (0.0012 g/cm3) at standard room temperature and pressure were used for density calculation. The theoretical density of the sample was calculated based on the composition using the following equation:
where ρ Thsample is the theoretical density of the sample, ρFe is the density of iron (7.87 gm/cc), ρCr is the density of chromium (7.19 gm/cc), CFe is the wt fraction of Fe in the sample and CCr is the weight fraction of Cr in the sample. The relative density was then estimated in terms of the percentage of theoretical density.
Vickers microhardness tests were performed on the spark plasma sintered samples using a LECO 100 M microhardness tester using a load of 0.5 kgf (4.91 N) and a loading time of 15 s. The same X-ray diffractometer and the SEM were used to analyze the phase constituents and grain size of the spark plasma sintered samples. On performing energy-dispersive spectroscopy (EDS) analysis, chromium oxide has been found present in the steel as Cr2O3. Unfortunately, due to the inherent limitations of energy precise, quantitative analysis could not be done. However, from stoichiometric analysis chromium oxide particles present, the oxygen content is found to be ~ 0.1 wt%, which is in the same range found in other alloys.
Corrosion characterization
Electrochemical corrosion testing on the spark plasma sintered samples was carried out using a potentiostat (Versa STAT MC, Ametek). Highly pure deionized water having a resistivity of 18.2 MΩ-cm was used for preparing electrolyte solutions for all the corrosion tests. A three-electrode system was used for the test. The working electrode was Fe–Cr samples with a surface area of 1 cm2. A shielded copper wire was soldered on one flat surface of the spark plasma sintered cylindrical specimen (12.5 mm in diameter and 7 mm in length) for electrical connectivity, and it was mounted using epoxy such that only one flat surface was exposed. The exposed surface was metallographically polished down to 0.5-μm finish. The polished specimen was washed with soap water, ultrasonically cleaned in methanol and dried in nitrogen stream before electrochemical corrosion testing. All specimens were subjected to the same polishing and cleaning procedure. The reference electrode was Ag/AgCl (199 mV vs. standard hydrogen electrode), and the counter electrode was a platinum wire having a surface area of 2 cm2. The corrosion tests were performed in three different aqueous solutions such as 0.5 M H2SO4, 0.05 M NaH2PO4 and 1 M NaOH. One ingot-metallurgy (wrought) HT-9 alloy (Fe–12Cr–1Mo–0.5W–0.5Ni–0.25V–0.2C), a high strength ferritic–martensitic stainless steel, was also investigated for comparison.
Corrosion potential was recorded for about 30 min. After reaching a steady-state open-circuit potential (OCP), linear polarization (LP) was carried out by scanning the potential within a potential window of ± 25 mV with respect to OCP with a scan rate of 1 mV/s. After LP, potentiodynamic polarization (PD) was performed by scanning the potential in the range of − 0.5 V to + 1.5 V with respect to OCP at a rate of 1 mV/s. In order to understand the passivation behavior of the specimens, potentiostatic tests were carried out. Freshly polished samples (up to 0.5-µm finish) were passivated for 1 h by applying a constant potential corresponding to the middle potential of the passive range from the anodic polarization plot. The electrochemical impedance spectroscopy (EIS) was carried out at the passivation potential by scanning the frequency in the range of 0.01–10,000 Hz. Mott–Schottky analyses was performed at 1 kHz by scanning the potentials in the cathodic direction at 50 mV intervals from the passivation potential to OCP. All the corrosion tests have been duplicated and at times triplicated to verify reproducibility. The tabulated values are the average values of the test runs, and the plots depict the best condition.
Results and discussion
Characteristics of the milled Fe–Cr powders
The milled powder batches were examined by SEM. Figure 1a–d shows the secondary electron (SE) SEM images of the powder samples ball milled for 10 h having different Cr contents (6, 9, 12 and 14 wt%), respectively.
Powder size analysis revealed that the mean powder size increased as a function of increasing Cr content as shown in Fig. 2. Also, the powder size distribution became progressively wider with increasing Cr content. It can be inferred that with increasing Cr during milling, the Fe–Cr alloy becomes more solid solution hardened [34], and consequently, both the powder size and powder size distribution increase.
Figure 3 shows XRD patterns of the various milled powder samples. Prominent peaks are observed near the diffraction angles (2θ) of 44.91°, 65.46° and 82.57°. These peaks correspond to the planes {110}, {200} and {211} of BCC Fe, respectively [35]. The lattice parameter of α-Fe is 2.87 Å and that of Cr is 2.89 Å. A slight shift of the peaks to the left is observed with increasing Cr, which signifies progressive dissolution of Cr into the Fe matrix. Also, from Table 1, it can be noted that with increasing the Cr concentration, the lattice parameter of the Fe–Cr powders increases which is another indication that Cr is getting incorporated into the matrix. Although the lattice parameter decreases, the strain does not vary significantly with change in the Cr content.
Characteristics of spark plasma-sintered Fe–xCr samples
Evaluation of density, microhardness and XRD pattern
In this section, density Vickers microhardness and XRD pattern of spark plasma sintered Fe–xCr alloys are presented and discussed. Figure 4 shows the variation of relative density of the sintered samples with increasing Cr content. With an increase in Cr concentration, the relative density keeps on decreasing. The reason behind this trend is that with the milled powder size increased with increasing Cr content. Bigger powder size results in lower compaction, and thus, during the consolidation process via SPS, the porosity level increased resulting in lower density of the higher-chromium counterparts.
Figure 5 shows the variation of microhardness with the increase in Cr content. It is known that with increasing Cr percentage, the alloy becomes more solid solution strengthened. The solid solution strengthening can be expressed by the following equation [34]:
where Ai is the strengthening coefficient of alloying elements Cr (8.5 MPa/wt%) [36] and \( C_{i} \) is the concentration of the alloying elements in wt%. So, with the increase in Cr, the microhardness of the material should increase.
However, the microhardness is found to increase significantly from 6 to 9 wt% Cr samples. But the hardness decreased as we increased the Cr content from 9 to 14 wt%. One of the reasons behind this is that due to the decrease in relative density, the porosity in the material increases, which plays a significant role in decreasing the microhardness of the subsequent materials. That effect of increase in porosity could not be counteracted by the solid solution strengthening along with other modes of strengthening mechanisms imparted by slightly smaller crystallize sizes.
Figure 6 shows the XRD pattern of the sintered specimens. The peaks clearly show the presence of ferrite (BCC). Since iron and chromium peaks are almost similar, they cannot be distinguished. A small peak is observed to the left of Fe (110) peak in higher %Cr alloys. This peak corresponds to chromium nitride (Cr2N) (200) peak.
Sintering kinetics
In order to understand the SPS kinetics, the model proposed by Young and Cutler [37] has been used. In their study, non-isothermal equations for volume and grain boundary diffusion were modified. The equations represent the relationship between the shrinkage (Y) and diffusion coefficient (D), where c represents the heating rate in units of K s−1 [37]:
where Y is the linear shrinkage (Δl/l0), Ω is the volume of vacancy (m3), γ is the surface energy (J/m2), DV is the volume diffusion coefficient given by DV = D V 0 exp(− QV/RT) (m2.s−1), bDB is the product of the grain boundary thickness ‘b’ and grain boundary diffusion coefficient ‘DB’ given by DB = D B 0 exp(− QB/RT) (m3 s−1), k is Boltzmann constant (J/K), R is the universal gas constant (J/K mol), T is the temperature (K) and a is the particle radius (m). QV and QB are the activation energy (J/mol) for volume diffusion and grain boundary diffusion, respectively. The activation energies for volume diffusion and grain boundary diffusion can be obtained from the slope of the plots between ln (YT(dY/dT)) versus 1/T and ln (Y2T(dY/dT)) versus 1/T, respectively.
Figure 7 depicts the displacement profile curves of the spark plasma sintered samples. The displacement profile arises due to linear shrinkage (i.e., axial shrinkage) caused due to the uniaxial applied load. Constant heating rate was used during SPS. The temperature at which densification begins (Ts) and the temperature at which maximum densification rate occurs (Tmax) are calculated from the displacement curve and its first derivative, respectively [38], and are listed in Table 2. It is found that densification starts at around 600 °C and reaches the maximum rate at 825 °C which is similar to the values obtained by previous studies [39].
From the slope of the ln (YT dY/dT) versus 1/T plots shown in Fig. 8a, the volume diffusion activation energy for the 10-h milled Fe–xCr sintered samples at 1000 °C was estimated. From the slope of the ln (Y2T dY/dT) versus 1/T plots shown in Fig. 8b, the grain boundary diffusion activation energy for the 10-h milled Fe–xCr sintered samples at 1000 °C alloy was estimated. The calculated data are tabulated in Table 2. The activation energy for volume diffusion and grain boundary diffusion of pure α–Fe is 251 and 174 kJ/mol, respectively [40]. The nanograin boundary diffusion for Fe powder was estimated to be 187 kJ/mol according to Lee et al. [41].
Considering the above scenario, one can hypothesize that in the initial phase of sintering the grain boundary diffusion is the dominant mechanism. The plausible reason for this could be preponderance of boundaries due to the nanocrystalline crystallite size produced during high-energy ball milling. However, during the process of SPS, various mechanisms can occur as reported in previous studies [15]. One of the reasons that in the initial phase, surface diffusion is the dominant mechanism because electric charge is concentrated mainly on the outer surface of the particles leading to capacitive nature of two nearby powder particles producing enough heat for the surface diffusion to take place. In the later stage when enough particles have joined with each other, the volume diffusion or the lattice diffusion might come into play as depicted in Fig. 7, but the exact mechanism of the final stage of densification is yet unknown. With increasing Cr content, it is seen that GB diffusion activation energy is decreasing, and thus, the higher-chromium alloy starts diffusion at lower temperatures compared to their lower Cr counterparts. Also, the temperature range for the GB diffusion increases with increasing Cr content.
Corrosion analysis
Open-circuit potential (OCP or simply corrosion potential) represents a mixed potential at which the anodic reaction rate is equal to the cathodic reaction rate. More negative OCP of a specimen indicates higher activity for corrosion in a given environment. OCP profiles (OCP versus time) of the Fe–xCr alloys and alloy HT-9 in 0.5 M H2SO4, 0.05 M NaH2PO4 solution and 1 M NaOH solution are shown in Fig. 9a–c, respectively. The OCP increased with the increase in the Cr content in the 0.5 M H2SO4 solution as shown in Fig. 9a. It is observed that a steady state of OCP was reached quickly for HT-9. A steady state of OCP was observed for Fe–9Cr after about 600 s. Fe–6Cr showed a decreasing trend, whereas Fe–12Cr and Fe14Cr specimens exhibited increasing trend of OCP with time. Since OCP is influenced by exchange current densities and Tafel slopes of both anodic and cathodic reactions, the variations in the OCP could be attributed to change of these parameters individually and in combination. The exchange current density and Tafel slope of cathodic reaction are catalyzed by surface. Therefore, these parameters will be influenced by the composition of the specimens. There are two possible cathodic reactions in the sulfuric acid solution which is not deaerated, namely:
The anodic reaction is given by M → Mn+ + ne-. If the kinetics of the cathodic reactions was the same for all the specimens in this study (which may not be true), the decrease in the OCP values with time and lower OCP of Fe–6Cr could be attributed to the absence of a passive layer in the 0.5 M H2SO4 solution that increased the exchange current density and decreased Tafel slope for anodic reaction. The increase in Cr content in the alloy increased the anodic Tafel slope and decreased the exchange current density. The increase in the OCP with time for Fe–12Cr and Fe–14Cr could be attributed to the formation and thickening of a passive film that subsequently increased the anodic Tafel slope.
In case of 0.05 M NaH2PO4 solution (Fig. 9b), due to the formation of complex oxide layer possibly containing PO 3-4 ions, the nature of OCP starts varying. Porosity in the materials starts playing an important role here. Since HT-9 is a wrought material, the porosity level in the material is very low and forms a relatively defect free surface layer. Therefore, it has a higher OCP value compared to other alloys. The spark plasma sintered materials show an increase in OCP values with the increase in Cr content. However, since Fe–14Cr has a higher porosity level than Fe–12Cr, the former has a lower OCP value. In the 1 M NaOH solution (Fig. 9c), the formation of hydroxide layer is more probable [42]. In this solution, Fe–6Cr and Fe–9Cr exhibit a surprising characteristic compared to the other alloys. This may be related to the formation of a thicker iron oxyhydroxide layer. Fe–12Cr and HT-9 alloys have the same amount of Cr, but the latter one having lower porosity has a higher OCP. Although Fe–14Cr has higher Cr content than Fe–12Cr, the porosity level in Fe–14Cr is higher than in Fe–12Cr resulting in lower OCP than the latter. Linear polarization resistance (Rp) is the resistance of the specimen to charge transfer across the electrochemical interface during the application of an external potential [43]. The lower the value of the Rp is, higher is the corrosion rate of the material. Table 3 shows the Rp values of the alloys in various solutions. The Rp values show a general trend of increase with increasing Cr content of Fe–Cr alloy samples in a particular solution. The pH of the solution influenced the Rp values significantly. The specimens showed higher Rp values in high pH solution. The corrosion resistance was low when the pH was low.
Figure 10a–c illustrates the potentiodynamic polarization behavior of the specimens in 0.5 M H2SO4, 0.05 M NaH2PO4 and 1 M NaOH solutions, respectively. All the specimens exhibited more or less similar cathodic polarization behavior in the 0.5 M H2SO4 solution. However, the anodic polarization behavior was widely different between different specimens which was a strong function of porosity level. The HT-9 showed good passivation behavior with a passive current density lower than 10 μA/cm2 between − 0.2 and 1.2 VAg/AgCl. The Fe–Cr spark plasma sintered specimens do not show passivation in 0.5 M H2SO4 solution. With increasing Cr, a passivation layer is formed on Fe–12Cr and Fe–14Cr as seen from the OCP results, but the absence of a passive region during anodic polarization could be attributed to not attaining the critical current density for achieving passivity. For example, HT-9 showed a critical current density of about 32 mA/cm2 to attain passivity. This current density was recorded at a potential of − 0.4 VAg/AgCl. On the other hand, spark plasma sintered Fe–12Cr and Fe–14Cr showed passivity only above 0.8 V when the current density was about 100 mA/cm2. The Fe–6Cr and Fe–9Cr specimens did not show passivity plausibly because the critical current density for passivation could not be achieved in the potential range during anodic potential scan at a rate of 1 mV/s. In 0.05 M NaH2PO4 solution, due to the buffering nature of the solution, the alloys behave differently. Distinct passive regions are revealed in the anodic polarization plots of specimens except for Fe–9Cr as shown in Fig. 10b. HT-9 showed the lowest passive current density. All the specimens showed a critical current density to attain the passivation which increased as the Cr content of the alloy decreased. The critical current density was recorded at around 0 VAg/AgCl in most of the specimens, and this value increased with the decrease in the Cr level. The critical current density was orders of magnitude larger than the passive current density in case of Fe–6Cr or Fe–14Cr, whereas HT-9 and Fe–12Cr showed almost similar order of critical current density and passive current density. HT-9 specimen showed a very low anodic current density at a wide range of anodic potentials, and the Fe–6Cr and Fe–14 Cr specimens showed an active–passive behavior in the NaH2PO4 solution. On the other hand, Fe–9Cr and Fe–12Cr specimens showed high current densities indicating the absence of a passive layer in the range of anodic potentials investigated. The higher critical current density requirement could be attributed to the relatively faster scan rate of the potential (1 mV/s). In 1 M NaOH solution, all the alloys showed similar passivation behaviors. It has been reported in previous studies [44] that formation of Cr(III) oxide and its slow dissolution rate in alkaline solution are responsible for the excellent passive behavior of high-Cr Fe-based alloys. The values of Ecorr, Icorr, anodic slope (βa) and cathodic slope (βc) are summarized in Table 4.
Icorr values were extrapolated from the cathodic and anodic polarization plots given in Fig. 10 and also calculated using the relation:
It is noted that the extrapolated corrosion rated matched well with the calculated values. The Icorr values increased with the decrease in the pH of the electrolyte for a given alloy. The Icorr value did not change with Cr content of the specimens in the 0.5 M H2SO4 solution. However, having more than 12% Cr was found to be advantageous in the NaH2PO4 solution. The Icorr values decreased with the increase in the Cr content of the specimens in the NaOH solution. From the Icorr values as shown in Table 4, it can be inferred that the HT 9 has the highest corrosion resistant alloy among the 5 alloys in any individual solution under consideration. The anodic and cathodic Tafel slopes of the specimens were in the range of 0.1–0.23. The HT-9 specimens showed lower corrosion rates along with shallower Tafel slopes. The spark plasma sintered specimens, on the other hand, showed relatively steeper Tafel slopes but higher corrosion rates. This observation indicated that the porosity present in the spark plasma sintered specimens played a significant role on the corrosion behavior.
Potentiostatic passivation was carried out on the Fe–xCr alloys and alloy HT-9 in 0.5 M H2SO4, 0.05 M NaH2PO4 solution and 1 M NaOH solution for 1 h, and the results are shown in Figs. 11a–c, respectively. Figure 11a shows the passive current transients recorded in the 0.5 M H2SO4 solution at a potential which is the middle potential of the passivation zone as obtained from Fig. 10a. It is interesting to note that during potentiodynamic polarization, a large current (in the order of 100 mA/cm2) is recorded on the Fe–Cr alloys which precluded a passivation behavior. On the other hand, under potentiostatic conditions a current decay behavior is observed which implied formation of a surface layer that resulted in passivation. Fe–6Cr and Fe–9Cr showed a delayed passivation. During the first 200–300 s of potentiostatic conditioning, the current decay is slow with an exponent of 0.07. After about 500 s, an abrupt current decay is observed. This behavior could be attributed to passivation by dissolution and reprecipitation mechanism. It can be argued that during the initial 500 s dissolution occurred because of the high current density which increased the ionic strength at the electrode/electrolyte interface. When the ionic strength reaches super-saturation because of slow convection or local change in the pH, precipitation of a surface layer occurs leading to abrupt current decay. The Fe–12Cr and Fe–14Cr specimens showed an instantaneous current decay behavior with the application of a constant potential with a decay exponent of about 0.68.
Figure 11b shows the current decay behaviors of the Fe–Cr alloys in the 0.05 M NaH2PO4 solution. A similar behavior is seen for the Fe–6Cr and Fe–9Cr alloy specimens like in the 0.5 M H2SO4 solution. Both of these alloys exhibit a delayed passivation, although Fe–6Cr initially showed a corrosion tendency. Figure 11c shows the current decay behaviors of Fe–Cr alloys in the 1 M NaOH solution. The passivation in alkaline solution is observed occur with three different slopes for all the specimens except for Fe–6Cr. In the absence of surface chemical analysis data, it is difficult to delineate the possible surface reactions for each slope. The overall reactions could be the formation of a Cr-rich oxide, Fe–Cr-containing spinel oxide, and conversion of the Fe–Cr-based oxide into its oxyhydroxide.
The passive current decay can be represented using the following equation [45]:
where I is the passivation current at time t, I0 is initial current density of the fresh specimen surface in the test environment and α is the passivation kinetic exponent. The value of α gives us an idea about how quickly the surface gets passivated in an environment. Higher the value of α, faster is the passivation kinetics. Negative value indicates passivity layer breakage. The values of the α for the different alloys in different solutions are summarized in Table 5.
From Table 5, we can deduce that the passivation kinetics of the alloys is slower in the low pH solution than in the alkaline pH. Passivation kinetics increases with increasing Cr content in all the solutions. Also, the passivation depends on the porosity level. Fe–12Cr showed faster passivation kinetics than HT-9 in spite of having almost similar Cr content. This could be attributed to the finer grain size of the spark plasma sintered Fe–12Cr (1.9 ± 0.2 µm) compared to HT-9 (11.3 ± 0.3 µm). Therefore, the passivation reaction could be associated with possible grain boundary diffusion of Cr.
EIS was carried out followed by the potentiostatic passivation, and the results are given in Figs. 12a–c in the form of Bode plots. Nyquist plots and phase angle versus frequency plots are included in the supporting information as shown in Fig. 15a–c and Fig. 16a–c, respectively. Figure 12a shows the impedance moduli of Fe–Cr alloys and HT-9 in 0.5 M H2SO4. The HT-9 specimen showed the highest impedance, and all Fe–Cr alloys other than Fe–6Cr showed almost similar impedance in the sulfuric acid solution. It is noted that the impedance results could not be directly related with the potentiostatic passive current decay behaviors. The impedance results of the specimens in the 0.05 M NaH2PO4 solution are shown in Fig. 12b. In this case, the Fe–9Cr shows the lowest impedance. This could be associated with the formation of defective surface layer during the potentiostatic conditioning that shows highly oscillating current decay behavior as shown in Fig. 12b. With the exception of Fe–9Cr, other Fe–Cr specimens show increasing impedance with the increase in the Cr content in the neutral pH condition. Here also, the HT-9 sample shows the highest impedance in this environment. Figure 12c shows the impedance characteristics of the specimens in the high pH solution. The impedance values are higher by an order of magnitude for all the specimens in the 1 M NaOH solution than in low pH solutions. Among Fe–Cr specimens, the Fe–6Cr specimen shows the highest impedance. No direct correlation between Cr content of Fe–Cr alloy, and the impedance magnitude is observed. The HT-9 specimen shows very high impedance in all the pH conditions.
Figure 13 shows the Mott–Schottky plots of Fe–Cr alloys and HT-9 in different pH solutions. The potentiostatically formed passive layers on these specimens showed both n-type and p-type semiconductivity depending on the potential range. This phenomenon indicates that the Fe–Cr alloys contained a duplex layer with an inner p-type layer (Cr-rich oxide or Fe1-xO) and an outer n-type layer (defective Fe2O3 type). The n-type semiconductivity could be attributed to the possible presence of oxygen vacancies in the oxide layer, and the p-type could be assigned to metal cation vacancies [46, 47]. The p-type behavior observed at high anodic potentials (oxygen evolution potential range) could also be attributed to possible formation of an inversion layer with enhanced hole concentration at the surface level due to large upward band bending [48]. In addition, oxygen evolution occurring on the specimen surface could result in further oxidation of M3+ cations to higher valence states that lead to introduction of cation vacancies and p-type conductivity [49]. If p-type semiconductivity is observed at low anodic potentials, it could be associated with the formation of Fe1-xO- or Cr2O3-type oxides that show inherent p-type semiconductivity [50].
Figure 13a shows the Mott–Schottky plots of Fe–Cr alloys in 0.5 M H2SO4 solution. Predominantly, an n-type surface layer is observed on these specimens in the sulfuric acid solution because of the positive slopes. Steeper is the slope of the 1/C2 vs. potential, the lower is the charge carrier density (less defective is the surface layer). The slope of the Mott–Schottky plots increased with the increase in the Cr content, in general. Figure 13b shows the Mott–Schottky plots in the NaH2PO4 solution. Both n-type and p-type behaviors can be noted in this environment. Interestingly, the Fe–6Cr showed predominantly the p-type behavior in the investigated potential range. The exact reason for this behavior is not known at present. One possible reason could be that the n-type layer of the duplex surface layer formed on this specimen became degenerate under the polarization and therefore exhibited only p-type semiconductivity.
Table 6 summarizes the charge carrier density of the passive films of different specimens in different pH solutions. Higher the charge carrier density is, higher is the electrical conductivity and defect concentration which may lead to lower corrosion resistance. The spark plasma sintered specimens showed higher charge carrier density than the wrought HT-9 specimen in all the pH conditions. This could be attributed to inherent porous nature of the bulk specimen and small grain sizes encountered in the spark plasma sintered specimens. Fine grain size promotes faster diffusion (short-circuit diffusion through grain boundaries) of metal that ionized forming more cations. When the cation formation occurs fast under potentiostatic conditions, not enough oxygen anions are available at the interface to form stoichiometric oxide which leads to inherent oxygen vacancies. No direct correlation is observed between the Cr content of Fe–Cr alloys and the charge carrier density.
Conclusions
The main objective of this study was to understand the microstructural evolution and corrosion property of Fe–Cr alloys processed via ball milling and SPS. From the work, the following salient features can be concluded.
-
1.
With increasing Cr content, the powder size increased and powder size distribution became broader. The lattice parameter and lattice strain showed only slight variation with change in Cr content.
-
2.
During SPS for 45 min at 1000 °C and 80 MPa pressure, with increasing Cr content the relative density decreased which is attributed to the increase in powder particle size during ball milling.
-
3.
Kinetics study indicates that in the initial stage of SPS, grain boundary diffusion is the dominant mechanism for the initial stage of consolidation process. Also, with increasing Cr content, it is seen that GB diffusion activation energy is decreasing, and thus in the higher Cr alloy, diffusion starts at lower temperatures compared to their lower-chromium counterparts. Also, the temperature range for the GB diffusion increases with increasing Cr percentages.
-
4.
Corrosion analysis shows that with increasing pH of the solution, the passive layer formation occurs faster in the wrought HT-9 compared to the spark plasma sintered materials with even higher chromium contents.
-
5.
Though at lower pH solution Fe–6Cr and Fe–9Cr alloys start passivating at longer time, at higher pH the passivation rate becomes almost similar for all the alloys
-
6.
The passive layers formed under potentiostatic conditions on these specimens exhibited both n-type and p-type semiconductivity.
-
7.
The spark plasma sintered specimens showed higher charge carrier density than the wrought HT-9 specimen in all the pH conditions.
References
Kohyama A, Hishinuma A, Gelles DS, Klueh RL, Diets W, Ehrich K (1996) Auger spectroscopy study of the stress enhanced impurity segregation in a Cr–Mo–V steel. J Nucl Mater 233–237:138–147
Garner FA, Toloczko MB, Sencer BH (2000) Comparison of swelling and irradiation creep behavior of fcc-austenitic and bcc-ferritic/martensitic alloys at high neutron exposure. J Nucl Mater 276:123–142
Klueh RL, Harries DH (2014) High-chromium ferritic and martensitic steel for nuclear applications. ASTM, West Conshohocken
Rajan KS (2014) Selection of materials for reactor internals, school of chemical and biotechnology. SASTRA University. www.nptel.ac.in/courses/103106101/Module%20-%204/Lecture%20-%202.pdf. Accessed 14 Sept 2018
Suryanarayana C (2001) Mechanical alloying and milling. Prog Mater Sci 46:1–184
Chen C-L, Dong Y-M (2011) Effect of mechanical alloying and consolidation process on microstructure and hardness of nanostructured Fe–Cr–Al ODS alloys. Mater Sci Eng A 528:8374–8380
Suryanarayana C, Ivanov E, Boldyrev VV (2001) The science and technology of mechanical alloying. Mater Sci Eng A 304–306:151–158
Fogagnolo JB, Velasco F, Robert MH, Torralba JM (2003) “Effect of mechanical alloying on the morphology, microstructure and properties of aluminum matrix composite powders. Mater Sci Eng A 342:131–143
Zawrah M, Shaw L (2003) Microstructure and hardness of nanostructured Al/Fe/Cr/Ti alloys through mechanical alloying. Mater Sci Eng A 355:37–49
Juarez R, Sunol JJ, Berlanga R, Bonastre J, Escoda L (2007) The effects of process control agents on mechanical alloying behavior of a Fe–Zr based alloy. J Alloys Compd 434–435:472–476
Oksiuta Z, Baluc N (2009) Effect of mechanical alloying atmosphere on the microstructure and Charpy impact properties of an ODS ferritic steel. J Nucl Mater 386–388:426–429
Wang XL, Wang GF, Zhang KF (2010) Effect of mechanical alloying on microstructure and mechanical properties of hot-pressed Nb–16Si alloys. Mater Sci Eng A 527:3253–3258
Guillon O (2014) Field-assisted sintering technology/spark plasma sintering: mechanisms, materials, and technology developments. Adv Eng Mater 16:830–849
Leuven KU, SPS process modeling. https://www.mtm.kuleuven.be/Onderzoek/Ceramics/research/sintering/spark-plasma
Suárez M, Fernández A, Menéndez JL, Torrecillas R, Kessel HU, Hennicke J, Kirchner R, Kessel T (2013) Challenges and opportunities for spark plasma sintering: a key technology for a new generation of materials in Sintering applications, Burcu Ertuğ, IntechOpen. https://doi.org/10.5772/53706
Schonbein CF, Faraday M (1836) On a peculiar voltaic condition of iron. Philos Mag 9(53–57):122–123
Oblonsky LJ, Davenport AJ, Ryan MP, Isaacs HS, Newman RC (1997) In situ X-ray absorption near edge structure study of the potential dependence of the formation of the passive film on iron in borate buffer. J Electrochem Soc 144:2398–2404
Toney MF, Davenport AJ, Oblonsky LJ, Ryan MP, Vitus CM (1997) Atomic structure of the passive oxide film formed on iron. Phys Rev Lett 79:4282–4285
Oblonsky LJ, Ryan MP, Isaacs HS (1998) In situ determination of the composition of surface films formed on Fe–Cr alloys. J Electrochem Soc 145:1922–1932
Schroeder V, Devine T (1999) Surface enhanced Raman spectroscopy study of the galvanostatic reduction of the passive film of iron. J Electrochem Soc 146:4061–4070
Virtanen S, Schmuki P, Buechler M, Isaacs HS (1999) Electrochemical behavior of Fe in phosphate solutions studied by in situ X-ray absorption near edge structure. J Electrochem Soc 146:4087–4094
Oblonsky LJ, Ryan MP, Isaacs HS (2000) In situ XANES study of the formation and reduction of the passive film formed on Fe in acetate solution. Corros Sci 42:229–241
Schmuki P, Virtanen S, Davenport AJ, Vitus CM (1996) Transpassive dissolution of Cr and sputter-deposited Cr oxides studied by in situ X-ray near-edge spectroscopy. J Electrochem Soc 143:3997–4005
Betova I, Bojinov M, Englund A, Fabricius G, Laitinen T, Makela K, Saario T, Sundholm G (2001) Contact electric impedance and resistance studies of the conduction mechanism in passive films on ferrous alloys. Electrochim Acta 46:3627–3640
Li S, Wang Y, Dai X, Liu F, Li J, Wang X (2016) Evaluation of hardening behaviors in ion-irradiated Fe–9Cr and Fe–20Cr Alloys by nanoindentation technique. J Nucl Mater 478:50–55
Ono K, Arakawa K, Shibasaki H, Kurata H, Nakamichi I, Yoshida N (2004) Release of helium from irradiation damage in Fe–9Cr ferritic alloy. J Nucl Mater 329–333:933–937
Heintze C, Hernandez-Mayoral M, Ulbricht A, Bergner F, Shariq A, Weissgarber T, Frelinghaus H (2012) Nanoscale characterization of Fe–9%Cr model alloys compacted by spark plasma sintering. J Nucl Mater 428:139–146
Liu J, Chen W (2018) Microstructure, mechanical properties and corrosion behavior of an Fe–10Cr–2.7B–5.5Al–13Mn alloy prepared by spark plasma sintering. J Alloys Compd 741:348–359
Kgoete FM, Popoola API, Fayomi OSI (2018) Influence of spark plasma sintering on microstructure and corrosion behaviour of Ti–6Al–4 V alloy reinforced with micron-sized Si3N4 powder. Def Technol 14:403–407
Marnier G, Keller C, Noudem J, Hug E (2014) Functional properties of a spark plasma sintered ultrafine-grained 316L steel. Mater Des 63:633–640
Zakeri M, Nazari A (2012) Neural Comput. Appl. 21:S723
Nelson JB, Riley DP (1945) An experimental investigation of extrapolation methods in the derivation of accurate unit-cell dimensions of crystals. Proc Phys Soc 57:160–177
Williamson GK, Hall WH (1953) X-ray line broadening from filed aluminium and wolfram. Acta Metall 1:22–31
Schmauder S, Kohler C (2011) Atomistic simulations of solid solution strengthening of α-iron. Comput Mater Sci 50(4):1238–1243
Chu XTA, Ta BN, Ngo LTH, Do MH, Nguyen PX, Nam DNH (2016) Microwave absorption properties of iron nanoparticles prepared by ball-milling. J Electron Mater 45:2311–2315
Totten GE, Xie L, Funatani K (2003) Handbook of mechanical alloy design. Marcel Dekker, New York
Young WS, Cutler IB (1970) Initial sintering with constant rates of heating. J Am Ceram Soc 53:659–663
Guyon J, Hazotte A, Monchoux JP, Bouzy E (2013) Effect of powder state on spark plasma sintering of TiAl alloys. Intermetallics 34:94–100
Pasebani S, Charit I, Butt DP, Cole JI, Wu Y, Burns J (2016) Sintering behavior of lanthana-bearing nanostructured ferritic steel consolidated via spark plasma sintering. Adv Eng Mater 18(2):324–332
Frost HJ, Ashby MF (1977) Fundamental aspects of structural alloy design. Plenum, New York
Lee JS, Choi JP, Lee GY (2013) Consolidation of hierarchy-structured nanopowder agglomerates and its application to net-shaping nanopowder materials. Materials 6:4046–4063
Reartes Garciela B, Morando Pedro J, Blesa Miguel A (1995) Reactivity of chromium oxide in aqueous solutions. 2. Acid dissolution. Langmuir 11:2277–2284
Toshev Y, Mandova V, Boshkov N, Stoychev D, Petrov P, Tsvetkova N (2006) Protective coating of zinc and zinc alloys for industrial applications. In: International conference on multi-material micro manufacture Grenoble, France
Hoppe HW, Haupt S, Strehblow HH (1994) Combined surface analytical and electrochemical study of the formation of passive layers on Fe/Cr alloys in 1 M NaOH. Surf Interface Anal 21:514–525
Burstein GT, Davis DH (1981) Reactions of scratched copper electrodes in aqueous solutions. J Electrochem Soc 128(33):2270–2276
Sanchez M, Gregori J, Alonso C, Garcia-Jareno JJ, Takenouti H, Vicente F (2007) Electrochemical impedance spectroscopy for studying passive layers on steel rebars immersed in alkaline solutions simulating concrete pores. Electrochim Acta 52:7634–7641
Scharrifi-Asl S, Mao F, Lu P, Kursten B, Macdonald DD (2015) Exploration of the effect of chloride ion concentration and temperature on pitting corrosion of carbon steel in saturated Ca(OH)2 solution. Corros Sci 98:708–715
Williamson J, Isgor OB (2016) The effect of simulated concrete pore solution composition and chlorides on the electronic properties of passive films on carbon steel rebar. Corros Sci 106:82–95
Wijayantha KGU, Saremi-Yarahmadi S, Peter LM (2011) Kinetics of oxygen evolution at α-Fe2O3 photoanodes: a study by photoelectrochemical impedance spectroscopy. Phys Chem Chem Phys 13:5264–5270
Carter CB, Norton MG (2013) Ceramic materials science and engineering, 2nd edn. Springer, New York, p 556
Acknowledgements
The work presented here is partly supported by the funding received from a Laboratory Directed Research & Development (LDRD) Program of the Idaho National Laboratory. The authors gratefully acknowledge Dr. Thomas Williams for his help with the XRD work and Nathan Jerred for his help with spark plasma sintering.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kundu, A., Shrestha, N., Korjenic, A. et al. A study on microstructural evolution and corrosion behavior of spark plasma sintered Fe–Cr alloy system. J Mater Sci 54, 14171–14188 (2019). https://doi.org/10.1007/s10853-019-03861-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03861-6