Abstract
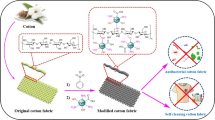
Silver nanoparticles (Ag NPs) have been deposited on the surface of cotton fabrics by a facile two-step method involving liquid precipitation and dielectric barrier discharge (DBD) cold plasma reduction. X-ray diffraction, X-ray photoelectron spectroscopy and high-resolution TEM show that Ag exists only in the metallic state form on the surface of the cotton fibers after DBD plasma treatment, demonstrating that DBD plasma is a promising technique for metallic oxide in situ reduction. The Ag NPs-modified cotton fabrics exhibit the significant antibacterial property against both Escherichia coli and Staphylococcus aureus, which have potential applications in the healthcare and medicine fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The traditional method to kill bacteria is mainly by the use of antibiotic drugs. However, the abuse of antibiotics has aroused many healthy problems [1, 2], especially the emergence of multidrug-resistant bacteria which has become one of the most severe public health threats worldwide [3]. Thus, in the past few decades, the development of different kinds of potent antibacterial and skin-innoxious fabrics has attracted considerable attention from researchers [4, 5]. Cotton fabrics are widely used in human daily life as well as in medical and healthcare fields due to their unique properties, such as water-absorbing, flexible, renewable and biodegradable [6, 7], but some drawbacks hinder the widespread application of cotton fabrics in medicine and healthcare; for example, the adherence of microorganisms to cotton fabrics possibly leading to severe infectious diseases [8]. Different methods of surface modification are used for the production of customized cotton fabrics with special properties, such as water repellency [9], self-cleaning [10], UV-blocking [11], as well as antibacterial effects [8, 12,13,14,15].
Ag nanoparticles (Ag NPs) have been acknowledged as effective antimicrobial agents [3], which are innoxious [16], stable in extreme environments and have a broad antimicrobial spectrum [17,18,19]. Compared with Ag ions and salts, Ag NPs can continuously release Ag+ and maintain the Ag ion concentration for a longtime [20,21,22,23]. Apart from that, Ag NPs can have a direct effect on the bacterial membrane. It was reported that Ag NPs can concentrate on the surface of bacterial membranes, subsequently breaking the balance of cell permeability, thereby resulting in the disruption of bacterial membranes [3]. When bacteria are exposed to Ag NPs, they will be influenced by both Ag+ and Ag NPs [20]. Therefore, Ag NPs have a better and longer acting antimicrobial effect than its ionic state.
Different techniques have been studied to prepare antibacterial fabrics decorated by functional nanoparticles [23]. Methods like chemical reduction [24], photodegradation deposition [25] and in situ biosynthesis [19] have been reported for preparing antibacterial fabrics with Ag NPs. However, chemical reducers, harsh reaction conditions, or long time-consuming reactions involved in the methods as mentioned above limit the large-scale production of antibacterial fabrics.
Hydrogen cold plasma under dielectric barrier discharge (DBD) is a promising technique employed for materials modification [26]. The DBD device contains two plane-parallel metal electrodes covered by a dielectric layer, respectively, and a proper electrodes gap width to ensure stable plasma operation, as shown in Fig. 1b. The working gas flows in the gap, and the discharge can be ignited by means of a high voltage alternating current power source [27]. The high reducibility of the H radicals or atoms produced in the plasma makes this method especially effective in the deoxidization of metal ions. It has been successfully used in reducing Pt- and Co-based catalysts [28] and for reducing metal oxides on the surface of TiO2 [29, 30]. H2 DBD plasma technique has the unique advantages of simplicity, mild reaction conditions and fast reaction rate. Taking our early research as an example, metallic oxides, like Ag oxide, could be totally reduced to their metallic state by H2 DBD plasma method at room temperature and atmospheric pressure in only a few seconds [31].
However, the application of this technique in textile modification is still in its infancy. In this paper, a facile and low-cost method used for the rapid preparation of Ag NPs-functionalized cotton fabrics has been studied. The surface morphology, physical and chemical structure, as well as Ag element content of cotton fabrics before and after treatment were investigated. The antibacterial properties of cotton fabrics after treatment were also studied.
Materials
In the present experiment, commercially available cotton fabrics (average fiber diameter 20 μm, area 5 cm × 5 cm) were used. The fabrics were ultrasonically cleaned with ethanol solution for 20 min and then washed repeatedly with distilled water to remove the impurities and dried in air before use. AgNO3 (AR) and ammonium hydroxide (AR) were obtained from Sinopharm Chemical Reagent Co., Ltd. All of the chemical reagents were used without further purification.
Precipitation reaction and reduction of Ag NPs by H2/Ar DBD plasma
The Ag NPs-modified cotton fabrics were prepared by a conventional impregnation method. Typically, the cotton fabric samples were immersed into AgNO3 solution and 0.3 M ammonium hydroxide solution was added dropwise into the solution with magnetic stirring at room temperature. The samples changed from white to dark due to the deposition of AgOH particles [part (a) of Fig. 1]. The resulting samples were subsequently dried at 60 °C for 30 min, followed by a DBD plasma treatment in a homemade reactor to in situ synthesize Ag NPs [part (b) of Fig. 1]. Before the DBD plasma treatment, the working gas (a mixture of Ar and H2 gases with the volume ratio of 1:1) was introduced into the reactor at a flow rate of 50 mL/min for 1 min to purge the reactor. The duration of the plasma treatment was limited to 5 s with the flow rate controlled at 30 mL/min. The input power was 22.5 W. The concentrations of AgNO3 in the cotton fabrics were variously 0.1 wt%, 0.15 wt%, 0.2 wt%, 0.25 wt% and 0.3 wt%, and the corresponding samples obtained were denoted as Ag1, Ag2, Ag3, Ag4 and Ag5, respectively.
Characterization
X-ray diffraction (XRD) patterns of the samples were obtained via a D/Max2550pc X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) at a scan rate of 4°/min in the range 10°–80°. X-ray photoelectron spectroscopy (XPS) was used to investigate the chemical changes arising out of the plasma treatment, using a VG ESCALAB MARK II spectrometer with an Mg Kα (1253.6 eV) X-ray source, and collected spectral data were calibrated by the C 1s peak at 284.6 eV. Field emission scanning electron microscopy (FESEM, Hitachi SU-70) was used to characterize the microstructures of the cotton fibers and Ag NPs particles. Energy dispersive spectroscopy (EDS) was performed on the SEM. The sample morphology was observed by transmission electron microscopy (TEM, JEOL-2000EX) and high-resolution transmission electron microscopy (HRTEM) at a 200 kV accelerating voltage.
Bacterial strains
The antibacterial activity of cotton fabric with Ag nanocomposites was tested against two bacterial strains viz. Escherichia coli (ATCC 11229) and Staphylococcus aureus (ATCC 6538). E. coli belongs to gram-negative bacteria, while S. aureus belongs to gram-positive bacteria. The strains were preserved in liquid nitrogen, kindly provided by Professor Wang Shoufeng, Department of Basic Medical Sciences, College of Medicine, Zhejiang University.
Antibacterial experiment
In this experiment, to determined minimum inhibitory concentration (MIC) of the functional cotton fabrics [32, 33], a serial dilution protocol was carried out. Under aseptic conditions, different concentrations of antibacterial agents in sterilized test tubes were mixed with a same volume bacterial solution (either E. coli or S. aureus) having a bacterial liquid concentration of about 5 × 105 CFU/mL standard. After mixing, these test tubes were placed in an incubator at 37 °C for 24 h. A test tube having only growth media and bacteria served as a control. For this test, 5 mg to 25 mg weights of functional cotton fabrics were immersed in 3 mL of bacterial solution. The density of the suspensions of the respective microorganisms was adjusted to 0.5 McFarland turbidity standards. The minimum concentration of the compound which inhibited the growth of the respective organisms was considered as the MIC.
Results and discussion
The XRD patterns for all Ag-deposited cotton fabric samples are shown in Fig. 2. The diffraction patterns exhibited essentially correspond to the pristine cotton fabric, and for all samples, no diffraction peaks for Ag were identified, which is likely due to the small weight percentage of Ag and its good dispersion on the surface of the cotton fibers.
X-ray photoelectron spectroscopy (XPS) was utilized to characterize the surface states of all samples. As shown in Fig. 3a, peaks identified as C, O, and Ag are present, verifying the existence of metallic Ag. To investigate the chemical composition of the cotton fabric samples, high-resolution XPS analyses of C 1s and Ag 3d were performed. For the peak assignments, for C1 (C–H or C–C bonds) located at 285.0 eV, the values of 286.7 eV and 288.1 eV were reported for a typical cellulose C 1s signature consisting of carbons with one or two bonds to oxygen (C2: C–O bonds and C3: O–C=O bonds) in the literature [34]. For the C1 s spectrum in Fig. 3b, the spectra of all samples contained these peaks mentioned above. In Fig. 3c, the Ag 3d spectrum exhibited two contributions, namely, 3d5/2 and 3d3/2 (resulting from the spin–orbit splitting), located at, respectively, 368.6 eV and 374.6 eV, and the spin energy separation was 6 eV, which indicated that Ag existed in its metallic state [31]. It should be noted that for the peaks located at low binding energies in all treated samples (Ag1 to Ag5), the chemical groups were introduced by the H2 DBD plasma treatment.
Figure 4a shows that these cotton fibers had typical diameters of about 20 μm, and the pristine cotton fabric presented a smooth surface, while the other samples after treatment exhibited different surface morphologies, with the formation of spherical or octahedral Ag NPs with a grain size of about 0.5 μm, as well as particles at the nanoscale. As the concentration of AgNO3 increased, more Ag NPs were deposited, and even assembled on the surface of the cotton fibers, indicating that the structure and amount of Ag NPs were greatly affected by the solvents.
Once the cotton fabric was immersed into the AgNO3 solution, ion exchange interaction and complexation between silver ions and chemical groups (–OH and –COOH) would take place on the surface of the cotton fabrics, and silver ions were bonded to the surface of the cotton fibers, introducing sites for precipitation of Ag2O [24].
During the period of DBD plasma treatment, according to Fig. 5, when a potential difference applied between two electrodes, electrons will gain energy and accelerate [35], which can give rise to collisions with heavy particles, e.g., ionization (“gas breakdown”) and excitation (producing Ar* and H2*) [36]. Therefore, in the plasma system, there were abundant excited H radicals or atoms with high reducibility. The Ag NPs were in situ synthesized on the surface of the cotton fabrics. For the present experiments, the whole process can mainly be explained as following reactions:
where e* is the electrons with high energy, and H2* and Ar* the excited state of H2 and Ar.
In Fig. 6c, the SEM images and corresponding EDX mapping diagrams of sample Ag3 revealed that the elemental Ag was well distributed on the surface of the cotton fibers, likewise suggesting the good distribution of Ag NPs. The actual amounts of Ag NPs in the different samples were estimated from the EDS data, as shown in Table 1, which are consistent with the observations from SEM.
TEM and high-resolution TEM tests were carried out to investigate the morphology and size of the Ag NPs. The Ag NPs were collected from sample Ag3 after ultrasonic processing for 20 min. Different morphologies of Ag NPs were observed, as shown in Fig. 7. Particles with a grain size of about 0.5 μm are in accordance with the SEM observations. Furthermore, Fig. 7b, c showed Ag NPs with lattice fringes of 0.235 nm that could be well indexed to the (111) plane of Ag. The Ag NPs with a size of about 10 nm had two possible forms of existence. They could either attach on the Ag NPs with larger grain size, or distribute on the surface of the cotton fibers. The results show that Ag+ ions were sufficiently reduced to Ag0 by H2 DBD plasma in only 5 s. Thus, this technique turns out to be a promising method to prepare metallic nanoparticles on the surface of fabrics.
Further strong ultrasonic processing was adopted to quantify the durability of Ag NPs. Morphologies of Ag NPs for sample Ag2 after strong ultrasonic processing (JP-020, 40 kHz, 120 W, Skymen Cleaning Equipment Co., Ltd) were show in Fig. 8a–c. According to Table 2, the contents of Ag element after different processing time were estimated by the EDS analysis. The content of Ag element was reduced rapidly at first, but with prolonging ultrasonic treatment time, the content was almost constant. The decline might result from the collapse and shedding of large particles, as shown in Fig. 8d. However, Ag NPs at nanoscale were the main factors impacting antibacterial effect. Even after 30-min ultrasonic processing, there were Ag NPs well dispersed on the fabrics, which indicated that Ag NPs with large particle size broke into smaller particles with excellent adhesion.
Minimum inhibitory concentration (MIC) measurements of the cotton fabric samples with Ag NPs were carried out to determine quantitatively the corresponding antibacterial activity. The MIC is defined as the minimum concentration of the samples that inhibits visible growth of the tested bacteria [37]. Both bacterial strains were incubated with different concentrations of samples for 24 h at 37 °C. It was observed that the cotton fabrics deposited with Ag NPs have a significant activity against the tested bacteria.
In this experiment, minimum inhibitory concentrations of the cotton fabrics and their corresponding Ag NPs for all samples are listed in Table 3. All samples showed full inhibition against E. coli and S. aureus except sample Ag1 due to its extremely low Ag content. The observed difference in sensitivity between E. coli and S. aureus possibly arose from the cell wall components. Compared with S. aureus, the cell wall of E. coli consists of an outer membrane with lipids, proteins and lipopolysaccharides, which could act as a protective layer. As a result, these samples were more positive against S. aureus [38]. Minimum inhibitory concentrations also varied for different samples, and increased from sample Ag2 to Ag5. The antimicrobial mechanism of Ag NPs has been explained in many literatures [3, 39,40,41]. It is widely accepted that silver ions play a exceedingly important role in antibacterial agents and that small Ag NPs with a higher superficial area and faster rate of Ag+ release than large Ag NPs would cause more serious damage to microorganisms. In this experiment, the sample Ag2 with the lowest minimum inhibitory concentration exhibited the best antibacterial property among all samples, due to its large surface area and fewer nanoclusters. However, particles observed with a scale of 0.5 μm were not conducive to antibacterial ability, which might cause a relatively higher MIC compared to the results of some other literature values [42, 43].
Conclusions
The present study offers a green, two-step, in situ method for preparing cotton fabrics decorated with different contents of Ag NPs, and the obtained samples showed certain antibacterial properties against both Escherichia coli and Staphylococcus aureus. Atmospheric pressure DBD cold plasma treatment was applied to fabric modification for the first time, to reduce the silver precursor prepared by liquid phase deposition into the metallic state. Our findings suggest that DBD cold plasma has promising applications for the in situ reduction in metallic oxides. It should be noted that this study has concentrated primarily on the unique advantages of the DBD plasma technique for surface modification, and further research on other details, such as the synthesis of size-adjustable and shape-controllable Ag NPs, will be summarized in our next study.
References
Bruce RL, Daniel ER (2006) Non-inherited antibiotic resistance. Nat Rev Microbiol 4:556–562
Davies J (1994) Inactivation of antibiotics and the dissemination of resistance genes. Science 264:375–382
Zheng K, Setyawati MI, Leong DT, Xie JC (2018) Antimicrobial silver nanomaterials. Coord Chem Rev 357:1–17
Durán N, Marcato PD, Souza GIHD, Alves OL, Esposito E (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 3:203–208
Ilana P, Guy A, Nina P, Geoffrey G, Serguei M, Aharon G (2008) Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology. https://doi.org/10.1088/0957-4484/19/24/245705
Brandt A, Grasvik J, Hallett JP, Welton T (2013) Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem 15:550–583
Park CH, Kang YK, Im SS (2010) Biodegradability of cellulose fabrics. J Appl Polym Sci 94:248–253
Li Z, Chen J, Cao W, Wei D, Zheng A, Guan Y (2018) Permanent antimicrobial cotton fabrics obtained by surface treatment with modified guanidine. Carbohydr Polym 180:192–199
Yu M, Gu G, Meng WD, Qing FL (2007) Superhydrophobic cotton fabric coating based on a complex layer of silica nanoparticles and perfluorooctylated quaternary ammonium silane coupling agent. Appl Surf Sci 253:3669–3673
Wang R, Wang X, Xin JH (2010) Advanced visible-light-driven self-cleaning cotton by Au/TiO2/SiO2 photocatalysts. ACS Appl Mater Int 2:82–85
Xin JH, Daoud WA, Kong YY (2004) A new approach to UV-blocking treatment for cotton fabrics. Text Res J 74:97–100
El-Shafei AM, Fouda MMG, Knittel D, Schollmeyer E (2008) Antibacterial activity of cationically modified cotton fabric with carboxymethyl chitosan. J Appl Polym Sci 110:1289–1296
Elnaggar ME, Shaheen TI, Zaghloul S, Elrafie MH, Hebeish AJI (2016) Antibacterial activities and UV protection of the in situ synthesized titanium oxide nanoparticles on cotton fabrics. Ind Eng Chem Res 55:2661–2668
Perelshtein I, Lipovsky A, Perkas N, Gedanken A, Moschini E, Mantecca P (2015) The influence of the crystalline nature of nano-metal oxides on their antibacterial and toxicity properties. Nano Res 8:695–707
Gao D, Lyu L, Lyu B, Ma J, Yang L, Zhang J (2017) Multifunctional cotton fabric loaded with Ce doped ZnO nanorods. Mater Res Bull 89:102–107
Lee HJ, Jeong SH (2005) Bacteriostasis and skin innoxiousness of nanosize silver colloids on textile fabrics. Text Res J 75:551–556
Ye J, Cheng H, Li H, Yang Y, Zhang S, Rauf A, Zhao Q, Ning GJ (2017) Highly synergistic antimicrobial activity of spherical and flower-like hierarchical titanium dioxide/silver composites. J Colloid Interface Sci 504:448–456
Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5:916–924
Shaheen TI, Abd El Aty AA (2018) In-situ green myco-synthesis of silver nanoparticles onto cotton fabrics for broad spectrum antimicrobial activity. Int J Biol Macromol 118:2121–2130
David E, Elizabeth MG, Courtney S, Sabber H (2018) Silver nanoparticle antibacterial efficacy and resistance development in key bacterial species. Biomed Phys Eng Express. https://doi.org/10.1088/2057-1976/aad5a7
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK et al (2007) Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol 3:95–101
Lalueza P, Monzón M, Arruebo M, Santamaría J (2011) Bactericidal effects of different silver-containing materials. Mater Res Bull 46:2070–2076
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83–96
Rehan M, Mashaly HM, Mowafi S, El-Kheir AA, Emam HE (2015) Multi-functional textile design using in situ Ag NPs incorporation into natural fabric matrix. Dyes Pigment 118:9–17
Rehan M, Barhoum A, Assche GV, Dufresne A, Gätjen L, Wilken R (2017) Towards multifunctional cellulosic fabric: UV photo-reduction and in situ synthesis of silver nanoparticles into cellulose fabrics. Int J Biol Macromol 98:877–886
Liu C, Li M, Wang J, Zhou X, Guo Q, Yan J, Li Y (2016) Plasma methods for preparing green catalysts: current status and perspective. Chin J Catal 37:340–348
Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P (2006) Atmospheric pressure plasmas: a review. Spectrochim Acta Part B 61:2–30
Kim SS, Lee H, Na BK, Song HK (2004) Plasma-assisted reduction of supported metal catalyst using atmospheric dielectric-barrier discharge. Catal Today 89:193–200
Di L, Xu Z, Wang K, Zhang X (2013) A facile method for preparing Pt/TiO2 photocatalyst with enhanced activity using dielectric barrier discharge. Catal Today 211:109–113
Di L, Xu Z, Zhang X (2013) Atmospheric-pressure cold plasma for synthesizing Ag modified Degussa P25 with visible light activity using dielectric barrier discharge. Catal Today 211:143–146
Cheng X, Dong P, Huang Z, Zhang Y, Chen Y, Nie X, Zhang X (2017) Green synthesis of plasmonic Ag nanoparticles anchored TiO2 nanorod arrays using cold plasma for visible-light-driven photocatalytic reduction of CO2. J CO2 Util 20:200–207
Tahir K, Ahmad A, Li B, Nazir S, Khan AU, Nasir T, Khan ZUH, Naz R, Raza M (2016) Visible light photo catalytic inactivation of bacteria and photo degradation of methylene blue with Ag/TiO2 nanocomposite prepared by a novel method. J Photochem Photobiol B Biol 162:189–198
Dobrucka R, Dlugaszewska J (2015) Antimicrobial activities of silver nanoparticles synthesized by using water extract of Arnicae anthodium. Indian J Microbiol 55:168–174
Tenhunen TM, Lewandowska AE, Orelma H, Johansson LS, Virtanen T, Harlin A, Österberg M, Eichhorn SJ, Tammelin T (2018) Understanding the interactions of cellulose fibres and deep eutectic solvent of choline chloride and urea. Cellulose 25:137–150
Raizer Y, Braun C (1992) Gas discharge physics. Appl Opt 31:2400–2401
Bogaerts A, Neyts E, Gijbels R, Mullen V (2002) Gas discharge plasmas and their applications. Spectrochim Acta Part B 57:609–658
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16
Prasad TNVKV, Kambala VSR, Naidu R (2011) A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr Nanosci 7:531–544
Jiang B, Tian C, Song G, Pan Q, Wang Z, Shi L, Qiao Y, Fu H (2012) A green route to synthesize novel Ag/C antibacterial agent. Mater Res Bull 47:458–463
Zaki S, Elkady MF, Farag S, Abd-El-Haleem D (2012) Determination of the effective origin source for nanosilver particles produced by Escherichia coli strain S78 and its application as antimicrobial agent. Mater Res Bull 47:4286–4290
Zhang W, Xiao B, Fang T (2018) Chemical transformation of silver nanoparticles in aquatic environments: mechanism, morphology and toxicity. Chemosphere 191:324–334
Li S, Wang QT, Yu HQ, Ben T, Xu HJ, Zhang JC, Du QY (2018) Preparation of effective Ag-loaded zeolite antibacterial materials by solid phase ionic exchange method. J Porous Mater 25:1797–1804
Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloid Surf B Biointerfaces 76:50–56
Acknowledgements
This work was supported by the opening foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases and Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Medical College, Zhejiang University, Grant No. 2017KF08, and the National Natural Science Foundation of China No. 50772098. The authors also gratefully acknowledge Associate Professor Wang Shoufeng, Department of Basic Medical Sciences, College of Medicine, Zhejiang University, Hangzhou, China, for providing the bacterial strains.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, Z., Wang, S., Yang, F. et al. A two-step preparation method for nanocrystalline Ag-decorated cotton fabrics and their antibacterial assessment. J Mater Sci 54, 10447–10456 (2019). https://doi.org/10.1007/s10853-019-03637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03637-y