Abstract
Poor charge transfer and separation rate are the major bottlenecks for the activity and stability of BiVO4 photoanode. Here, we introduced oxygen vacancies into MoO3/BiVO4 heterojunction film by post-annealing the film in argon-saturated environment for improving its photoelectrochemical (PEC) water oxidation activity and stability. In comparison with the normal MoO3/BiVO4 film, the MoO3/BiVO4 film with oxygen vacancies is of better PEC water oxidation performance. Specifically, a higher photocurrent density of 4.1 mA/cm2 in 0.1 M Na2SO4 at 1.1 V versus SCE was achieved on the MoO3/BiVO4 film with oxygen vacancies, which is about 200% improved over the normal MoO3/BiVO4 film (1.83 mA cm−2, at 1.1 V versus SCE). In addition, the MoO3/BiVO4 film with oxygen vacancies shows more stable activity and faster kinetics for water oxidation, without significant activity loss for 5 h reaction at 1.23 V versus RHE. The enhanced performance on such a MoO3/BiVO4 film photoanode can be attributed to that the oxygen vacancies accelerate the charge transfer and separation rate between film/electrolyte interface, and thus improve the water oxidation activity and restrain the anodic photocorrosion simultaneously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, BiVO4 has been widely studied as one of the most promising photoanode materials for photoelectrochemical (PEC) water splitting [1]. The advantages of BiVO4 for PEC water splitting can be generalized as follows: First, BiVO4 is of suitable band gap (~ 2.4 eV); thus, it can absorb about 11% light from the solar spectrum [2]. Second, the valence band of BiVO4 is located at a more positive potential (~ 2.40 V versus RHE) than the water oxidation potential (1.23 V versus RHE), allowing for solar water oxidation [3]. And third, the raw materials for the production of BiVO4 photoanode are in abundance and of low cost. Under standard AM 1.5 G solar light irradiation, a theoretical photocurrent of 7.6 mA/cm2 can be obtained on BiVO4 photoanode with a high solar-to-hydrogen conversion efficiency \( (\eta_{\text{STH}} ) \) of 9.3% [4, 5], very close to the \( \eta_{\text{STH}} \) of commercial requirement for PEC water splitting (10%). Unfortunately, the actual water oxidation activity on BiVO4 photoanode was impeded by its poor charge transport and separation property [1, 6]. In addition, the photostability of BiVO4 was suffered from anodic photocorrosion that involves the loss of V5+ ions from BiVO4 lattice by dissolution [7]. Therefore, the modifications of BiVO4 activity and stability are the forefront of PEC water splitting field.
In general, the charge transport property of BiVO4 is related to its composition and morphology, and the separation property of BiVO4 was affected by its surface feature. Accordingly, composition and morphology tuning are developed as two effective strategies in recent years for addressing the charge transport issue of BiVO4. Typically, W and Mo co-substituting the partial sites of V in BiVO4 that could improve the conductivity of BiVO4 and introduce polarons into BiVO4 lattice, and thus, enhanced bulk charge migration property is observed on W and Mo co-doped BiVO4 photoanode [8, 9]. Furthermore, proper oxygen evolution catalysts or photocatalyst couplings have been demonstrated to be effective approaches for improving the charge separation property of BiVO4. For example, FeOOH or Co-Pi (Cobalt-Phosphate) coated on BiVO4 is capable of enhancing the separation of photo-generated holes and electrons on BiVO4 surface [10, 11]. Owing to the matched band potentials between WO3 and BiVO4, WO3/BiVO4 heterojunction photoanode is of higher charge separation efficiency through the physical separation of surface charge [12]. On the other side, BiVO4 is not thermodynamically stable enough against photocorrosion, which is the primary cause of BiVO4 anodic photocorrosion. In the work of Bard et al., an inert layer of amorphous TiO2 coupled on BiVO4 film shows the function of protecting BiVO4 from photocorrosion via changing the oxidation state at BiVO4/electrolyte interface [13]. Additionally, Choi et al. reported the use of a V5+-saturated electrolyte that can inhibit the photooxidation-coupled dissolution of BiVO4 photoanode [7]. Significantly, the degree of BiVO4 anodic photocorrosion critically depends on the relative rate of photocorrosion compared with the rates of interfacial charge transfer and surface charge separation [7, 10]. It reveals that when the BiVO4 photoanode has faster interfacial charge transfer rate or surface charge separation rate than its photocorrosion rate, the BiVO4 anodic photocorrosion can be kinetically suppressed. In summary, both the PEC activity and stability of BiVO4 are determined by its charge transfer and separation properties. It is noteworthy that the reported works mainly focus on the modification of individual property of BiVO4 (activity or stability), few regard to the improvements of PEC activity and stability simultaneously.
Both experimental and calculational works have revealed that oxygen vacancies can effectively modulate the electronic properties of photocatalysts, and thus improve their bulk charge transport and surface separation efficiency [14]. Normal BiVO4 is of poor PEC activity due to its low carrier mobility of ~ 0.044 cm2/V s [15], while BiVO4 with oxygen vacancies shows much higher PEC activity [16], indicating higher carrier mobility in BiVO4 with oxygen vacancies. As remarkable charge transfer material, MoO3 with oxygen vacancies possesses high carrier mobility up to 1100 cm2/V s [17]. Interestingly, the band potentials between MoO3 and BiVO4 are favorable to separate the surface charge of BiVO4 [18]. Inspired by this information, MoO3/BiVO4 heterojunction photoanode with oxygen vacancies is expected to have high water oxidation activity and stability. The bulk charge transport in BiVO4, surface charge separation on BiVO4, and the charge transfer and separation between BiVO4 and MoO3 interface all can be improved by the oxygen vacancies effect in theory.
Herein, oxygen vacancies were introduced into the MoO3/BiVO4 heterojunction film by post-annealing the film in argon-saturated environment. Subsequently, the MoO3/BiVO4 film with oxygen vacancies was investigated as photoanode for water oxidation. Due to the existence of oxygen vacancies in MoO3 and BiVO4, a high photocurrent density of 4.1 mA/cm2 was achieved on the MoO3/BiVO4 photoanode in 0.1 M Na2SO4 at 1.1 V versus SCE under irradiation of simulated solar light (100 mW/cm2). Additionally, the MoO3/BiVO4 film photoanode with oxygen vacancies shows high stability for water oxidation, without significant loss of photoactivity for 5 h reaction.
Experimental section
Material synthesis
The MoO3/BiVO4 heterojunction film with and without oxygen vacancies was prepared using a drop-casting method on FTO glass substrate as described in our previous work with annealing modifications [18, 19]. Firstly, Bi(NO3)3.5H2O (Aladdin, 99.0%) was dissolved in a solvent containing concentrated HNO3 (Aladdin, 68%) and ethylene glycol (EG, Aladdin) to form a Bi precursor solution of 25 mM. NH4VO3 (Sigma-Aldrich, 97%) was dissolved in EG as V precursor solution with a concentration of 25 mM. Molybdenum powder (Aladdin, 99.9%) was firstly dissolved in H2O2 (Aladdin, 30 wt%) and then mixed with EG to form 5 mM Mo precursor solution. Secondly, the V and Bi precursor solutions were mixed with same volume for the preparation of BiVO4 film. 20 μL of mixed precursor solution was dropped on the FTO glass substrate (1.0 cm × 1.5 cm, sheet resistance < 15 Ω) that had been cleaned and sonicated with distilled water and ethanol. Next, the mixed precursor solution on FTO glass was dried at 150 °C for 60 min and then annealed at 500 °C for 120 min in air to form a BiVO4 film. Finally, 10 μL of Mo precursor was dropped on the BiVO4 film and then annealed at 500 °C for 120 min in air to form the MoO3/BiVO4 heterojunction film. For the introduction of oxygen vacancies, the MoO3/BiVO4 heterojunction film was further annealed at 400 °C for 40 min in an argon-saturated environment and then be marked as MoO3/BiVO4(Ar). Based on subsequent PEC measurements, such an annealing condition was regarded as the optimal after investigating various annealing temperatures and time (temperature: 350 °C, 400 °C and 450 °C; time: 20 min, 40 min, 60 min and 100 min). For comparison, the BiVO4 and MoO3 films were treated using the same post-annealing condition in argon-saturated environment and then be marked as BiVO4(Ar) and MoO3(Ar), respectively. In addition, MoO3/BiVO4(O2) film was prepared via post-annealing the MoO3/BiVO4 film in O2-saturated atmosphere at 500 °C for 2 h.
Material characterization
Scanning electron microscopy (SEM, Zeiss Supra 55 VP) and transmission electron microscope (TEM, JEOL, JEM-2010) were used to observe the morphology and microstructure of the as-prepared films. X-ray diffraction (XRD) patterns of the as-prepared films were recorded on a PANalytical X’pert PRO diffractometer equipped with Cu-Ka radiation at a scanning rate of 2°/min. Chemical state and compositions of the as-prepared films were characterized using X-ray photoelectron spectroscopy (XPS, ESCALAB 250, Thermo Scientific). UV–Vis absorption spectrum was collected on a Shimadzu UV-2600/2700 spectrophotometer. The Raman spectrum was measured using a Renishaw in Via Raman microscope with 514.5 nm argon ion laser. The electron paramagnetic resonance (EPR) measurements were taken on Bruker EMX-plus operating in the X-band (9.52 GHz) with a microwave power of 2 mW. The content of V in the electrolyte (0.1 M Na2SO4) that was used for PEC stability testing was measured by inductively coupled plasma technique (ICP, Thermo ICAP6300 Duo).
Electrochemical and photoelectrochemical measurements
The electrochemical and photoelectrochemical measurements were taken in a three-electrode cell (quartz cube cup, 5.0 × 5.0 × 5.0 cm3). The as-prepared films with an area of 1.0 cm2 were used as working electrode. The counter electrode was a Pt wire with a diameter of 0.5 mm (99.99%, CHI Instrument), and the reference electrode was saturated calomel electrode (SCE, Shanghai INESA Scientific Instrument). 0.1 M Na2SO4 was mainly employed as the electrolyte for electrochemical and photoelectrochemical measurements. The pH of electrolyte was checked using a benchtop pH meter (PHS-3C, Shanghai INESA Scientific Instrument). A CH Instruments 660E electrochemical workstation was used for electrochemical and photoelectrochemical measurements.
The irradiation source was a 300 W xenon lamp with AM 1.5G filter (Beijing China Education Au-light Co., Ltd.). For light measurements, the lamp was positioned to provide irradiation of 100 mW/cm2 on the films. The light irradiation power was measured by a thermopile detector (Beijing China Education Au-light Co., Ltd.). The photoelectrochemical measurements of the films were mainly conducted using back-side irradiation (through the FTO glass substrate to the film). IPCE measurements were taken using a full solar simulator (Beijing China Education Au-light Co., Ltd. 300 W xenon lamp) with an AM 1.5 filter and a motorized monochromator (Oriel Corner-stone 130 1/8 m). A typical monochromatic light used for the IPCE measurements can be found in Fig. S8. Light power was measured using a handheld optical power meter with a UV enhanced silicon photodetector (Newport, Models 1916C and 818-UV). The IPCE is expressed by following equation:
where j is the measured photocurrent density at a specific wavelength (mA cm−2), λ is the wavelength of incident light (nm), and Plight is the measured light power density at that wavelength (mW/cm2).
The electrochemical impedance spectroscopy (EIS) was performed in 0.1 M Na2SO4 solution under irradiation (100 mW/cm2) at 1.23 V versus RHE with an AC amplitude of 5 mV, frequency of 10 mHz–100 kHz. Zview software was used to fit the measured EIS spectrum and get the equivalent circuit. To convert the potential (versus SCE) to RHE (NHE at pH 0), the following equation was used.
Mott–Schottky measurements were taken in 0.1 M Na2SO4 solution using an impedance versus applied potential method at a frequency of 1000 Hz. Mott–Schottky plots were created and fitted to ideal semiconductor behavior:
here C is the space charge layer capacitance, ε is the relative dielectric constant, ε0 is the permittivity of free space, e is the elemental charge, A is the surface area of sample, Nd is the concentration of charge carriers, Va is the applied potential, Vfb is the flat band potential, k is Boltzmann constant, and T is temperature.
To investigate whether the photocurrents on MoO3/BiVO4(Ar) film photoanode originated from the water oxidation, the Faraday efficiency of O2 evolution [η(O2)] was measured in a single gas-tight cell at 1.23 V versus RHE. Prior to measurement, the solution (0.1 M Na2SO4) was degassed by bubbling Ar for 0.5 h. The amount of generated O2 was detected using an Agilent 7890B gas chromatograph. The value of [η(O2)] was calculated according to Eq. (4).
Titration of KMnO4 solution measurement
The titration of KMnO4 solution was applied to detect the quantity of H2O2 that formed during PEC water oxidation. Firstly, 1 mM KMnO4 solution was prepared and calibrated with 1 mM Na2C2O4 solution. Secondly, 10 mL of electrolyte (0.1 M Na2SO4) was collected immediately after one hour of PEC stability testing and acidified with 5 mL of 3 M H2SO4, and then titrated with standard KMnO4 solution. Finally, the concentration of formed H2O2 was calculated on the basis of used volume of KMnO4 standard solution. To reduce the experimental error, thrice parallel titration was carried out. The specific reaction equations related to the titration are as follows:
Results and discussion
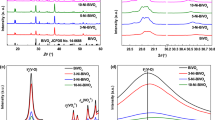
The morphology and microstructure of the as-prepared BiVO4(Ar) and MoO3/BiVO4(Ar) film were observed using SEM and TEM. As shown in Fig. 1a, the BiVO4(Ar) film is composed of randomly oriented nanoflakes and the thickness of film is about 500 nm (inset of Fig. 1a). For the MoO3/BiVO4(Ar) film, well-distributed MoO3 nanoparticles were coupled with the BiVO4 nanoflakes to form MoO3/BiVO4 heterojunctions (Fig. 1b, c). From the SEM image observation for the MoO3/BiVO4 film (shown in Fig. S1), it is found that the film is of pretty similar morphology and microstructure before and after post-annealing in argon-saturated environment. Similar observations have been found in previous works related to photocatalysts with oxygen vacancies [16]. The HRTEM image shown in Fig. 1e reveals the distance of one kind of lattice fringe to be 0.31 nm, which is consistent with the interplanar spacing of the (121) planes of BiVO4, and the other kind of lattice fringe to be 0.39 nm which is consistent with the interplanar spacing of the (200) planes of MoO3. Elemental mapping shows that the MoO3/BiVO4(Ar) film possesses a homogeneous spatial distribution of Bi, V, O, and Mo species (Fig. 1e), which further confirms the successful formation of MoO3/BiVO4 heterojunctions. Figure 1d displays the X-ray diffraction (XRD) pattern of the as-prepared MoO3/BiVO4 and MoO3/BiVO4(Ar) film. As expected, the characteristic diffraction peaks of MoO3 (JCPDS 05-0508) and BiVO4 (JCPDS 14-0688) were observed in both MoO3/BiVO4 and MoO3/BiVO4(Ar) XRD patterns. In addition, a diffraction peak at 31.64° indexed to M9O26 (JCPDS 05-0441) was observed in the XRD patterns. It is not surprising to find the characteristic diffraction peak of M9O26 in MoO3, because MoO3 is a typical non-stoichiometric oxide [17, 20]. Significantly, the diffraction peak of M9O26 is more obvious in the XRD pattern of MoO3/BiVO4(Ar) film compared with that in the XRD pattern of MoO3/BiVO4 film, indicating higher proportion of M9O26 in MoO3/BiVO4(Ar) film. Similar result was also observed in the XRD pattern of the MoO3(Ar) film that post-annealed in argon-saturated environment (shown in Fig. S2), hinting oxygen deficit is more obvious in the post-annealed film.
The existence of oxygen vacancies in MoO3/BiVO4(Ar) film was characterized by Raman, XPS and EPR technique. In the Raman spectra (Fig. 2a), the peak around 210 cm−1 is attributed to the external mode of BiVO4, while the peaks around 330 and 365 cm−1 are assigned to the asymmetric and symmetric deformation modes of the VO43− tetrahedron (δas(VO43−) and δs(VO43−)), respectively [21]. Furthermore, a peak around 825 cm−1 was observed, which corresponds to anti-symmetric stretching of V-O. It is noteworthy that the peak location of anti-symmetric V-O stretching for MoO3/BiVO4(Ar) film was shifted to higher wave number (825 cm−1) relative to the MoO3/BiVO4 film (823 cm−1). In the previous reports related to BiVO4 with oxygen vacancies, similar change on the peak of anti-symmetric V-O stretching was observed [16, 22], suggesting the existence of oxygen vacancies in MoO3/BiVO4(Ar) film. Additionally, the Bi 4f and V 2p peaks in the XPS spectrum of MoO3/BiVO4(Ar) film both show shift to lower binding energy compared with those in MoO3/BiVO4 film (Fig. 2b, c). Such changes can be attributed to the introduction of oxygen vacancies that results in partial reduction of Bi3+ and V5+ ions. [14] Simultaneously, a shift of the Mo 3d peaks to lower binding energy was observed in the XPS spectrum of MoO3/BiVO4(Ar) film (Fig. 2d), confirming the presence of oxygen vacancies in MoO3. Furthermore, the MoO3/BiVO4(Ar) film was further characterized by EPR spectroscopy to detect the presence of oxygen vacancies in the film. As recorded in Fig. 2e, the EPR spectrum for MoO3/BiVO4 film and MoO3/BiVO4 (Ar) film both shows a signal center at g = 1.978, which is consisted to the g value for paramagnetic V4+ [23].
However, the EPR signal of MoO3/BiVO4 (Ar) film shows markedly increased than that of MoO3/BiVO4 film, confirming the presence of oxygen defects in MoO3/BiVO4(Ar) film [24]. According to the XRD patterns, Raman, XPS and EPR spectrum, we can conclude that the oxygen vacancies were successfully introduced into MoO3/BiVO4 film by post-annealing the film in argon-saturated atmosphere.
To optimize the PEC activity of MoO3/BiVO4(Ar) film, different post-annealing temperatures and time were investigated in the argon-saturated atmosphere. The LSV measurements indicate that the MoO3/BiVO4 film post-annealed at 400 °C for 40 min in argon-saturated atmosphere is of the optimal PEC water oxidation activity (shown in Fig. S3a and S3b). Thus, this post-annealing condition was used to treat the MoO3/BiVO4 film for subsequent investigation.
Figure 3a, b shows the LSV and i-t curves of the MoO3/BiVO4 film with and without oxygen vacancies that measured in 0.1 M Na2SO4 under AM 1.5G irradiation. Both under front and back irradiation, the MoO3/BiVO4(Ar) film shows enhanced photocurrent density. A higher photocurrent density of 4.10 mA cm−2 is achieved on the MoO3/BiVO4(Ar) under back irradiation at 1.1 V versus SCE, which is over 200% enhancement compared with the MoO3/BiVO4 film (1.83 mA cm−2) at the same condition. The Faraday efficiency of O2 evolution on MoO3/BiVO4(Ar) film electrode is close to 100%, indicating that the photocurrent on the film is mainly originated from the PEC water oxidation (shown in Fig. S4). In addition, the onset potential of photocurrent was negatively shifted on the MoO3/BiVO4(Ar) film (0.03 V versus SCE) compared with the MoO3/BiVO4 film (0.23 V versus SCE), revealing its faster water oxidation kinetics. For comparison and confirmation, the PEC water oxidation activity on MoO3/BiVO4(O2) film that was prepared via post-annealing the MoO3/BiVO4 film in O2 atmosphere at 500 °C for 2 h was investigated. In general, the MoO3/BiVO4(O2) film is of similar activity as the MoO3/BiVO4 film (see in Fig. S3d), both lower than that of the MoO3/BiVO4(Ar) film. Besides, higher photocurrent was observed on the MoO3 and BiVO4 film with oxygen vacancies (Fig. S5), further demonstrating the positive effect of oxygen vacancies on the water oxidation activity of photoanode. To clarify the role of oxygen vacancies, the charge separation and injection efficiency of MoO3/BiVO4(Ar) and MoO3/BiVO4 film were quantificationally evaluated based on the LSV measurements in a hole scavenger (Na2SO3) containing solution (see Fig. S6) and the absorbed photon flux of films (see Fig. 5c). In comparison with the MoO3/BiVO4 film, higher charge separation and injection efficiency were achieved on the MoO3/BiVO4(Ar) film (Fig. 3c, d). The charge separation and injection efficiency on the MoO3/BiVO4(Ar) film are, respectively, 34.5% and 72.0% at 1.23 V versus RHE, while those on the MoO3/BiVO4 film are 30.0% (separation efficiency) and 46.0% (injection efficiency), respectively. It is generally accepted that the charge-injection efficiency of photoanode is related to its surface reaction kinetics. Comparatively speaking, the charge-injection efficiency for the MoO3/BiVO4 film with oxygen vacancies is particularly improved, indicating that the oxygen vacancies have more obvious influence on the water oxidation kinetics of MoO3/BiVO4 film.
The influence of oxygen vacancies on the PEC water oxidation kinetics of MoO3/BiVO4 photoanode was investigated by electrochemical impedance spectroscopy (EIS). As shown in Fig. 4a, two slightly depressed semicircles are observed in the Nyquist plots that measured on the MoO3/BiVO4 and MoO3/BiVO4(Ar) film electrode under irradiation. It is well known that the arc of semicircle in this typical Nyquist plot is relevant to the charge separation and transfer kinetics at electrode/electrolyte interface [25]. The semicircle arcs of MoO3/BiVO4 (Ar) film electrode are much smaller than those of MoO3/BiVO4 film electrode, suggesting faster charge separation and transfer kinetics during PEC water oxidation. The equivalent circuit in the light of measured impedance data was shown in Fig. 4b. In the equivalent circuit, Rs represents the resistance of solution, Rss is the surface state resistance related to the charge transfer from the valence band or conduction band to the surface of semiconductor electrode [25], Rsc is the space charge separation resistance [18], and CPEss and CPEsc are the constant phase elements for the electrolyte/electrode interface and electrode surface, respectively. The fitted values of Rsc and Rss for the MoO3/BiVO4(Ar) film electrode are 30.45 Ω and 186.5 Ω, respectively, which are much lower than those for the MoO3/BiVO4 film electrode (Rsc: 49.21Ω; Rss: 894.9Ω, shown in Table 1). The EIS result demonstrates that the MoO3/BiVO4 film with oxygen vacancies has faster interfacial charge transfer and separation rate during PEC water oxidation. Figure 4c shows the Mott–Schottky plots for the MoO3/BiVO4 and MoO3/BiVO4(Ar) film electrode in 0.1 M Na2SO4 under 100 mW/cm2 simulated solar light irradiation. Positive slope was observed in the Mott–Schottky plots for both MoO3/BiVO4 and MoO3/BiVO4(Ar) film electrode, showing typical feature of n-type semiconductor. In comparison with the MoO3/BiVO4 film electrode, the MoO3/BiVO4(Ar) film electrode shows a lower slope in its Mott–Schottky plot. From the SEM observations, we know that the MoO3/BiVO4 film both with and without oxygen vacancies has similar morphology and microstructure (see Fig. S1). Under the same measurement conditions, the slope of Mott–Schottky plot is inversely proportional to the charge carrier density of film electrode (see the Mott–Schottky equation in Experimental section) [26, 27]. Accordingly, it can infer that the MoO3/BiVO4 film with oxygen vacancies is of higher charge density than the normal MoO3/BiVO4 film under irradiation.
a Nyquist plots of the MoO3/BiVO4 and MoO3/BiVO4(Ar) film electrode. The EIS measurements were performed in 0.1 M Na2SO4 at 1.23 V versus RHE under 100 mW/cm2 simulated solar light illumination. The solid line in Fig. 4a is the fitted data from Zview software using the proposed equivalent circuit model. b An equivalent circuit for the film electrode. c Mott–Schottky plots for the MoO3/BiVO4 and MoO3/BiVO4(Ar) film electrode in 0.1 M Na2SO4 at a frequency of 1000 Hz under AM 1.5G illumination
The above results suggest that the introduction of oxygen vacancies improves the PEC water oxidation activity of MoO3/BiVO4 film obviously. To investigate the relation between the PEC activity and the wavelength of the incident light quantitatively, incident photon-to-current conversion efficiency (IPCE) measurements were taken for the films. Figure 5a is the IPCE spectrum for the MoO3/BiVO4 and MoO3/BiVO4(Ar) film that measured in 0.1 M Na2SO4 at 1.23 V versus RHE. For both the MoO3/BiVO4 and MoO3/BiVO4(Ar) films, the photoresponse ranges are observed from 320 to 520 nm, which are consistent with their UV–Vis absorption spectrum (Fig. 5b). However, the MoO3/BiVO4(Ar) shows higher IPCE than the MoO3/BiVO4 film, further confirming their PEC activity difference. The photocurrent density of MoO3/BiVO4 and MoO3/BiVO4(Ar) film was, respectively, integrated to 0.78 mA/cm2 and 2.02 mA/cm2 using their IPCE spectrum data, which are close to the photocurrent value from their LSV results (the data shown in Table S1). Significantly, the MoO3/BiVO4 film has similar optical absorption behavior regardless of oxygen vacancies (Fig. 5b). Based on the absorbed photon flux spectra (Fig. 5c), the total absorbed photocurrents of MoO3/BiVO4 and MoO3/BiVO4(Ar) film are integrated to be 5.91 mA cm−2 and 6.01 mA cm−2, respectively. Combined with the PEC activity results and optical absorption spectra, it can be concluded that the dramatically enhanced PEC water oxidation activity on MoO3/BiVO4(Ar) film cannot be caused by the slight difference of light absorption.
For the water oxidation on photoanode, the stability is an important parameter to estimate the performance of photoanode. Figure 6a shows the PEC stability curve of the MoO3/BiVO4 and MoO3/BiVO4(Ar) film in 0.1 M Na2SO4 at 1.23 V versus RHE. For the MoO3/BiVO4 film, the photocurrent density drops from 0.63 mA cm−2 to 0.14 mA cm−2 after 10000 s of irradiation. The PEC activity decay of MoO3/BiVO4 film is mainly ascribed to the anodic photocorrosion that originated from the photooxidation-coupled dissolution of V5+ on the BiVO4 film. As a contrast, stable photocurrent (~ 2.0 mA cm−2) was obtained on the MoO3/BiVO4(Ar) film under a longer irradiation time of 18000 s. It is necessary to point out that the PEC activity on MoO3/BiVO4 (Ar) film shows decreased trend when in excess of 5 h reaction. However, about 90% PEC activity can be recovered by re-annealing the MoO3/BiVO4 (Ar) film at 400 °C for 40 min in argon-saturated atmosphere (MoO3/BiVO4 (Ar) 2nd cycle in Fig. 6a). After the PEC stability testing, the concentration of V in Na2SO4 solution which was used as electrolyte for PEC stability testing was detected by ICP technique. As shown in Table 2, the concentration of V is 3.28 μM in the Na2SO4 solution which was used for the MoO3/BiVO4 film stability testing, confirming that the attenuation of PEC activity is initiated by V5+ dissolving. A lower V concentration of 0.90 μM was detected in the MoO3/BiVO4(Ar) film that used Na2SO4 solution. Additionally, we further detected the concentration of H2O2 that may be formed through a two-electron pathway on the films during PEC stability testing [28]. After 3600 s of PEC stability testing, 10 mL of 0.1 M Na2SO4 electrolyte was sampled immediately for KMnO4 titration to detect the concentration of H2O2. The concentration of H2O2 from the electrolyte that was used for MoO3/BiVO4 film PEC stability testing is 6.53 μM, and for the MoO3/BiVO4(Ar) film is 2.24 μM (Table 3), revealing weaker H2O2 formation on the MoO3/BiVO4(Ar) film and thus weaker anodic photocorrosion. Actually, the anodic photocorrosion rate of BiVO4 was influenced by its interfacial charge transfer and surface recombination rate [7, 10]. The degree of BiVO4 anodic photocorrosion can be kinetically suppressed through the improvement of its interfacial charge transfer rate. Combined with PEC results above (the charge separation and injection efficiency and EIS), it can be reasonably inferred that the MoO3/BiVO4 film with oxygen vacancies is capable of restraining the anodic photocorrosion of BiVO4, because of its improved interfacial charge transfer and surface charge separation rate.
The flat band potential of MoO3(Ar) and BiVO4(Ar) was, respectively, determined to be 0.56 V versus RHE and 0.06 V versus RHE by Mott–Schottky measurement (shown in Fig.S7a and 7b). Based on the semi-empirical theory that the conduction band potential of n-type semiconductors is often more negative by about 0.1 V than its flat band potential [29], the conduction band potential of MoO3(Ar) and BiVO4(Ar) is inferred to be 0.46 V versus RHE and -0.04 V versus RHE, respectively. Meanwhile, the band gap of MoO3(Ar) film is determined to be 3.05 eV (see Fig.S7c and 7d). On the basis of results obtained above, a possible mechanism for the improved PEC water oxidation performance on the MoO3/BiVO4(Ar) film was proposed (Fig. 6b). For the MoO3/BiVO4 film with oxygen vacancies, effective heterojunctions are formed at MoO3-x/BiVO4-x interface through their physical coupling and band potentials matching. Under irradiation with solar light, the photo-generated electrons and holes on BiVO4-x surface are orderly separated and transferred. Specifically, the electrons on BiVO4 surface are transferred to MoO3 due to the conduction band potential difference between BiVO4 and MoO3, while the holes physically stayed on BiVO4 for driving the water oxidation reaction. Duo to the presence of oxygen vacancies in MoO3 and BiVO4, the interfacial charge transfer and separation rate were accelerated. As a result, the PEC water oxidation activity on MoO3/BiVO4(Ar) film was improved as well as the anodic photocorrosion was restrained.
Conclusions
In summary, oxygen vacancies were introduced into the MoO3/BiVO4 film photoanode to improve its PEC water oxidation activity and stability. In comparison with MoO3/BiVO4 film, a higher photocurrent density of 4.1 mA/cm2 was achieved on the MoO3/BiVO4 film with oxygen vacancies at 1.1 V versus SCE in 0.1 M Na2SO4. In addition, the MoO3/BiVO4 photoanode with oxygen vacancies shows higher water oxidation stability, without significant loss of photoactivity for 5 h reaction. The enhanced performance on such a MoO3/BiVO4 film can be attributed to that the oxygen vacancies accelerate the charge transfer and separation rate between film/electrolyte interface and thus improve the PEC water oxidation activity and restrain the anodic photocorrosion.
References
Tan HL, Amal R, Ng YH (2017) Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: a review. J Mater Chem A 5:16498–16521
Jia Q, Iwashina K, Kudo A (2012) Facile fabrication of an efficient BiVO4 thin film electrode for water splitting under visible light irradiation. Proc Natl Acad Sci 109:11564–11569
Walsh A, Yan Y, Huda MN, Al-Jassim MM, Wei SH (2009) Band edge electronic structure of BiVO4: elucidating the role of the bis and Vd orbitals. Chem Mater 21:547–551
Kim TW, Choi KS (2014) Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343:990–994
Chen Z, Jaramillo TF, Deutsch TG, Kleiman-Shwarsctein A, Forman AJ, Gaillard N, Garland R, Takanabe K, Heske C, Sunkara M (2010) Accelerating materials development for photoelectrochemical hydrogen production: standards for methods, definitions, and reporting protocols. J Mater Res 25:3–16
Zhao Z, Li Z, Zou Z (2011) Electronic structure and optical properties of monoclinic clinobisvanite BiVO4. Phys Chem Chem Phys 13:4746–4753
Lee DK, Choi KS (2018) Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat Energy 3:53–60
Park HS, Kweon KE, Ye H, Paek E, Hwang GS, Bard AJ (2011) Factors in the metal doping of BiVO4 for improved photoelectrocatalytic activity as studied by scanning electrochemical microscopy and first-principles density-functional calculation. J Phys Chem C 115:17870–17879
Luo W, Yang Z, Li Z, Zhang J, Liu J, Zhao Z, Wang Z, Yan S, Yu T, Zou Z (2012) Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ Sci 4:4046–4051
Seabold JA, Choi KS (2012) Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst. J Am Chem Soc 134:2186–2192
Abdi FF, Krol R (2012) Nature and light dependence of bulk recombination in Co-Pi catalyzed BiVO4 photoanodes. J Phys Chem C 116:9398–9404
Rao PM, Cai L, Liu C, Cho IS, Lee CH, Weisse JM, Yang P, Zheng X (2014) Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett 14:1099–1105
Eisenberg D, Ahn HS, Bard AJ (2014) Enhanced photoelectrochemical water oxidation on bismuth vanadate by electrodeposition of amorphous titanium dioxide. J Am Chem Soc 136:14011–14014
Wang G, Yang Y, Han D, Li Y (2017) Oxygen defective metal oxides for energy conversion and storage. Nano Today 13:23–39
Abdi FF, Savenije TJ, May MM, Dam B, van de Krol R (2013) The origin of slow carrier transport in BiVO4 thin film photoanodes: a time-resolved microwave conductivity study. J Phys Chem Lett 4:2752–2757
Wu JM, Chen Y, Pan L, Wang P, Cui Y, Kong DC, Wang L, Zhang X, Zou JJ (2018) Multi-layer monoclinic BiVO4 with oxygen vacancies and V4+ species for highly efficient visible-light photoelectrochemical applications. Appli Catal B Environ 221:187–195
Balendhran S, Deng J, Ou JZ, Walia S, Scott J, Tang J, Wang KL, Field MR, Russo S, Zhuiykov S, Strano MS, Medhekar N, Sriram S, Bhaskaran M, Kalantar-zadeh K (2013) Enhanced charge carrier mobility in two-dimensional high dielectric molybdenum oxide. Adv Mater 25:109–114
He H, Zhou Y, Ke G, Zhong X, Yang M, Bian L, Lv K, Dong F (2017) Improved surface charge transfer in MoO3/BiVO4 heterojunction film for photoelectrochemical water oxidation. Electrochim Acta 257:181–191
He H, Berglund SP, Rettie A, Chemelewski W, Xiao P, Zhang Y, Mullins CB (2017) Synthesis of BiVO4 nanoflake array films for photoelectrochemical water oxidation. J Mater Chem A 24:9371–9379
Bouzidi A, Benramdane N, Tabet-Derraz H, Mathieu C, Khelifa B, Desfeux R (2003) Effect of substrate temperature on the structural and optical properties of MoO3 thin films prepared by spray pyrolysis technique. Mater Sci Eng, B 97:5–8
Yoon H, Mali MG, Choi JY, Kim MW, Choi SK, Park H, Al-Deyab SS, Swihart MT, Yarin AL, Yoon SS (2015) Nanotextured pillars of electrosprayed bismuth vanadate for efficient photoelectrochemical water splitting. Langmuir 31:3727–3737
Tan H, Suyanto A, De Denko AT, Saputera WH, Amal R, Osterloh FE, Ng Y (2017) Enhancing the photoactivity of faceted BiVO4 via annealing in oxygen-deficient condition. Part Part Syst Charact 34:1600290
Zapart MB, Zapart W, Wyslocki B, Zhukov AP (1988) EPR of V4+ ions in SbVO4. Ferroelectrics 80:55–58
Zheng JY, Lyu HY, Xie C, Wang RL, Tao L, Wu HB, Zhou HJ, Jiang SP, Wang SY (2018) Defect-enhanced charge separation and transfer within protection layer/semiconductor structure of photoanodes. Adv Mater 30:1801773
Bohra D, Smith WA (2015) Improved charge separation via Fe-doping of copper tungstate photoanodes. Phys Chem Chem Phys 17:9857–9866
Parmar KPS, Kang HJ, Bist A, Dua P, Jang JS, Lee JS (2012) Photocatalytic and photoelectrochemical water oxidation over metal-doped monoclinic BiVO4 photoanodes. Chemsuschem 5:1926–1934
Ma M, Zhang K, Li P, Jung MS, Jeong MJ, Park JH (2016) Dual oxygen and tungsten vacancies on a WO3 photoanode for enhanced water oxidation. Angew Chem Int Ed 128:11998–12002
Fuku K, Miyase Y, Miseki Y, Funaki T, Gunji T, Sayama K (2017) Photoelectrochemical hydrogen peroxide production from water on a WO3/BiVO4 photoanode and from O2 on an Au cathode without external bias. Chem Asian J 12:1111–1119
Matsumoto Y, Omae M, Sato E, Watanabe I (1986) Photoelectrochemical properties of the Zn-Ti-Fe spinel oxides. J Electrochem Soc 133:711–716
Acknowledgements
The authors acknowledge National Basic Research Program of China (973 Program: 2014CB846003), National Natural Science Foundation of China (41702037), Sichuan Science and Technology Program (2017JY0146 and 2018JY0462), Research Fund of Southwest University of Science and Technology (15zx7104 and 15zx7123).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Yang, M., Du, J. et al. MoO3/BiVO4 heterojunction film with oxygen vacancies for efficient and stable photoelectrochemical water oxidation. J Mater Sci 54, 671–682 (2019). https://doi.org/10.1007/s10853-018-2863-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2863-6