Abstract
In recent years, interfacial doping with other atoms, molecules, and nanoparticles in molybdenum disulfide (MoS2) has been proven as a new route to explore the potential application of 2D materials in microelectronical devices. In this paper, we utilized a one-step chemical vapor deposition approach to synthesize monolayer MoS2(1−x)Se2x nanosheets in atmospheric pressure using MoO3, S, and Se powders as precursors. AFM and visible-light microscopy showed that the as-grown nanosheets were single layers, their surface was atomic flat, and the maximum grain size was over 100 μm. XPS characterization demonstrated that the concentration of selenium in MoS2(1−x)Se2x nanosheets was affected by the amount of selenium powder in the doping process. The back-gate FETs were fabricated to investigate the electrical properties of monolayer MoS2(1−x)Se2x nanosheets with different Se contents. The field effect properties of MoS2(1−x)Se2x (x = 0.65) transistors indicated that a moderate mobility was achieved, and ohmic contact was obtained at the interface of the MoS2(1−x)Se2x channel and metal electrodes. Characterization using high-resolution transmission electron microscopy showed that the microstructure of as-grown MoS2(1−x)Se2x (x = 0.65) had a regular hexagonal lattice structure, which revealed that it was a single-crystalline two-dimensional material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a member of two-dimensional transition metal dichalcogenides (TMDs) [1, 2], molybdenum disulfide (MoS2) has been widely studied due to its unique material properties [3,4,5,6]. Unlike graphene, which has no bandgap, molybdenum disulfide is a semiconductor and its bandgaps are around 1.2–1.8 eV depending on the number of layers. When MoS2 is decreased from few layers to a single layer (around 0.65 nm), MoS2 will change from an indirect semiconductor to a direct semiconductor [7, 8]. Due to its outstanding physical and chemical properties, MoS2 has great prospects in the fields of microelectronics [9, 10], optoelectronics [11, 12], and energy storage [13,14,15]. In order to achieve the desired physical and chemical properties of MoS2, some progress has been made in experimental and theoretical calculation of molybdenum disulfide doping [16,17,18,19]. Dolui et al. investigated the impact of substituted elements on the characteristics of MoS2 by first-principles calculations [19]. Other studies have reported that the bandgap was clearly modulated when substituted elements such as Se or W were introduced during the preparations of MoS2 [20,21,22,23]. However, there are still many major challenges in preparing the MoS2(1−x)Se2x alloy. For example, it is still hard to synthesize large-area and layer-controlled MoS2(1−x)Se2x nanosheets. The effect of the Se/S source ratio on the concentration and uniformity of substituted selenium in the samples has not been fully investigated. In addition, the performance of electrical devices fabricated by MoS2(1−x)Se2x is still not optimal.
Here, we reported the synthesis of large-area MoS2(1−x)Se2x monolayers by one-step atmospheric pressure chemical vapor deposition (APCVD). During the preparation process, the ratio of selenium and sulfur source not only determined the elemental composition of the samples, but also affected the grain size of the MoS2(1−x)Se2x monolayers. The content and distribution of selenium in the triangular MoS2(1−x)Se2x domains were characterized by XPS and EDX spectra. The central positions and intensities of Raman peaks corresponding to MoS2(1−x)Se2x nanosheets with different Se contents clearly demonstrated variation of vibration modes in the alloy. High-resolution transmission electron microscopy (HRTEM) images demonstrated that as-grown samples were single crystals. To measure the electrical properties, synthesized MoS2(1−x)Se2x nanosheets were used as channels in field effect transistors. With different Se content, these exquisite devices showed promising electrical performances. Our research provides the possibility for the application of MoS2(1−x)Se2x in the micro–nano-electronic field.
Results and discussion
MoS2(1−x)Se2x nanosheets were grown on SiO2/Si substrates, using a three-temperature-zone tube furnace (Fig. 1a). Due to the different melting points, selenium powders and sulfur powders were placed separately in different upstream regions. During the experiment, sulfur and selenium vapor reacted with MoO3 after being transported by the carrier gas in downstream zone III to where the substrates were located. Here, the carrier gas was a mixture gas of hydrogen and argon (8% H2). As a reduction agent, the introduced H2 could convert MoO3 to MoO3-x, which facilitated the further reaction (see Supporting Information (SI): SI-1).
The images from the visible-light microscope in Fig. 1b–f showed the isolated triangular domains of MoS2(1−x)Se2x nanosheets, which ranged in size from a few microns to more than 100 μm. The grain size of the samples was closely related to the ratio of selenium and sulfur powders. The total amount of selenium and sulfur powders used in the experiments was 20 mg. As the ratio of selenium powder increased, the size of MoS2(1−x)Se2x triangular domains clearly decreased (Fig. 1b–f). The major reason for this phenomenon is that the chemical activity of sulfur is stronger than selenium, and thus crystallization of the MoS2(1−x)Se2x nanosheets is more difficult at the Se-rich reaction process compared to the S-rich atmosphere [24,25,26]. Topography of MoS2(1−x)Se2x nanosheets was characterized by an atomic force microscope (AFM). The triangular domain in Fig. 1g had a homogenous contrast and no obvious particles, which revealed that the surface of the prepared sample was clean and atomic flat. The white curve in Fig. 1g was the cross-sectional scan of the sample (marked by a red line), and its height was ~ 0.8 nm, indicating that the MoS2(1−x)Se2x nanosheet was a single layer [27]. In the process of MoS2(1−x)Se2x growth, the amount of hydrogen flow was the main factor to obtain these monolayer samples (see SI: Fig. S1 and Fig. S2).
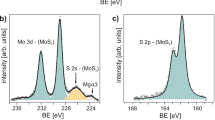
The chemical states and Se contents in MoS2(1−x)Se2x nanosheets were confirmed by X-ray photoelectron spectroscopy (XPS). Figure 2a showed that the S 2p had two peaks at ~ 162.8 and ~ 164.0 eV, which were attributed to S 2p3/2 and S 2p1/2, respectively. The binding energy of Se 3p3/2 was observed at ~ 161.2 eV. According to the above-mentioned results, x in MoS2(1−x)Se2x could be calculated by the following formula:
where IS and ISe were the areas under the peaks of S 2p3/2 and Se 3p3/2, respectively; and FS and FSe represented the relative symmetric factors for S 2p3/2 (0.4453) and Se 3p3/2 (0.8493), respectively [28]. It was notable that x in MoS2(1−x)Se2x went up as the ratio of selenium powders rose during the experiments (Fig. 2b). With increase in the Se content in MoS2(1−x)Se2x nanosheets, the peak of Se 3p3/2 was obvious, while the intensities of S 2p3/2 and 2p1/2 became weak and begin to disappear. The peaks at ~ 229.7 and ~ 232.8 eV corresponded to Mo4+ 3d5/2 and Mo4+ 3d3/2, respectively (Fig. 2c), in agreement with previously reported values [7]. There was no obvious peak of Mo6+ 3d5/2 at ~ 236 eV, indicating that MoO3 was completely reduced to MoS2(1−x)Se2x. The doublet peaks of Se 3d5/2 and Se 3d3/2 were evident at ~ 55.2 and ~ 56.1 eV, as long as selenium was contained in the nanosheets (Fig. 2d) [29].
Raman spectroscopy was an effective method to analyze the phonon vibration mode properties of the TMDs. While MoS2(1−x)Se2x was produced from APCVD, the two sets of composition-dependent peaks were obtained in the Raman spectra [30], corresponding to an MoSe2-related feature at low frequency (200–300 cm−1) and an MoS2 typical feature at high frequency (350–410 cm−1) (Fig. 3a). There were two typical peaks of MoS2 centered at ~ 401.1 cm−1 (E 12g ) and ~ 380.5 cm−1(A1g), which were attributed to in-plane vibration of S atoms and out-of-plane vibration of S and Mo atoms, respectively. The inset in Fig. 3b showed the schematic of two vibration modes. These MoS2-like modes shifted to low frequency by increasing the Se content in MoS2(1−x)Se2x. This result suggested that the introduction of Se atoms distorted the Mo-S bonds and softened the vibrations of E 12g and A1g modes of MoS2. As the Se atoms occupied the parts of sulfur sites, MoSe2-like peaks of monolayer MoS2(1−x)Se2x appeared and became noticeable between 200 and 300 cm−1. When selenium was completely substituted for sulfur, the out-of-plane A1g mode of MoSe2 was identified at 241.1 cm−1.
The field effect transistors (FETs) were fabricated by electron beam lithography (EBL) to demonstrate the electrical properties of the synthesized MoS2(1−x)Se2x monolayers with different Se contents (x = 0, 0.43, 0.65). 10 nm Ti and 100 nm Au were deposited as the electrodes of FETs by electron beam evaporation and magnetron sputtering, respectively. The Ids−Vgs transfer characteristic curves (Fig. 4a, c, e) suggested that all the MoS2(1−x)Se2x devices exhibited n-type transport behavior. The high Ion/Ioff ratios of ~ 107 were extracted from semilogarithmic coordinates in the inset shown in Fig. 4a, c, e. Carrier mobility μ was calculated by the following formula: [3, 31]
where L and W were channel length of 2 μm and width of 1 μm, respectively; and Cox was the SiO2 gate capacitance, which was estimated to be approximately 1.16 × 10−8 Fcm−2. As Se contents rose from x = 0 to x = 0.65, carrier mobility of devices gradually improved from ~ 0.12 to ~ 3.72 cm2 V−1 s−1. The much lower carrier mobility of x = 0 and x = 0.43 was possibly attributed to the following reasons: first, poor contact existed between MoS2(1−x)Se2x channels and the Au electrodes, causing serious contact resistance. Second, introduced Se caused inevitable lattice distortion or defects in the MoS2(1−x)Se2x samples reducing the efficiency of carrier transport. Third, organic or inorganic impurity derived from the process of fabricating and testing FETs might have resulted in the degradation of device performance. The contact between the channel and electrodes could be characterized by Ids−Vds curves (Fig. 4b, d, f). The contact of x = 0 and x = 0.43 belonged to the Schottky contact (Fig. 4b, d), but when x = 0.65, the linear curves in Fig. 4f indicated that ohmic contact was formed between the MoS2(1−x)Se2x channel and the Au electrodes. The ohmic contact improved the transport of carrier mobility in monolayer MoS2(1−x)Se2x (x = 0.65) FETs. Another reason for obtaining the best electrical properties in x = 0.65 samples was that the bandgap of MoS2(1−x)Se2x decreased with the rise in selenium contents [32], which made it easier for carriers to be exciting from the top of the valence band to the conduction band, thus improving the electrical properties. To further promote the performance of monolayer MoS2(1−x)Se2x FETs, elaborating the high-k top-gating [33] and tuning the contact between channels and electrodes [34] should be considered in future research.
a, c, e The source-drain current to the gate voltage (Ids−Vgs) and b, d, f the source-drain current to the source-drain voltage (Ids−Vds) for FETs in which the MoS2(1−x)Se2x nanosheets were used as channels. a, b for x = 0; c, d for x = 0.43; e, f for x = 0.65. The insets of a, c, e were semilogarithmic coordinates of Ids−Vgs curves and the inset of b was the visible-light microscope image of the FETs
In order to further explore the microstructure of the monolayer MoS2(1−x)Se2x nanosheets (x = 0.65) with optimal electrical properties, transmission electron microscopy (TEM) combined with energy-dispersive X-ray spectroscopy (EDX) was conducted to investigate its lattice structure and element distribution. In the image of atomic-resolution high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM) (Fig. 5a), different atoms could be identified by Z contrast (Z = atomic number) [20]. The Mo sites were brighter than most bi-chalcogen (X2, X = S, Se) sites because the atomic number of Mo (42) was larger than those of Se (34) and S (16). Figure 5a revealed that Se existed in the lattice of MoS2 in the form of substitution doping. Two predominant lattice spacings measured from the high-resolution TEM image (Fig. 5b) were ~ 0.28 nm and ~ 0.16 nm, representing (100) and (110) planes, respectively [35, 36]. This result was consistent with selected area electron diffraction (SAED) patterns (Fig. 5c) which confirmed that the sample was the single crystal of a hexagonal structure [37]. EDX mapping was used to characterize the distribution of the elements in the samples (and EDX spectrum of MoS2(1−x)Se2x could be seen in SI: Fig. S3). The identical and similar triangles of Fig. 5d–f indicated the ideal element uniformity in the as-grown MoS2(1−x)Se2x monolayer. Combined with images of high-resolution TEM and EDX mapping, x = 0.65 of samples had no fatal defects in the lattice arrangement, and the distribution of elements in single samples was homogeneous. Single-crystalline microstructures were important reasons that MoS2(1−x)Se2x (x = 0.65) could obtain distinctive electrical properties.
a HAADF-STEM image of the MoS2(1−x)Se2x nanosheet (x = 0.65). b High-resolution TEM images of the MoS2(1−x)Se2x nanosheet (x = 0.65). c The bright spots in selected area electron diffraction (SAED) pattern reveal that the sample is a single crystal. EDX mappings of d Mo, e S and f Se in the MoS2(1−x)Se2x nanosheet
Conclusion
To summarize, we have synthesized the triangular and uniform domains of MoS2(1−x)Se2x monolayer nanosheets by a simple APCVD approach. The impact of the ratio of selenium and sulfur powder on the size and Se contents of MoS2(1−x)Se2x was investigated in detail. XPS confirmed that the Se contents in MoS2(1−x)Se2x nanosheets increased with increasing selenium powders. The electrical properties of the samples with different selenium contents were also discussed in this paper. The results showed that optimal electrical properties were obtained when the Se concentration of the nanosheets reached the maximum (x = 0.65). According to the microstructure of MoS2(1−x)Se2x (x = 0.65), HRTEM combined with EDX showed that the as-grown nanosheets were single crystals and the distribution of Mo, S and Se was uniform in the triangular domain. This work provides a feasible and convenient method to synthesize high-quality and large-area MoS2(2-x)Se2x monolayers with controllable stoichiometry.
Experimental details
Growth of monolayer MoS2(1−x)Se2x nanosheets
MoS2(1−x)Se2x nanosheets were grown on SiO2/Si substrates, utilizing a three-temperature-zone tube furnace. Sulfur and selenium powders were placed in upstream zone I (150 °C) and zone II (300 °C), respectively, due to their different melting points; the total amount of selenium and sulfur powders was 20 mg. Silicon oxide substrates were immersed in acetone and isopropanol for ultrasonic cleaning for 10 min as pretreatment. SiO2 substrates dried by nitrogen were placed face down on ceramic boats with 10 mg MoO3 powder in downstream zone III. The growth temperature of MoS2(1−x)Se2x in zone III was about 700 °C to 800 °C. The carrier gas was a mixture of argon–hydrogen (8% H2) gas with 65 sccm during growth.
Characterizations of monolayer MoS2(1−x)Se2x nanosheets
The topography and thickness of as-grown MoS2(1−x)Se2x nanosheets were characterized by visible-light microscopy (Olympus) and atomic force microscopy (AFM, 5600LS). X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250Xi) was performed with a monochromatic Al Kα X-ray source to investigate the elemental composition of the MoS2(1−x)Se2x nanosheets. The phonon vibration mode properties of MoS2(1−x)Se2x were characterized by Raman spectrum (ThermoFisher DXR) at 532 nm excitation wavelength. HAADF-STEM (Titan Cubed Themis G2 300) was used to identify the atomic phase of MoS2(1−x)Se2x nanosheets at 100 keV. High-resolution transmission electron microscopy combined with EDX spectrum (Talos F200X) was applied at 200 keV to characterize the crystalline structure of the MoS2(1−x)Se2x nanosheets.
Fabrication and characterization of MoS2(1−x)Se2x FETs
The source and drain electrodes of the monolayer MoS2(1−x)Se2x FETs were fabricated by electron beam lithography. The electrode materials were 10 nm Ti and 100 nm Au deposited by electron beam evaporation and magnetron sputtering deposition, respectively. The back-gate electrodes were fabricated by etching p++ SiO2 substrates with BOE solution. The electrical properties of the FETs were measured using a semiconductor parameter analyzer (Agilent B1500A).
References
Zheng B, Wang Z, Qi F et al (2017) CVD growth of large-area and high-quality HfS2 nanoforest on diverse substrates. Appl Surf Sci 435:563–567
Hu P, Wang L, Yoon M et al (2013) Highly responsive ultrathin GaS nanosheet photodetectors on rigid and flexible substrates. Nano Lett 13:1649–1654
Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A (2011) Single-layer MoS2 transistors. Nat Nanotechnol 6:147–150
Cain JD, Shi F, Wu J, Dravid VP (2016) Growth mechanism of transition metal dichalcogenide monolayers: the role of self-seeding fullerene nuclei. ACS Nano 10:5440
Ling X, Lee Y, Lin Y, Fang W, Yu L, Dresselhaus M, Kong J (2014) Role of the seeding promoter in MoS2 growth by chemical vapor deposition. Nano Lett 14:464–472
Antonelou A, Sygellou GL, Leftheriotis G, Dracopoulos V, Yannopoulos SN (2016) Facile, substrate-scale growth of mono- and few-layer homogeneous MoS2 films on Mo foils with enhanced catalytic activity as counter electrodes in DSSCs. Nanotechnology 27:045404
Eda G, Yamaguchi H, Voiry D, Fujita T, Chen M, Chhowalla M (2011) Photoluminescence from chemically exfoliated MoS2. Nano Lett 11:5111
Feng Y, Zhang K, Wang F et al (2015) Synthesis of large-area highly crystalline monolayer molybdenum disulfide with tunable grain size in a H2 atmosphere. ACS Appl Mater Inter 7:22587–22593
Desai SB, Madhvapathy SR, Sachid AB et al (2016) MoS2 transistors with 1-nanometer gate lengths. Science 354:99–102
Radisavljevic B, Whitwick MB, Kis A (2012) Small-signal amplifier based on single-layer MoS2. Appl Phys Lett 101:66
Tsai DS, Liu KK, Lien DH et al (2013) Few-layer MoS2 with high broadband photogain and fast optical switching for use in harsh environments. ACS Nano 7:3905–3911
Chen X, Yang H, Liu G et al (2018) Hollow spherical nanoshell arrays of 2D layered semiconductor for high-performance photodetector device. Adv Funct Mater 28:1705153
Chhetri M, Gupta U, Yadgarov L, Rosentsveig R, Tennec R, Rao CNR (2015) Beneficial effect of Re doping on the electrochemical HER activity of MoS2 fullerenes. Dalton Trans 44:16399
Cao X, Shi Y, Shi W, Rui X, Yan Q, Kong J, Zhang H (2013) Preparation of MoS2-coated three-dimensional graphene networks for high-performance anode material in lithium-ion batteries. Small 9:3433–3438
Wang Z, Li Q, Xu H et al (2018) Controllable etching of MoS2 basal planes for enhanced hydrogen evolution through the formation of active edge sites. Nano Energy 49:634–643
Du Y, Liu H, Neal AT, Si M, Ye PD (2013) Molecular doping of multilayer MoS2 field-effect transistors: reduction in sheet and contact resistances. IEEE Electron Device Lett 34:1328–1330
Fang H, Tosun M, Seol G, Chang TC, Takei K, Guo J, Javey A (2013) Degenerate n-doping of few-layer transition metal dichalcogenides by potassium. Nano Lett 13:1991–1995
Eshun K, Xiong HD, Yu S, Li Q (2015) Doping induces large variation in the electrical properties of MoS2 monolayers. Solid State Electron 106:44–49
Dolui K, Rungger I, Pemmaraju CD, Sanvito S (2013) Possible doping strategies for MoS2 monolayers: an ab initio study. Phys Rev B 88:4192–4198
Feng Q, Zhu Y, Hong J et al (2014) Growth of large-area 2D MoS2(1−x)Se2x semiconductor alloys. Adv Mater 26:2648–2653
Zhang W, Li X, Jiang T, Song J, Lin Y, Zhu L, Xu X (2015) CVD synthesis of Mo(1−x)WxS2 and MoS2(1−x)Se2x alloy monolayers aimed at tuning the bandgap of molybdenum disulfide. Nanoscale 7:13554–13560
Mann J, Ma Q, Odenthal PM et al (2014) 2-Dimensional transition metal dichalcogenides with tunable direct band gaps: MoS2(1–x)Se2x monolayers. Adv Mater 26:1399–1404
Li H, Zhang Q, Duan X et al (2015) Lateral growth of composition graded atomic layer MoS2(1−x)Se2x nanosheets. J Am Chem Soc 137:5284–5287
Lu X, Utama MIB, Lin J et al (2014) Large-area synthesis of monolayer and few-layer MoSe2 films on SiO2 substrates. Nano Lett 14:2419
Li Y, Zhang K, Wang F, Feng Y, Li Y, Han Y, Tang D, Zhang B (2017) Scalable synthesis of highly crystalline MoSe2 and its ambipolar behavior. ACS Appl Mater Inter 9:36009–36016
Huang JK, Pu J, Hsu CL et al (2014) Large-area synthesis of highly crystalline WSe2 monolayers and device applications. ACS Nano 8:923–930
Feng Q, Mao N, Wu J, Xu H, Wang C, Zhang J, Xie L (2015) Growth of MoS2(1−x)Se2x (x = 0.41–1.00) monolayer alloys with controlled morphology by physical vapor deposition. ACS Nano 9:7450–7455
Kiran V, Mukherjee D, Jenjeti RN, Sampath S (2014) Active guests in the MoS2/MoSe2 host lattice: efficient hydrogen evolution using few-layer alloys of MoS2(1-x)Se2x. Nanoscale 6:12856–12863
Zheng B, Chen Y, Qi F et al (2017) 3D-hierarchical MoSe2 nanoarchitecture as a highly efficient electrocatalyst for hydrogen evolution. 2D Mater 4:025092
Gong Q, Cheng L, Liu C et al (2015) Ultrathin MoS2(1−x)Se2x alloy nanoflakes for electrocatalytic hydrogen evolution reaction. ACS Catal 5:2213–2219
Das S, Chen HY, Penumatcha AV, Appenzeller J (2012) High performance multilayer MoS2 transistors with scandium contacts. Nano Lett 13:100–105
Susarla S, Kutana A, Hachtel JA et al (2017) Quaternary 2D transition metal dichalcogenides (TMDs) with tunable bandgap. Adv Mater 29:1702457
Feng W, Zheng W, Cao W, Hu P (2014) Back gated multilayer InSe transistors with enhanced carrier mobilities via the suppression of carrier scattering from a dielectric interface. Adv Mater 26:6587–6593
Chuang HJ, Chamlagain B, Koehler M et al (2016) Low-resistance 2D/2D ohmic contacts: a universal approach to high-performance WSe2, MoS2, and MoSe2 transistors. Nano Lett 16:1896
Li H, Duan X, Wu X et al (2014) Growth of alloy MoS2xSe2(1−x) nanosheets with fully tunable chemical compositions and optical properties. J Am Chem Soc 136:3756–3759
Dai T, Fan X, Ren Y et al (2018) Layer-controlled synthesis of wafer-scale MoSe2 nanosheets for photodetector arrays. J Mater Sci 53:8436–8444
Li Y, Wang F, Tang D et al (2018) Controlled synthesis of highly crystalline CVD-derived monolayer MoSe2 and shape evolution mechanism. Mater Lett 216:261–264
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2017YFB0405600), Natural Science Foundation of Tianjin City (Grant Nos. 18JCZDJC30500, 17JCYBJC16100, and 17JCZDJC31700), and National Natural Science Foundation of China (Grant Nos. 61404091, 61274113, 61505144, 51502203, and 51502204).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, D., Wang, F., Zhang, B. et al. Field effect properties of single-layer MoS2(1−x)Se2x nanosheets produced by a one-step CVD process. J Mater Sci 53, 14447–14455 (2018). https://doi.org/10.1007/s10853-018-2617-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2617-5