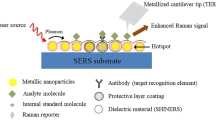
Abstract
In recent years, the unique properties of magnetic functional nanomaterials have received considerable attentions and show promising applications in separation, detection, diagnosis, catalysis, environment remediation and so on. Specifically, introducing magnetic nanomaterials (MNPs) into traditional sensing techniques greatly simplifies detection operation and improves sensing performances, which makes magnetic nanomaterial-based sensing techniques become a hot research topic. Compared with other sensing techniques such as chromatography, fluorescence, mass spectrum and electrochemistry, surface-enhanced Raman scattering (SERS) displays unique properties of high-sensitivity, fingerprint specificity and nondestructive detection. The introduction of MNPs in SERS has proven to be an efficient way to resolve several critical challenges in practical SERS analysis leading to highly efficient target separation and enrichment, high-sensitive detection and precise outcomes analysis. This makes the MNPs involved SERS analysis a powerful technique with very appealing and promising application in various branches of analytical science. In this review, we first briefly introduced the preparation, encapsulation and surface modification of magnetic nanoparticles, assembly of magnetic nanoparticle–plasmonic substrates and then discussed their applications in SERS analysis, including biomedical application, environmental analysis, food safety and chemical reaction monitoring. Finally, we presented some outlooks on further developments of magnetic nanoparticles in SERS applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The past few decades have witnessed the rapid development of analytical science, and there is no doubt that nanomaterials play a vital role in this process. Functional nanomaterials not only greatly improve the sensing performance of traditional analytical methods, but also accelerate the birth of some new analytical techniques. Among various nanomaterials, magnetic nanoparticles (MNPs) with unique magnetism and excellent physicochemical properties have attracted much attention recently [1] and have shown great potential in catalysts [2], sensors [3], adsorbents [4], wastewater treatments [5], semiconductors [6], pigments, magnetic data storage devices [7], magnetic resonance imaging (MRI) [8], bio-separations [9] and medicines [10]. Specifically, the superior adsorption capacity, magnetic separation/concentration ability and magnetic resonance imaging utility of MNPs arouse intensive interests for researchers in various branches of analytical science [11,12,13]. As the properties and application of MNPs are closely related to the size, shape and surface ligand of MNPs, thus manipulating the size, shape, component, surface ligand of MNPs is greatly desired, and much effort has been devoted to the controllable preparation of MNPs in the past decades, and several reviews are available on this issue [14,15,16,17,18]. However, the direct application of bare or single-component MNPs is limited due to their shortcomings including poor stability/dispersity, relative inertness and poor surface functionality. Hence, in most cases, the modified MNPs or MNP-based composite structure was employed. The MNP-based composites not only overcome the shortcoming of bare single MNPs, but also incorporate new properties of the second material in catalysis, optical, spectroscopy, electrochemistry and so on, which greatly extended the application area of magnetic particle-based hybrid. The successful preparation of modified MNPs or MNP-based hybrid also promotes the application of MNPs in other analytical techniques in addition to separation and MRI. Considering this, efficiently coupling these materials to various analytical techniques such as electroanalytical chemistry, chromatography and spectroscopy is vital to take full advantages of the unique properties of MNPs for advanced sensing performance.
As a powerful analytical technique [19], surface-enhanced Raman scattering (SERS) has been widely employed for molecule sensing (environmental pollutants [20], biomarkers [21], pesticides [22], explosives [23] and drugs [24, 25]), bio-imaging [26], diagnostic [27], reaction monitoring [28] and so on due to its incomparable superiority of the unique fingerprint spectroscopy, nondestructive data acquisition [29] and single-molecule sensitivity (single-molecule SERS has been reported with enhancement factor up to 1014–1015 in total [30]). Even so, the application of SERS in practical analysis still encounters several critical challenges, such as (1) the interferences from complex matrix of real sample; (2) the high cost of SERS analysis due to the utilization of noble metal as substrate and its low recyclability; (3) the limited concentration/enrichment effect of the substrate for target; (4) and the complex or sophisticated procedure for fabricating enhancement hot spots. Fortunately, the unique properties of MNPs may provide efficient solutions to these challenges. By simply manipulating magnetic force, we can easily achieve: (1) facilely and fast separate analyte from sample matrix to reduce the interference; (2) recycling utilization of magnetic substrate; (3) effective and simultaneous separation and enrichment/concentration ability, which is beneficial to detect low-abundance analyte; and (4) ordered arrangement or controllable aggregation of substrates under magnetic attraction, which can generate a favorable density of “hot spots” for subsequent SERS enhancements. Therefore, rational designed MNP–plasmonic nanostructures and efficiently coupling MNPs with SERS could greatly improve the sensing ability of SERS and extended their application fields. Given that many solutions have been given in the fabrication of MNPs–Au, MNPs–Ag and multifunctional MNPs–Au/Ag-based hybrid in the past decades, it is also convenient to introduce MNPs in SERS analysis.
Though much of work has been done to prepare various MNPs and huge progresses have been made to apply MNPs in SERS, the research on MNPs preparation and its application in various fields including SERS still continue with full passion and tempting potential and a large space is still available to explore the application of MNPs in SERS analysis. Considering this, it is quite necessary to summarize the recent advances of MNPs in SERS analysis. Previously, lots of review articles on preparation and applications of MNPs have been published; however, most of these reviews focus on biomedical related application [31, 32] (including diagnosis, drug delivery) and pollutant treatment [33]. Reviews on preparation and application of MNP biosensors are also reported, while most presented the general introduction of MNPs in sample pretreatment and application in specific analyte detection [34, 35] using different sensing techniques (typically electrochemical methods, MRI, SPR), in which SERS is seldom involved. Very recently, reviews focus on application of MNPs in fluorescence detection [36] and electrochemical sensing [37] are also available. Nevertheless, reviews on application of MNPs in SERS, an important, powerful analytical technique with high sensitivity and promising application, have not been reported despite the fact that many good original works have been published. On the other hand, reviews about SERS and its application were also widely reported, which often pay attention to the plasmonic substrate fabrication and their application in specific molecule sensing [38] or biomedical diagnosis, while few special focus on MNPs. Some comprehensive reviews on biosensing may involve MNPs and SERS [11, 39, 40], but with quite limited, fragmented space, and they did not clearly correlate MNPs with SERS. To grasp the whole picture of MNPs in SERS, the reader needs lots of time and great effort to search among volume reviews on MNPs and SERS. Therefore, there is a large space and great necessity to summarize the progress on application of MNPs in SERS. The introduction and discussion of these achievements will provide the appropriate background for developing novel materials and analysis methods for the detection of diverse analytes in real sample with SERS and MNPs.
To better understand the research progress in this area and shed a light on future research road, a critical review is present here. First, recent progress on preparation methods of MNPs including the preparation, encapsulation and surface modification of MNPs is briefly reviewed. Then, assembly of MNP–plasmonic substrates for efficient SERS is introduced. After that, application of MNPs in SERS analysis is intensively discussed. Finally, the outlook about the MNPs and their application in SERS is presented.
Preparation of magnetic nanoparticles
Preparation method
Synthesis of MNPs with desirable properties and high potential applications are greatly demanded. Up to now, there are several typical and newly emerging methods to prepare MNPs with various morphologies and properties. These preparation methods include co-precipitation, thermal decomposition, hydrothermal, sol–gel, microemulsion, flow injection technique, aerosol/vapor, sonochemical technique and electrochemical methods [14,15,16,17,18, 41,42,43,44,45,46,47,48]. The characters of these methods are listed in Table 1.
Seeing the above preparation methods, co-precipitation is the most facile and convenient wet chemical method to prepare MNPs with reproducible quality once the reaction conditions are well controlled, but the size and shape control are not so good. Thermal decomposition is the most well-known technique to prepare uniform-sized superparamagnetic MNPs by heating iron precursors in solvents (or aqueous solution) at high temperature under autogenous pressure. Sol–gel method can obtain pure amorphous phases MNPs with good monodispersity and controlled microstructure. Microemulsion route can be used to prepare the size- and shape-controlled MNPs, yet the presence of surfactants in the reaction may cause the aggregation of prepared MNPs which need additional washing progress and stabilization treatment. Flow injection technique is a novel strategy with several superiorities to fabricate small-sized MNPs, such as high mixing homogeneity, high reproducibility and precise control of reaction conditions. Sonochemical technique has been widely employed to synthesis highly monodispersive nanoparticles with different morphologies, but has some limitations in the size control and large-scale production. Electrochemical method is a cost-effective and easily available way, which does not need high temperature to fabricate MNPs; however, as-prepared MNPs are often poorly ordered, and the electrodeposition is only available to execute on conducting substrates. Generally speaking, the chosen of proper method depends on the desire properties what we need, so the advantages and shortcomings of each method should be balanced. All in all, there is still a great need to develop a cost-effective, convenient and efficient approach to produce monodispersed MNPs with uniform size and controllable shape and surface properties for different applications.
Encapsulation and surface modification
Although MNPs have many unique properties, bare MNPs are easily oxidized, eroded by acids or bases, and they have fewer active groups, and thus, it is hard to keep their stability without aggregation or precipitation for a long time [49]. These issues have severely restricted the practical applications of MNPs in many fields. Consequently, encapsulation and surface modification of bare MNPs with appropriate materials is necessary to resolve these issues.
Organic polymer materials
To avoid agglomeration, endowing the bare magnetic particles with new properties, organic polymers are often employed to encapsulate MNPs. Polymer as coating will prevent nanoparticles from oxidation, enhance compatibility and reduce susceptibility to leach [15]. As a result, encapsulated MNPs display improved monodispersity, enhanced chemical stability and reduced toxicity [50]. In general, polymers can be self-polymerized to form a shell on the surface of MNPs, either chemically anchored or physically adsorbed, which could create repulsive forces to balance the van der Waals attractive forces and the magnetic [51].
Usually, three typical polymerizations methods are employed to prepare polymer-coated MNPs: seed precipitation polymerization [52], cross-linking of polymers [53,54,55] and inverse emulsion polymerization [56]. The thickness of stable polymeric shells created by these strategies around the nanoparticles can be turned by changing the monomer concentration, polymerization temperature, polymerization time and water/surfactant ratio.
Organic polymers as coating shell endow the MNPs’ surface with functional groups, such as carboxyl (–COOH), amino (–NH2), hydroxy (–OH) and sulfhydryl (–SH). The most frequently used polymers include noncytotoxic folic acid [57, 58], poly(acrylic acid) (PAA) [59], multifunctional poly(styrene-alt-maleic acid) (PSMA) [60], poly(methacrylic acid) (PMAA) [61], thermosensitive poly(N-isopropylacrylamide) (PNIPAM) [62, 63], poly(ethylenimine) (PEI) [64,65,66] and conductive and electromagnetic wave absorptive polyaniline (PANI) [67]. Moreover, poly(pyrrole) (PPy) [68], poly(ethylene glycol) (PEG) [69], polyesters and their copolymers are also involved. The introduced functional groups can easily bond biomolecules or substances with opposite charge, and it is a quite effective way to bond electronegative noble metallic nanoparticles on shell surface to generate hot spots for Raman signals enhancement.
However, the introduction of organic polymers may cause additional Raman peaks or signal background in the Raman spectrogram, which would have a large impact on the analysis of unknown samples. Moreover, inherent shortcomings of organic polymers would also accompany by the introduction. And additional washing progresses and further treatments are necessary to remove excess polymers. Consequently, these vital influences should take into consideration in the modification step as well as take effective treatments before the polymer-modified MNPs was used for SERS analysis.
Inorganic materials
Inorganic matrixes have early been explored to wrap MNPs to improve their performances. These inorganic material modifications could shield the nanoparticles, avoid oxidation and aggregation, enhance compatibility and obtain new functional groups [70]. Ordinarily, porous natural matrixes (silica and carbon [71,72,73]), noble metal nanoparticles (antibacterial silver [74], biocompatible gold [75]), photocatalytic metal-oxide semiconductor (ZnO [76] and TiO2 [77]) and graphene are used to wrap MNPs. These inorganic shells employed for the encapsulation of bare MNPs show incomparable advantages like economical, green environmental, nontoxic. Furthermore, new functions introduced by inorganic modification, such as porous silica and carbon, are physicochemical stable interlayers to further grow SERS-active nanoparticles, and carbon has strong adsorption capacity; roughed Ag and Au can generate tremendous enhancement effects; ZnO and TiO2 are photocatalytic interlayers to adsorb and degrade organic pollutions in wastewater; graphene with large surface area could increase the adsorption ability and generate chemical enhancement effect for SERS signals. Nevertheless, these inorganic coatings still have some problems: Au and Ag nanoparticles are prone to agglomerate, and Ag is easily oxidized; graphene materials are usually overlapped and cannot coat the MNPs uniformly and so on. These issues should be solved to better shield the core and improve their applications in various fields like flexible SERS substrates. Specifically, silica is an effective and widely used material to coat MNPs due to its high chemical and thermal stability, good compatibility and non-toxicity property [70]. The Stöber process is a typical method for wrapping MNPs with SiO2, which have been reported in the extensive literature [78]. In this approach, the thickness of coated SiO2 shell could be changed by adjusting the reaction time, the concentration of ammonia catalyst, TEOS precursor, etc. There are still some other methods to challenge for the preparation of uniform silica shells with controlled thickness, such as microemulsion method [79], aerosol pyrolysis [80] and sonochemical deposition [81]. The silica-coated MNPs can be easily modified by silanization reagent to endow it with functional group such as –SH or –NH2, which facilitates loading SERS-active Au or Ag nanoparticles on the MNPs surface. It should be mentioned that it is far from enough to purely use inorganic nanomaterials wrapped MNPs for SERS application. Hence, further modification steps are also demanded to enable the MNPs to bond various Au or Ag nanoparticles in a narrow distribution.
As both organic polymer and inorganic coating have their advantages, the choice of the modified material really depends on the specific application. In many cases, the combination usage of inorganic and organic polymeric materials would realize better performance in application, such as biocompatibility, stability and monodispersity of MNPs, and uniform distribution of Au or Ag nanoparticles on the surface. Whatever the capping layer is, the main object is to protect MNPs from oxidation and aggregation and facilitate its surface functionalization. But, the polymer or inorganic material coating process may complicate the preparation and degrade the crystal quality of MNPs, and importantly, thick coating will greatly influence the magnetic susceptibility of MNPs.
Assembly of MNP–plasmonic substrates
Based on the effective modification methods to overcome several limitations of bare MNPs, fabricating of assembly of MNP–plasmonic SERS substrates with intrinsic hot spots would be much easy and controllable. Combining the magnetic-induced aggregation, multidimensional hot spots can be generated. This will greatly improve the performance of MNP–plasmonic SERS substrates, including sensitivity and reproductivity. Normally, in situ growth and ex situ assembly are widely used to prepare the multicomponent substrates, and the example of the preparation of Fe3O4/Au substrates is shown in Fig. 1a [82].
a Three different techniques for the synthesis of Fe3O4@Au core–shell nanoparticles. Adapted from Ref. [75]. Copyright: 2015 American Scientific Publishers. Used with permission. b Plasmonic hot spots formed on Fe3O4@Au (a) and calculated electric field intensity distribution (b–e). Adapted from Ref. [82]. Copyright: 2016 Elsevier B.V. Used with permission. c Synthesis and functionalization of star-shaped Fe3O4@Au NPs. Adapted from Ref. [87]. Copyright: 2013 Royal Society of Chemistry. Used with permission. d The synthesis procedure of Fe3O4@Ag/PEI/Au@Ag core–shell–satellite microspheres. Adapted from Ref. [89]. Copyright: 2015 Royal Society of Chemistry. Used with permission
The in situ method means directly growing plasmonic nanoparticles onto the surface of modified MNPs. The most well-known way is reducing metallic irons into nanoparticles in the presence of reducing agents, which can directly deposit onto MNPs. Choi et al. [83] used butylamine to reduce AgNO3 in ethanol to directly deposit Ag onto the Fe3O4@SiO2 particles. Du et al. [84] developed a one-pot solvothermal route to synthesis Ag/Au/Fe3O4 composite in the presence of PVP and trisodium citrate. Physical deposition is also a widely accept approach, and Gao et al. [85] fabricated flowerlike Fe3O4/Ag via magnetron sputtering Ag nanoparticles onto immobilized Fe3O4 microflowers. Furthermore, photochemical deposition is a possible way to deposit silver nanostructures on ZnO/Fe3O4 composite under UV light irradiation [76]. The in situ approach has the advantages of inexpensive and convenient, but the disadvantages are also obvious: the shape, size and distribution of plasmonic nanoparticles on MNPs are hard to control. For example, the morphology of MNPs–Au composite is often limited in sphere.
In ex situ method, pre-synthesized MNPs and plasmonic nanoparticles are assembled to construct multicomponent MNP-based substrates. In this approach, the surface properties of the capping materials of MNPs are crucial to the bonding of nanoparticles. For example, negatively charged PMAA or positively charged branched PEI is often used to increase the negative or positive charge of the surface of MNPs through layer-by-layer assembly technique. Then, opposite charged noble metal particles can be easily and stably anchored onto the modified MNPs as efficient SERS substrates [86]. The ex situ strategy is effective to produce high-performance MNP–plasmonic SERS substrates, while the size, shape and attachment density of plasmonic particles–MNPs can be flexibly tuned. However, sometimes, ingenious design and sophisticated skill is need to control the interaction between MNPs and plasmonic particles to form highly efficient SERS substrate.
Additionally, the morphology and structure of SERS substrates is closely related to the enhancement effect; hence, it is important to fabricate MNP-based SERS substrates with various and multidimensional hot spots (Fig. 1b). Plasmonic structures with sharp protrusions or tips in all directions would also produce multiple intrinsic hot spots. And seed-mediated growth method plays an important role in producing desired substrates with large enhancement effects. For example, star-shaped MNP–Au nanoparticles could be formed by the growth of Au seeds in the presence of HAuCl4, PVP and DMF (Fig. 1c) [87, 88]. Besides, layer-by-layer assembly technique is a good approach to form multidimensional hotspots. Wang et al. [89] presented a core–shell–satellite 3D Fe3O4@Ag/Au@Ag substrate by the growth of Au seeds to form Ag shell and then attach Au@Ag (Fig. 1d). Polymer PEI was used between each step to attach Au and Au@Ag nanoparticles.
By rational designing the arrangement of MNPs and plasmonic particles, the efficient coupling between plasmonic particles could generate high enhancement effect, together with the facile separation, effective enrichment, concentration effect, the MNPs–Au/Ag hybrid could greatly promote the sensing performance of SERS. For practical analysis, it is still needed to fabricate novel MNPs–plasmonic particle hybrid with high enhancement ability, good stability and multiple functionality.
Applications in SERS
MNPs have been widely used in SERS research due to its incomparable superiorities: (1) unique size offered bio-imaging function, drug deliver capacity, stability and reproducibility; (2) the magnetic properties improve the efficiency of sample pretreatment and enrichment and achieve the control of targeting movement; (3) diverse morphologies facilitate the formation of abundant hot spots to improve SERS sensitivity; and (4) intrinsic excellent properties of surface functionalized MNPs possess. MNP-based SERS substrates with outstanding characteristics can achieve high-sensitivity recognition and rapid detection. And this is a rapid growing and very promising research branch in analytical science. Here, the application of MNPs in SERS analysis for biomedicine, food safety, environment, reaction monitoring is briefly introduced.
Biomedical application
MNPs have been widely used in biomedical field. SERS also exhibits good biomedical application values as a high-sensitive and nondestructive single molecular detection technique. A hybrid of MNPs–plasmonic particle may hold the function of diagnosis, drug delivery and treatment in one solid, which is not only beneficial for precise early diagnosis of diseases [90,91,92], but also make it easier to monitor the drug fate and evaluate treatment effect [93]. Generally, the labeled MNPs are used for cancer diagnostic and target detection (e.g., for nucleic acid (DNA/RNA) [59, 94,95,96,97], protein [64, 66, 98,99,100,101,102] and even bacteria [96, 103–106]).
As an example of MNPs application in cancer diagnostic study, Rong et al. [107] designed a sandwich magnetic immune complexes sensor to detect cancer biomarkers. In this work, capture antibody-coated Ag decorated Fe3O4 MNPs were employed as the carcinoembryonic antigen (CEA) concentration platform and the signal magnification substrate. Meanwhile, the SERS tags and Ag-coated Au nanorods were decorated with testing antibody. Because the plasmon peak of resonant Raman dye diethylthiatricarbocyanineiodide (DTTC) is near to the excitation wavelength, the Raman signals were greatly enhanced. Moreover, the high-sensitive sensors can detect CEA with a low LOD of 4.75 fg/mL (Fig. 2a) and a well-defined dynamic linear range between 10 fg/mL and 100 ng/mL (Fig. 2b). Similarly, through changing antigens and corresponding antibodies in the sandwich magnetic core–shell-based immune complexes, Lin et al. [108] tested CEA in human serum and Qiu et al. [61] detected CEA-expressed A549 cells at a very low abundance (~ 10 cells/mL). Ge et al. [109] determined human epididymis protein 4 (HE4) of ovarian cancer. Interestingly, Yang et al. [110] developed a label-free Fe3O4/Au/Ag nanocomposite for the detection of lung cancer biomarker (adenosine) in urine samples and achieved on-site screen adenosine by magnetically assisted SERS protocol (Fig. 2c). The substrate shows a good stability, excellent reproducibility, time efficiency (within 20 min to test one sample), high sensitivity with a LOD down to 10−10 M and a good linear range from 5 mM to 0.5 nM (Fig. 2d–f).
a SERS spectra of CEA at various concentrations under 785 nm excitation. b Plot of the Raman intensity at 1238 cm−1 versus the logarithmic concentration of CEA. Adapted from Ref. [107]. Copyright: 2016 Elsevier B.V. Used with permission. c Schematic illustration of array setup and procedures in the detection of adenosine by Fe3O4/Au/Ag nanocomposites with a portable Raman. SERS spectra of aqueous adenosine recorded after mixing with Fe3O4/Au/Ag nanocomposites d using confocal Raman (excitation laser at 633 nm) and e using a portable stabilized R. laser analyzer (excitation laser at 785 nm, 300 mW). f Linear correlation of Raman intensities (at 730 cm−1) with the logarithm of adenosine concentrations from 0.5 nM to 5 mM. Adapted from Ref. [110]. Copyright: 2014 American Chemical Society. Used with permission
For the DNA/RNA detection, Wu et al. [96] achieved Bacillus thuringiensis special gene fragment detection by Fe3O4 magnetic beads and Au/Ag core–shell nanorods based on the biotin–streptavidin-specific interaction with a good linear responsive range of 0.1 pM to 1 nM and a LOD of 0.14 pM. Pang et al. [95] reported that miRNA let-7b can be specifically captured by MNPs within a PE tube without any PCR pre-amplification treatment and quantitative detection of miRNA from 1 fM to 1 nM with a LOD as low as 0.3 fM (15 zeptomole, 50 μL) (Fig. 3a, b). Li et al. [59] demonstrated the sensitive detection of a specific single-stranded DNA (ssDNA) sequence on the basis of the SERS liquid chip. In their work, the corresponding probe DNA was grafted to the poly (styrene-co-acrylic acid)/Ag/SiO2 composite SERS tags, and the capture DNA was attached to the Fe3O4/PAA core–shell nanospheres. Quantitative detection of target ssDNA was achieved based on the well-defined linear correlation between the SERS signal intensity and the target ssDNA quantity in the range of 10 nM to 10 pM, and the LOD was approximately 10 pM.
a SERS intensities (1586 cm−1) versus let-7b concentrations. Inset: SERS spectra and linear relationship between the SERS intensity and the miRNA concentration. b Specificity of miRNA assay. Adapted from Ref. [95]. Copyright: 2015 Elsevier B.V. Used with permission. SERS spectra of d MGITC detecting E. coli, e DACITC detecting S. typh and f PPY detecting MRSA with the concentration range 104 to 101 CFU/mL, and corresponding histogram of the intensity of the characteristic peak. Adapted from Ref. [103]. Copyright: 2017 American Chemical Society. Used with permission
Protein detection is one of the earliest works in the development of MNPs application in biomedicine. Wang et al. [66] employed Fe3O4@PEI@Ag versatile SERS substrate to detect adsorbed p-ATP molecules and human immunoglobulin (lgG) with a LOD as low as 10−11 M and 10−14 g/mL, respectively. Besides, the model of Raman intensities with the logarithmic concentrations has good curve fitting characteristics, and corresponding coefficient of determinations is R2 = 0.995 of p-ATP and R2 = 0.992 of lgG. Balzerova et al. [100] presented the determination of IgG in blood. Alula et al. [76] prepared Ag@ZnO/Fe3O4 composites to determine creatinine in urine sample and quantitative analyses in aqueous solution. Yang et al. [101] presented an aptamer-modified MNPs–AuNPs satellite to detect prostate specific antigen (PSA) assay based on highly specific biorecognition, which possesses high sensitivity of LOD as low as 5 pg/mL and good repeatability with the recoveries between 97.63% and 103.84%.
Immobilized MNPs as high-performance SERS substrates can also be employed for the detection and identification of bacteria. Zhang et al. [105] utilized the SERS peak of mercaptobenzoic acid (MBA) at 1582 cm−1 or 5,5′-dithiobis (2-nitrobenzoic acid) (DNTB) at 1333 cm−1 to measure S. typhimurium or S. aureus in the range from 102 CFU/mL to 107 CFU/mL, respectively. The LOD of S. aureus is 35 CFU/mL, and S. typhimurium is 15 CFU/mL. Similarly, Kearns et al. [103] achieved simultaneous detection of multiple pathogens by combining lectin (Con A)-functionalized Ag@MNPs extraction with the unique Raman reporters of biorecognition antibody-functionalized AgNPs. In detail, malachite green isothiocyanate (MGITC), 7-dimethylamino-4-methylcoumarin-3-isothiocyanate (DACITC) and 4-(1H-pyrazol-4-yl)-pyridine (PPY) as Raman reporters were used to detect Escherichia coli, Salmonella typhimurium and methicillin-resistant Staphylococcus aureus, respectively. And LODs of single component are 101 CFU/mL (Fig. 3d, e), but quantitative analysis is not clarified.
To summary, MNP-based SERS substrates have realized accurate, fast and highly sensitive detection of biomarkers on the basis of specific biorecognition. However, most of current investigations are limited in the detection of target molecules in simple matrixes, and most of the cells, aptamers or bacteria pathogens are obtained in special artificial cultures. That means much of work need to be done on the real practice in complex matrixes, where detected substances are extracted from natural environment or cells, tissues and organs of living animals. Moreover, some limitations of MNP-based SERS substrates should be mentioned that the real-time vivo detections are restricted in the penetration ability of Raman laser and signal receiver. Additionally, antifouling ability, stability, biocompatibility and multiplex sensing ability of MNP-based SERS substrates still need to be improved in practice biomedical analysis.
Food safety
SERS analytical techniques have been proven to be an applicable approach for scrutinizing food safety [111], especially for the detection of bacterial pathogens [112,113,114], antibiotics [57, 115], pesticide residues [65, 89, 116,117,118,119,120,121,122] and banned food additives [123,124,125].
Sun et al. [123] designed a versatile core–satellite Fe3O4@SiO2/Au 3D magnetic microsphere (the Au nanoparticle gap was sub-10 nm) for charge selective detection of food dye molecules. This is one of the earliest reports on magnetic-based SERS-active hybrid for simultaneous selective enrichment, magnetic separation and SERS-based detection of multiple food dyes.
Much of work has been done for the detection of pesticide residues, commonly thiram, one of the typical dithiocarbamate fungicides. Yang et al. [117] directly grafted Au nanoparticles onto the magnetic network nanostructure surface with the assistance of inositol hexakisphosphate (IP 6), which exhibited sufficient adsorption, enrichment, separation and Raman enhancement for the on-site detection of trace thiram in multiple vegetable peels as low as 10−15 M by a portable Raman spectrometer, and a good linear relationship in the range of 500 nM to 50 fM (Fig. 4a–c). Liu et al. [116] introduced multifunctional Fe3O4@GO@Ag ternary complex to detect pesticide residues on the fruit peels based on a surface magnetic solid-phase extraction (SMSPE) method. SMSPE exhibits great advantage in simple pretreatment. The combined method displays fast detection (within 20 min), high efficiency and a high-sensitivity detection with LOD of 0.48 and 40 ng/cm2 for thiram and thiabendazole, respectively. The two LODs are much lower than the prescribed standards of the US Environmental Protection Agency (USEPA).
a SERS spectra of thiram with Au dotted magnetic network nanostructure (Au-MNN). b SERS spectra of cabbage peel spiked with thiram by the enhancement of Au-MNN. c The linear correlation of Raman intensities (at 1373 cm−1) with thiram concentrations from 50 fM to 500 nM in ethanol. Adapted from Ref. [116]. Copyright: 2013 Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim. Used with permission. d Raman spectra for E. coli O157:H7 capture in apple juice using MNP. e Stacked spectra showing E. coli O157:H7 concentration-dependent Raman shift signature of the MGITC reporter at vibrational frequency zones of 1180, 1370 and 1620 cm−1. f Plot representing SERS intensity at 1620 cm−1 vs. Log CFU/mL of target E. coli O157:H7. Adapted from Ref. [114]. Copyright: 2014 Elsevier B.V. Used with permission
Bacterial pathogens are great threats to food safety. Wang et al. [112] used a magnetic SERS immunoassay, pathogens modified MNPs@SiO2 and corresponding antibodies functionalized Au nanoparticles, to detect various pathogens in selected food matrices. The LOD of both Staphylococcus aureus and Salmonella entericaserovar Typhimurium can reach 103 CFU/mL in spinach wash, and the LOD was also successfully achieved in peanut butter. Najafi et al. [114] applied Fe3O4/Au nanoclusters for the detection of Escherichia coli O157 in apple juice with a LOD down to 102 CFU/mL less than an hour (Fig. 4d–f). What is more, the capture efficiency for E. coli O157 using MNPs was approximately 84–94% in liquid food matrix.
Chen et al. [126] first achieved the highly sensitive and selective SERS detection of NO2− in pond, urine and pickles. In their experiment, Fe3O4@SiO2/Au MNPs was modified by 4-aminothiophenol (4-ATP), which was used to react with NO2− to form a complex containing diazonium salt and azo bonds. Subsequently, three SERS peaks of the complex were used to analyze the concentration of nitrite ions. The corresponding LODs of NO2- were 15.63, 13.69 and 17.77 μM. These LODs are much lower than the standard (<1.0 mg/L (71.4 μM) in drinking water) of USEPA.
MNP-based SERS techniques have greatly improved the efficiency of food safety analysis, which used to be tedious, complex and time-consuming. Even so, novel structured MNPs–plasmonic substrate is still in great demand to achieve sensitive detection of low-abundance analyte and reduce the background from complicated food matrix. Besides, the design of substrate and the explanation of the results should consider the fact that the analyte might metabolize to new chemical substances during food processing, which is largely neglected in the past. Finally, quantitative reproducibility of SERS analysis is also needed to be improved.
Reaction monitoring
SERS is a real-time detection and characterization technique. The unique advantage to provide the fingerprint of molecule structure makes SERS a promising tool to monitor some important course of chemical reactions, including discriminating intermediate product of organic reaction, monitoring oxidation–reduction reaction, in situ characterizing catalytic reaction [76, 127,128,129,130,131,132].
Mezni et al. [127] studied an interesting phase transition of iron oxide shell (Fig. 3a). The phase of iron oxide (lepidocrocite (γ-FeOOH), magnetite (Fe3O4), maghemite (γ-Fe2O3) or hematite (α-Fe2O3), (Fig. 5a, b) can be discriminated by monitoring the formation of diiron-oxo bonds during the phase transition through the strong SERS effect of Au/Fe3O4 substrate, in which the gold cores also play a role as plasmonic nanoheaters to induce the thermal phase transition.
a Schematic of the transition of the iron oxide shell from the magnetite (Fe3O4) phase to the hematite (α-Fe2O3) phase under the irradiation of different laser intensities. b Phase transition properties of the Fe3O4-decorated AuNPs revealed by SERS spectra excited at 638 nm with laser intensity ranging from 0.1 to 100%. Adapted from Ref. [127]. Copyright: 2013 American Chemical Society. Used with permission
Additionally, by introducing porous carbon [128] and catalytic semiconductor titania [77, 129, 130] shield MNPs as SERS substrates, other multifunctional applications including catalysis, adsorption and degeneration process can also be monitored by SERS. For example, Lv et al. [129] developed core–satellite Fe3O4/Au@TiO2 substrate to monitor photoreduction in Cr(VI), and this platform with selectivity can quantitatively determinate Cr(VI) with evident sensitivity lasting one month. Cai et al. [128] employed Fe3O4/C/Au nanoparticles for in situ SERS monitoring the catalytic reaction of p-nitrothiophenol (p-NTP) to p-aminothiophenol (p-ATP), and the experiment found that the catalytic rate could be turned by the external magnetic field either present or absent. Shen et al. [77] synthesized Fe3O4@TiO2@Ag/Au microsphere substrate for in situ SERS monitoring of catalytic reduction of 4-nitrophenol. The excellent SERS-active substrate not only shows efficient catalytic performances, but also exhibits recyclability to self-cleaning for photocatalytical activity of TiO2, and the magnetism of Fe3O4 endows the substrates convenient separation and concentration ability.
It can be seen that MNP-based SERS substrates could be used to monitor surface plasmon-driven catalytic reaction and Raman laser-induced photocatalytic reaction. And real-time monitoring course of reaction would provide a perfect opportunity to better understand the process and mechanism of chemical reactions, which has a great impact on electrochemistry, pharmaceutical production and environmental remediation. Additionally, the catalytic property endows the substrates with recyclability to self-cleaning.
Environmental analysis
Environment pollution has become a worldwide problem, which has caused great threaten to public health. Considering this issue, accurate and fast detection methods for various pollutants are of great importance for environment monitoring and remediation. SERS-based techniques have received considerable attentions in this area due to its high sensitivity, nondestructive detection, ability to provide plenty of molecule structure information and in situ detection ability. The magnetic nanomaterials usually have high adsorption toward pollutant materials, and they are easily separated from the samples by magnetic forces. The combination of MNPs with SERS has shown powerful capacity for environment monitoring. The representative MNP-based SERS substrates applied in the field are noble metal nanoparticles decorated magnetic particles with strong adsorption capacity or abundant hotspots, such as nanotube-like [67], nanoclusters [133], 3D flowerlike [134], Fe3O4 nanostructured hollow microspheres, GO [135, 136] and rGO [137]. The target molecules analyzed by SERS included explosives [23, 138], pesticides [58, 138,139,140,141], heavy metal ions (e.g., Hg2+, Cr6+ and As3+) [142,143,144], organic dyes and persistent organic pollutants [72, 145,146,147,148,149,150,151,152,153,154], and pathogenic bacteria and pathogens [155].
Complex unknown sample together with the inefficient use of hot spots makes SERS discriminating and detecting target species in real environmental samples still a great challenge [155]. Undeniably, typical solid MNP-based substrates cover overwhelming majority for real environmental monitoring. But several of interesting structures obtained by immobilized MNPs are quite amazing to practice. A removable 3D macroscale superlattice array of Au nanorods doped with MNPs is obtained by evaporative self-assembly. The superlattice arrays not only possess fast separation and enrichment ability, but also achieve excellent sensitivity for SERS detection of thiram, diquat and polycyclic aromatic hydrocarbons with LOD down to ~ nM [20]. Poly(N-isopropylacrylamide) (pNIPAM), thermoresponsive glue, encapsulated Ag NPs and MNPs into a colloidal substrate could adsorb analytes in solution when it is swelled (low temperature) and reversibly generate hot spots upon collapse (high temperature or drying). While the substrate concentrated into small space region, it could detect PCP with a LOD as low as 10−12 M [62] (Fig. 6). In addition, Wang et al. [63] used multifunctional pNIPAM-co-acrylamide net incorporated with Au and MNPs to fabricate a composite substrate with magnetism, themosensitivity, SERS effect and drug delivery ability. This multifunctional composite substrate provides a new synthetic strategy to fabricate substrates combined with probing and label-free detection functions together. What is more, burgeoning magnetic platform with selective adsorption, catalysis and detection in the field is worth expected.
Representative TEM images of a the encapsulated magnetic nanoparticles after pNIPAM polymerization, b the magnetite@pNIPAM nanohybrid materials containing silver seeds and c the final magnetite–Ag@pNIPAM composite microgels. d SERS spectra of 1NAT in magnetite–Ag@pNIPAM in the swollen (4 °C, blue) and collapsed (60 °C, red) states, for different excitation laser lines (532, 633 and 785 nm). e Comparison of intensities of the band at 1368 cm−1 at low (blue) and high (red) temperature (average intensity and standard deviation for five measurements, green bars). f Optical image of the magnetite–Ag@pNIPAM microgels before and after exposure to a permanent magnet. g Detection limits for 1NAT in dilute dispersions of magnetite–Ag@pNIPAM after concentration of the material using a permanent magnet. Adapted from Ref. [62]. Copyright: 2011 American Chemical Society. Used with permission
In short, MNP-based SERS substrates provide the rapid extraction capacity for environmental pollutants detection with high sensitivity. But the coexistence of various pollutants with unrecognizable Raman signals would seriously interfere the selective detection. Moreover, not all of the pollutants have distinguished Raman signals; hence, proper designation of MNP-based substrates and effective modification of probe molecule are necessary to solve the problem. Furthermore, poor reproducibility and stability also restricted the application of MNP-based SERS substrates in environmental analysis. Therefore, significant efforts are still required to further develop more sensitive, stable and specific SERS substrates for these types of pollutants.
Conclusion and outlooks
Applications of functionalized MNPs have been introduced to many fields over the past decades. Taking its SERS application as an example, MNPs could facilely separate SERS substrate from matrix to reduce background response and meanwhile achieve the capture and enrich target, manipulate hot spots simultaneously, which greatly improve the sensing performance of SERS with LOD down to femto-mole level. This makes the MNPs involved SERS analysis a powerful technique for real sample analysis. The introduction and discussion of these achievements will provide the appropriate background for developing novel materials and analysis methods for the detection of diverse analytes in real sample with SERS.
Although great achievement has been done on MNPs involved SERS analysis, several issues should be considered in future research. (1) It is still necessary to develop a robust synthesis and modification system to prepare stable, well-dispersed, highly uniformed MNPs with desired properties in a simple and low-cost way for its further application. (2) Rational design and fabrication of highly active, multifunctional MNPs–Au/Ag substrate is in great demand as certain analytes at trace concentration are still difficult to detect, and the reliable analysis of real samples still poses challenges. (3) Most MNP–Au/Ag substrates are prepared in laboratory with low yield and complex process. To extend the application of MNPs involved SERS in laboratory and in field, a low-cost, high-yield batch fabrication approach for MNPs–Au/Ag substrate should be developed. (4) For real sample practice, the requirements on sensitivity, reproducibility, specificity, stability should always be kept in mind, while the portable detection system should also be considered. So far, it remains a great challenge to realize discriminating and quantitative detecting target species in complex real sample in various phases. Although a long way to go, it is believed that combination of MNPs with SERS and other advanced material or technique will have a bright future.
References
Talelli M, Aires A, Marciello M (2016) Protein-modified magnetic nanoparticles for biomedical applications. Curr Org Chem 19:1–1
Ma JQ, Guo SB, Guo XH, Ge HG (2015) A mild synthetic route to Fe3O4@TiO2-Au composites: preparation, characterization and photocatalytic activity. Appl Surf Sci 353:1117–1125
Baghayeri M (2015) Glucose sensing by a glassy carbon electrode modified with glucose oxidase and a poly(p-phenylenediamine)-based nanocomposite. RSC Adv 5:18267–18274
Wang YX, Wang SH, Niu HY, Ma YR, Zeng T, Cai YQ, Meng ZF (2013) Preparation of polydopamine coated Fe3O4 nanoparticles and their application for enrichment of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A 1283:20–26
Baikousi M, Bourlinos AB, Douvalis A et al (2012) Synthesis and characterization of γ-Fe2O3/carbon hybrids and their application in removal of hexavalent Chromium ions from aqueous solutions. Langmuir 28:3918–3930
Murray CB, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715
Majetich SA, Jin Y (1999) Magnetization directions of individual nanoparticles. Science 284:470–473
Zou J, Zhang W, Poe D et al (2010) MRI manifestation of novel superparamagnetic iron oxide nanoparticles in the rat inner ear. Nanomedicine 5:739–754
Ranzoni A, Sabatte G, Ijzendoorn LJV, Prins MWJ (2012) One-step homogeneous magnetic nanoparticle immunoassay for biomarker detection directly in blood plasma. ACS Nano 6:3134–3141
Zhang LY, Wang TT, Yang L, Liu C, Wang CG, Liu HY, Wang YA, Su ZM (2012) General route to multifunctional uniform yolk/mesoporous silica shell nanocapsules: a platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Chem Eur J 18:12512–12521
Cheng H-W, Luo J, Zhong C-J (2015) SERS nanoprobes for bio-application. Front Chem Sci Eng 9:428–441
Šefčovičová J, Tkac J (2015) Application of nanomaterials in microbial-cell biosensor constructions. Chem Pap 69:42–53
Damborska D, Bertok T, Dosekova E, Holazova A, Lorencova L, Kasak P, Tkac J (2017) Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim Acta 6:1–19
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144–157
Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Hyeon T (2003) Chemical synthesis of magnetic nanoparticles. Chem Commun 34:927–934
Ramimoghadam D, Bagheri S, Hamid SBA (2014) Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J Magn Magn Mater 368:207–229
Wang LP, Tang SK (2011) Progress of application of supercritical fluid technology in preparation of magnetic iron oxide nanoparticles. Chem Ind Eng P 30:339–344
Wang H, Jiang X, Lee ST, He Y (2014) Silicon nanohybrid-based surface-enhanced Raman scattering sensors. Small 10:4455–4468
Tang SY, Li Y, Huang H et al (2017) Efficient enrichment and self-assembly of hybrid nanoparticles into removable and magnetic SERS substrates for sensitive detection of environmental pollutants. ACS Appl Mater Interface 9:7472–7480
Guo MD, Dong J, Xie W, Tao L, Lu WB, Wang Y, Qian WP (2015) SERS tags-based novel monodispersed hollow gold nanospheres for highly sensitive immunoassay of CEA. J Mater Sci 50:3329–3336. https://doi.org/10.1007/s10853-015-8825-3
Rubira RJG, Camacho SA, Aoki PHB, Paulovich FV, Oliveira ON, Constantino CJL (2016) Probing trace levels of prometryn solutions: from test samples in the lab toward real samples with tap water. J Mater Sci 51:3182–3190. https://doi.org/10.1007/s10853-015-9628-2
Bao ZJY, Liu X, Chen Y, Wu YC, Chan HLW, Dai JY, Lei DY (2014) Quantitative SERS detection of low-concentration aromatic polychlorinated biphenyl-77 and 2,4,6-trinitrotoluene. J Hazard Mater 280:706–712
Aoki PHB, Furini LN, Alessio P, Aliaga AE, Constantino CJL (2013) Surface-enhanced Raman scattering (SERS) applied to cancer diagnosis and detection of pesticides, explosives, and drugs. Rev Anal Chem 32:55–76
Benjaber S, Peveler WJ, Quesadacabrera R et al (2016) Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules. Nat Commun 7:12189–12195
Han Y, Lei SL, Lu JH, He Y, Chen ZW, Ren L, Zhou X (2016) Potential use of SERS-assisted theranostic strategy based on Fe3O4/Au cluster/shell nanocomposites for bio-detection, MRI, and magnetic hyperthermia. Mater Sci Eng C Mater Biol Appl 64:199–207
Ngo HT, Gandra N, Fales AM, Taylor SM, Vo-Dinh T (2016) Sensitive DNA detection and SNP discrimination using ultrabright SERS nanorattles and magnetic beads for malaria diagnostics. Biosens Bioelectron 81:8–14
Chen P, Zhao AW, Wang J, He QY, Sun HH, Wang DP, Sun M, Guo HY (2018) In-situ monitoring reversible redox reaction and circulating detection of nitrite via an ultrasensitive magnetic Au@Ag SERS substrate. Sensor Actuat B-Chem 256:107–116
Schlücker S (2014) Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew Chem Int Edit 53:2–42
Hurst SJ, Fry HC, Gosztola DJ, Rajh T (2011) Utilizing chemical Raman enhancement: a route for metal oxide support-based biodetection. J Phys Chem C 115:620–630
Cardoso VF, Francesko A, Ribeiro C, Bañobre-López M, Martins P, Lanceros-Mendez S (2017) Advances in magnetic nanoparticles for biomedical applications. Adv. Healthcare Mater. 1700845
Wu W, Wu ZH, Yu T, Jiang CZ, Kim WS (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16:023501
Zhou Q, Li J, Wang M, Zhao D (2016) Iron-based magnetic nanomaterials and their environmental application. Crit Rev Sci Technol 46:783–826
Tran VT, Kim J, Tufa LT, Oh S, Kwon J, Lee J (2018) Magnetoplasmonic nanomaterials for biosensing/imaging and in vitro/in vivo biousability. Anal Chem 90:225–239
Cristea C, Tertis M, Galatus R (2017) Magnetic nanoparticles for antibiotics detection. Nanomaterials 7(119):7060119
Xia H, Ruijie Tong R, Song Y, Xiong F, Li J, Wang S, Fu H, Wen J, Li D, Zeng Y, Zhao Z, Wu JJ (2017) Synthesis and bio-applications of targeted magnetic-fluorescent composite nanoparticles. Nanopart Res 19(4):149
Wang T, Zhou Y, Lei C, Luo J, Xie S, Pu H (2017) Magnetic impedance biosensor: a review. Biosens Bioelectron 90:418–435
Liu Y, Zhou H, Hu Z, Yu G, Yang D, Zhao J (2017) Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: a review. Biosens Bioelectron 94:131–140
Farka Z, Juřík T, Kovář D, Trnková L, Skládal P (2017) Nanoparticle-based immunochemical biosensors and assays: recent advances and challenge. Chem Rev 117(15):9973–10042
Xiao D, Lu T, Zeng R, Bi Y (2016) Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta 183(10):2655–2675
Sayed FN, Polshettiwar V (2015) Facile and sustainable synthesis of shaped iron oxide nanoparticles: effect of iron precursor salts on the shapes of iron oxides. Sci Rep 5:9733–9747
Mou XL, Wei XJ, Li Y, Shen WJ (2012) Tuning crystal-phase and shape of Fe2O3 nanoparticles for catalytic applications. CrystEngComm 14:5107–5120
Kolen’ko YV, Bañobre-López M, Rodríguez-Abreu C et al (2014) Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J Phys Chem C 118:8691–8701
Lee J, Kwon SG, Park JG, Hyeon T (2015) Size dependence of metal-insulator transition in stoichiometric Fe3O4 nanocrystals. Nano Lett 15:4337–4342
Hufschmid R, Arami H, Ferguson RM, Gonzales M, Teeman E, Brush LN, Browning ND, Krishnana KM (2015) Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale 7:11142–11154
Park J, An K, Hwang Y et al (2004) Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater 3:891–895
Marini S (2015) Magnetic nanocomposites for heavy metals removal from stormwater. PhD dessetation, Università degli Studi di padova
Gutiérrez L, Costo R, Grüttner C et al (2015) Synthesis methods to prepare single- and multi-core iron oxide nanoparticles for biomedical applications. Dalton T 44:2943–2952
Wang LY, Sun Y, Wang J, Wang J, Yu AM, Zhang HQ, Song DQ (2011) Preparation of surface plasmon resonance biosensor based on magnetic core/shell Fe3O4/SiO2 and Fe3O4/Ag/SiO2 nanoparticles. Colloid Surf B 84:484–490
Stolnik S, Dunn SE, Garnett MC et al (1994) Surface modification of poly(lactide-co-glycolide) nanospheres by biodegradable poly(lactide)- poly(ethylene glycol) copolymers. Pharm Res 11:1800–1808
Cornell RM, Schertmann U (1997) The iron oxides: structure, properties, reactions, occurrence and uses. Corros Sci 39:1499–1500
Bazile D, Prud’homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M (1995) Stealth me. PEG-PLA nanoparticles avoid uptake by the mononuclear phagocyte system. J Pharm Sci 84:493–498
Peracchia MT, Gref R, Minamitake Y, Domb A, Lotan N, Langer R (1997) PEG coated nanoparticles from amphiphilic diblock and multiblock copolymer: investigation of their encapsulation and release characteristics. J Control Release 46:223–231
Jeong B, Bae YH, Kim SW (2000) Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymer. J Control Release 63:155–163
Lamprecht A, Ubrich N, Perez MH, Lehr CM, Hoffman M, Maincent P (1999) Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm 184:97–105
Sah H (1999) Protein behavior at the water/methylene chloride interface. J Pharm Sci 88:1320–1325
Zengin A, Tamer U, Caykara T (2014) Extremely sensitive sandwich assay of kanamycin using surface-enhanced Raman scattering of 2-mercaptobenzothiazole labeled gold@silver nanoparticles. Anal Chim Acta 817:33–41
Shan YF, Yang Y, Cao YQ, Huang ZR (2015) Facile solvothermal synthesis of Ag/Fe3O4 nanocomposites and their SERS applications in online monitoring of pesticide contaminated water. RSC Adv 5:102610–102618
Li JM, Ma WF, You LJ, Guo J, Hu J, Wang CC (2013) Highly sensitive detection of target ssDNA based on SERS liquid chip using suspended magnetic nanospheres as capturing substrates. Langmuir 29:6147–6155
Liu TM, Yu JS, Chang CA et al (2014) One-step shell polymerization of inorganic nanoparticles and their applications in SERS/nonlinear optical imaging, drug delivery, and catalysis. Sci Rep 4:5593–5603
Qiu YC, Deng D, Deng QW, Wu P, Zhang H, Cai CX (2015) Synthesis of magnetic Fe3O4-Au hybrids for sensitive SERS detection of cancer cells at low abundance. J Mater Chem B 3:4487–4495
Contreras-Cáceres R, Abalde-Cela S, Guardia-Girós P, Fernández-Barbero A, Pérez-Juste J, Alvarez-Puebla RA, Liz-Marzán LM (2011) Multifunctional microgel magnetic/optical traps for SERS ultradetection. Langmuir 27:4520–4525
Wang C, Wang Y, Jin Y, Xu T, Yuan L, Fang J (2015) Multifunctional nanocomposite with magnetism, thermosensitivity and surface enhanced Raman scattering effect. J Nanosci Nanotechnol 15:6784–6789
Lou L, Yu K, Zhang ZL, Huang R, Zhu JZ, Wang YT, Zhu ZQ (2012) Dual-mode protein detection based on Fe3O4-Au hybrid nanoparticles. Nano Res 5:272–282
Cai WY, Wang X, Yan YX (2014) Controllable fabrication and sensitive detection based on SERS substrates with Au nanocubes coated Fe3O4. Mater Res Bull 52:1–5
Wang CW, Xu JW, Wang JF, Rong Z, Li P, Xiao R, Wang SQ (2015) Polyethylenimine-interlayered silver-shell magnetic-core microspheres as multifunctional SERS substrates. J Mater Chem C 3:8684–8693
Yan MQ, Shen Y, Zhang GY, Bi H (2016) Multifunctional nanotube-like Fe3O4/PANI/CDs/Ag hybrids: an efficient SERS substrate and nanocatalyst. Mater Sci Eng, C 58:568–575
Ren GH, Shang MY, Zou HZ, Wang WQ (2016) Fe3O4@SiO2-SO3H@PPy@Au spheres: fabrication, characterization and application in SERS. Mater Chem Phys 173:333–339
Zhai YM, Zhai JF, Wang YL, Guo SJ, Ren W, Dong SJ (2009) Fabrication of iron oxide core/gold shell submicrometer spheres with nanoscale surface roughness for efficient surface-enhanced Raman scattering. J Phys Chem C 113:7009–7014
Li CY, Ma C, Wang F, Xi ZJ, Wang ZF, Deng Y, He NY (2012) Preparation and biomedical applications of core-shell silica/magnetic nanoparticle composites. J Nanosci Nanotechnol 12:2964–2972
He R, Cheng YC, Jin T, Jiang M, Chen C, Xu GJ (2014) Plasmonic core/satellite heterostructure with hierarchical nanogaps for Raman spectroscopy enhanced by shell-isolated nanoparticles. Adv Optical Mater 2:788–793
Gan Z, Zhao A, Zhang M, Tao W, Guo H, Gao Q, Mao R, Liu E (2013) Controlled synthesis of Au-loaded Fe3O4@C composite microspheres with superior SERS detection and catalytic degradation abilities for organic dyes. Dalton T 42:8597–8605
Ye Y, Chen J, Ding Q, Lin D, Dong R, Yang L, Liu J (2013) Sea-urchin-like Fe3O4@C@Ag particles: an efficient SERS substrate for detection of organic pollutants. Nanoscale 5:5887–5895
Prucek R, Tuček J, Kilianová M et al (2011) The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 32:4704–4713
Salihov SV, Ivanenkov YA, Krechetov SP et al (2015) Recent advances in the synthesis of Fe3O4@Au core/shell nanoparticles. J Magn Magn Mater 394:173–178
Alula MT, Yang J (2014) Photochemical decoration of magnetic composites with silver nanostructures for determination of creatinine in urine by surface-enhanced Raman spectroscopy. Talanta 130:55–62
Shen JH, Zhou Y, Huang JF et al (2017) In-situ SERS monitoring of reaction catalyzed by multifunctional Fe3O4@TiO2@Ag–Au microspheres. Appl Catal B-Environ 205:11–18
Hui C, Shen CM, Tian JF et al (2011) Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 3:701–705
Wang CW, Xu SP, Zhang KH, Li M, Li QJ, Xiao R, Wang SQ (2017) Streptomycin-modified Fe3O4–Au@Ag core-satellite magnetic nanoparticles as an effective antibacterial agent. J Mater Sci 52:1357–1368. https://doi.org/10.1007/s10853-016-0430-6
Guo B, Yim H, Khasanov A, Stevens J (2010) Formation of magnetic FexOy/silica core-shell particles in a one-step flame aerosol process. Aerosol Sci Tech 44:281–291
Morel AL, Nikitenko SI, Gionnet K et al (2008) Sonochemical approach to the synthesis of Fe3O4@SiO2 core-shell nanoparticles with tunable properties. ACS Nano 2:847–856
Du JJ, Xu JW, Sun ZL, Jing CY (2016) Au nanoparticles grafted on Fe3O4 as effective SERS substrates for label-free detection of the 16 EPA priority polycyclic aromatic hydrocarbons. Anal Chim Acta 915:81–89
Choi J-Y, Kim K, Shin KS (2010) Surface-enhanced Raman scattering inducible by recyclable Ag-coated magnetic particles. Vib Spectrosc 53:117–120
Du QJ, Tan LF, Li B, Liu TL, Ren J, Huang ZB, Tang FQ, Meng XW (2014) One-pot gradient solvothermal synthesis of the Ag/Au–Fe3O4 composite nanoparticles and their applications. RSC Adv 4:56057–56062
Gao Q, Zhao AW, Gan ZB et al (2012) Facile fabrication and growth mechanism of 3D flower-like Fe3O4 nanostructures and their application as SERS substrates. CrystEngComm 14:4834–4842
Yang LB, Bao ZY, Wu YC, Liu JH (2012) Clean and reproducible SERS substrates for high sensitive detection by solid phase synthesis and fabrication of Ag-coated Fe3O4 microspheres. J Raman Spectrosc 43:848–856
Quaresma P, Osório I, Dória G et al (2013) Star-shaped magnetite@gold nanoparticles for protein magnetic separation and SERS detection. RSC Adv 4:3659–3667
Reguera J, Aberasturi DJ, Winckelmans N, Langer J, Balsd S, Liz-Marzán LM (2016) Synthesis of Janus plasmonic-magnetic, star-sphere nanoparticles, and their application in SERS detection. Faraday Discuss 191:47–59
Wang CW, Li P, Wang JF, Rong Z, Pang YF, Xu JW, Xiao R, Wang SQ (2015) Polyethylenimine-interlayered core-shell-satellite 3D magnetic microspheres as versatile SERS substrate. Nanoscale 7:18694–18707
Tian Y, Chen LJ, Zhang J, Ma ZF, Song CN (2012) Bifunctional Au-nanorod@Fe3O4 nanocomposites: synthesis, characterization, and their use as bioprobes. J Nanopart Res 14:998–1009
Yuen C, Liu Q (2012) Magnetic field enriched surface enhanced resonance Raman spectroscopy for early malaria diagnosis. J Biomed Opt 17:017005–017013
Jun BH, Noh MS, Kim J et al (2010) Multifunctional silver-embedded magnetic nanoparticles as SERS nanoprobes and their applications. Small 6:119–125
Chen Y, Chen H, Shi J (2013) In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater 25:3144–3176
Fan CZ, Zhu SM, Xin HY, Tian YC, Liang EJ (2017) Tunable and enhanced SERS activity of magneto-plasmonic Ag-Fe3O4 nanocomposites with one pot synthesize method. J Opt 19:015401–015408
Pang YF, Wang CW, Wang J, Sun ZW, Xiao R, Wang SQ (2016) Fe3O4@Ag magnetic nanoparticles for microRNA capture and duplex-specific nuclease signal amplification based SERS detection in cancer cells. Biosens Bioelectron 79:574–580
Wu L, Xiao XY, Chen K et al (2017) Ultrasensitive SERS detection of Bacillus thuringiensis special gene based on Au@Ag NRs and magnetic beads. Biosens Bioelectron 92:321–327
Chen QS, Yang MX, Yang XJ, Li HH, Guo ZM, Rahma MH (2018) A large Raman scattering cross-section molecular embedded SERS aptasensor for ultrasensitive Aflatoxin B1 detection using CS-Fe3O4 for signal enrichment. Spectrochim Acta A 189:147–153
Zhou X, Xu WL, Wang Y, Kuang Q, Shi YF, Zhong LB, Zhang QQ (2010) Fabrication of cluster/shell Fe3O4/Au nanoparticles and application in protein detection via a SERS method. J Phys Chem C114:19607–19613
Kong XM, Yu Q, Lv ZP, Du XZ (2013) Tandem assays of protein and glucose with functionalized core/shell particles based on magnetic separation and surface-enhanced Raman scattering. Small 9:3259–3264
Balzerova A, Fargasova A, Markova Z, Ranc V, Zboril R (2014) Magnetically-assisted surface enhanced Raman spectroscopy (MASERS) for label-free determination of human immunoglobulin G(IgG) in blood using Fe3O4@Ag nanocomposite. Anal Chem 86:11107–11114
Yang K, Hu YJ, Dong N, Zhu GC, Zhu TF, Jiang NJ (2017) A novel SERS-based magnetic aptasensor for prostate specific antigen assay with high sensitivity. Biosens Bioelectron 94:286–291
Chaloupková Z, Balzerová A, Bařinková J, Medříková Z, Šácha P, Beneš P, Ranc V, Konvalinka J, Zbořil R (2018) Label-free determination of prostate specific membrane antigen in human whole blood at nanomolar levels by magnetically assisted surface enhanced Raman spectroscopy. Anal Chim Acta 997:44–51
Kearns H, Goodacre R, Jamieson LE, Graham D, Faulds K (2017) SERS detection of multiple antimicrobial-resistant pathogens using nanosensors. Anal Chem 89:12666–12673
Zhang L, Xua JJ, Mi L, Gong H, Jiang SY, Yu QM (2012) Multifunctional magnetic–plasmonic nanoparticles for fast concentration and sensitive detection of bacteria using SERS. Biosens Bioelectron 31:130–136
Zhang H, Ma X, Liu Y, Duan N, Wu SJ, Wang ZP, Xu BC (2015) Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens Bioelectron 74:872–877
Wang CW, Wang JF, Li M, Qu XY, Zhang KH, Rong Z, Xiao R, Wang SQ (2016) A rapid SERS method for label-free bacteria detection using polyethylenimine-modified Au-coated magnetic microspheres and Au@Ag nanoparticles. Analyst 141:6226–6238
Rong Z, Wang CW, Wang JF, Wang DG, Xiao R, Wang SQ (2016) Magnetic immunoassay for cancer biomarker detection based on surface-enhanced resonance Raman scattering from coupled plasmonic nanostructures. Biosens Bioelectron 84:15–21
Lin Y, Xu GH, Wei FD, Zhang AX, Yang J, Hu Q (2016) Detection of CEA in human serum using surface-enhanced Raman spectroscopy coupled with antibody-modified Au and γ-Fe2O3@Au nanoparticles. J Pharm Biomed 121:135–140
Ge M, Wei C, Xu MM, Fang CW, Yuan YX, Gu RN, Yao JL (2015) Ultra-sensitive magnetic immunoassay of HE4 based on surface enhanced Raman spectroscopy. Anal Methods 7:6489–6495
Yang TX, Guo XY, Wu YP, Wang H, Fu SY, Wen Y, Yang HF (2014) Facile and label-free detection of lung cancer biomarker in urine by magnetically assisted surface-enhanced Raman scattering. ACS Appl Mater Interface 6:20985–20993
Ouyang L, Zhu LH, Jiang JZ, Tang HQ (2014) A surface-enhanced Raman scattering method for detection of trace glutathione on the basis of immobilized silver nanoparticles and crystal violet probe. Anal Chim Acta 816:41–49
Wang YL, Ravindranath S, Irudayaraj J (2011) Separation and detection of multiple pathogens in a food matrix by magnetic SERS nanoprobes. Anal Bioanal Chem 399:1271–1278
Guven B, Basaran-Akgul N, Temur E, Ur Tamer, Boyac IH (2011) SERS-based sandwich immunoassay using antibody coated magnetic nanoparticles for Escherichia coli enumeration. Analyst 136:740–748
Najafi R, Mukherjee S, Hudson J Jr, Sharma A, Banerjee P (2014) Development of a rapid capture-cum-detection method for Escherichia coli O157 from apple juice comprising nano-immunomagnetic separation in tandem with surface enhanced Raman scattering. Int J Food Microbiol 18:89–97
Ashley J, Wu KY, Hansen MF, Schmidt MS, Boisen A, Sun Y (2017) Quantitative detection of trace level cloxacillin in food samples using magnetic molecularly imprinted polymer extraction and surface-enhanced Raman spectroscopy nanopillars. Anal Chem 89:11484–11490
Liu ZG, Wang Y, Deng R, Yang LY, Yu SH, Xu SP, Xu WQ (2016) Fe3O4@graphene oxide@Ag particles for surface magnet solid-phase extraction surface-enhanced Raman scattering (SMSPE-SERS): from sample pretreatment to detection all-in-one. ACS Appl Mater Interface 8:14160–14168
Yang TX, Guo XY, Wang H, Fu SY, Yu J, Wen Y, Yang HF (2014) Au dotted magnetic network nanostructure and its application for on-site monitoring femtomolar level pesticide. Small 10:1325–1331
Zhang XL, Niu CY, Wang YQ, Zhou SM, Liu J (2014) Gel-limited synthesis of dumbbell-like Fe3O4–Ag composite microspheres and their SERS applications. Nanoscale 6:12618–12625
Zheng HH, Zou BF, Chen L, Wang YQ, Zhang XL, Zhou SM (2015) Gel-assisted synthesis of oleate-modified Fe3O4@Ag composite microspheres as magnetic SERS probe for thiram detection. CrystEngComm 17:6393–6398
Guo HY, Zhao AW, Wang RJ et al (2015) Generalized green synthesis of Fe3O4/Ag composites with excellent SERS activity and their application in fungicide detection. J Nanopart Res 17:1–10
Tang XH, Don RL, Yang LB, Liu JH (2015) Fabrication of Au nanorod-coated Fe3O4 microspheres as SERS substrate for pesticide analysis by near-infrared excitation. J Raman Spectrosc 46:470–475
Tang XH, Cai WY, Yang LB, Liu JH (2013) Highly uniform and optical visualization of SERS substrate for pesticide analysis based on Au nanoparticles grafted on dendritic α-Fe2O3. Nanoscale 5:11193–11199
Sun ZL, Du JJ, Yan L, Chen S, Yang ZL, Jing CY (2016) Multifunctional Fe3O4@SiO2-Au satellite structured SERS probe for charge selective detection of food dyes. ACS Appl Mater Interface 8:3056–3062
Hu HB, Wang ZH, Pan L, Zhao SP, Zhu SY (2010) Ag-coated Fe3O4@SiO2 three-ply composite microspheres: synthesis, characterization, and application in detecting melamine with their surface-enhanced Raman scattering. J Phys Chem C 114:7738–7742
Yu SH, Liu ZG, Wang WX, Jin L, Xu WQ, Wu YQ (2018) Disperse magnetic solid phase microextraction and surface enhanced Raman scattering (Dis-MSPME-SERS) for the rapid detection of trace illegally chemicals. Talanta 178:498–506
Chen JH, Pang S, He LL, Nugen SR (2016) Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy. Biosens Bioelectron 85:726–733
Mezni A, Balti I, Mlayah A, Jouini N, Smiri LS (2013) Hybrid Au-Fe3O4 nanoparticles: plasmonic, surface enhanced raman scattering, and phase transition properties. J Phys Chem C 117:16166–16174
Cai WY, Tang XH, Sun B, Yang LB (2014) Highly sensitive in situ monitoring of catalytic reactions by surface enhancement Raman spectroscopy on multifunctional Fe3O4/C/Au NPs. Nanoscale 6:7954–7958
Lv B, Sun ZL, Zhang JF, Jing CY (2017) Multifunctional satellite Fe3O4-Au@TiO2 nano-structure for SERS detection and photo-reduction of Cr(VI). Colloid Surf A 513:234–240
Qin SH, Cai WY, Tang XH, Yang LB (2014) Sensitively monitoring photodegradation process of organic dye molecules by surface-enhanced Raman spectroscopy based on Fe3O4@SiO2@TiO2@Ag particle. Analyst 139:5509–5515
Ding QQ, Zhou HJ, Zhang HM, Zhang YX, Wang GZ, Zhao HJ (2016) 3D Fe3O4@Au@Ag nanoflowers assembled magnetoplasmonic chains for in situ SERS monitoring of plasmon-assisted catalytic reactions. J Mater Chem A 4:8866–8874
Wu Y, Yang H, Zhu L, Xie AJ, Li SK, Song JM, Shen YH (2014) Multifunctional SERS substrates of Fe3O4@Ag2Se/Ag: construction, properties and application. Anal Methods 6:7083–7087
Ye M, Wei ZW, Hu F et al (2015) Fast assembling microarrays of superparamagnetic Fe3O4@Au nanoparticle clusters as reproducible substrates for surface-enhanced Raman scattering. Nanoscale 7:13427–13437
Gao Q, Zhao AW, Gan ZB et al (2012) Facile fabrication and growth mechanism of 3D flower-like Fe3O4 nanostructrues and their application as SERS substrates. CrystEngComm 14:4834–4842
Chen FH, Wang YW, Chen QT, Han LF, Chen ZJ, Fang SM (2014) Multifunctional nanocomposites of Fe3O4–graphene–Au for repeated use in simultaneous adsorption, in situ SERS detection and catalytic reduction of 4-nitrophenol in water. Mater Res Express 1:299–308
Ding GH, Xie S, Zhu YM, Liu Y, Wang L, Xu FG (2015) Graphene oxide wrapped Fe3O4@Au nanohybrid as SERS substrate for aromatic dye detection. Sensor Actuat B-Chem 221:1084–1093
Zhang LL, Bao ZW, Yu XX et al (2016) Rational design of α–Fe2O3/reduced graphene oxide composites: rapid detection and effective removal of organic pollutants. ACS Appl Mater Interface 8:6431–6438
An Q, Zhang P, Li JM, Ma WF, Guo J, Hu J, Wang CC (2012) Silver-coated magnetite-carbon core–shell microspheres as substrate enhanced SERS probes for detection of trace persistent organic pollutants. Nanoscale 4:5210–5216
Song J, Chen ZP, Jin JW, Chen Y, Yu RQ (2014) Quantitative surface-enhanced Raman spectroscopy based on the combination of magnetic nanoparticles with an advanced chemometric model. Chemom Intell Lab 135:31–36
Song D, Yang R, Wang CW, Xiao R, Long F (2016) Reusable nanosilver-coated magnetic particles for ultrasensitive SERS-based detection of malachite green in water samples. Sci Rep 6:22870–22879
Niu CY, Zou BF, Wang YQ, Cheng L, Zheng HH, Zhou SM (2016) Highly sensitive and reproducible SERS performance from uniform film assembled by magnetic noble metal composite microspheres. Langmuir 32:858–863
Sun ZL, Du JJ, Lv B, Jing CY (2016) Satellite Fe3O4@SiO2–Au SERS probe for trace Hg2+ detection. RSC Adv 6:73040–73044
Du JJ, Jing CY (2011) Preparation of Fe3O4@Ag SERS substrate and its application in environmental Cr(VI) analysis. J Colloid Interface Sci 358:54–61
Du JJ, Cui JL, Jing CY (2014) Rapid in situ identification of arsenic species using a portable Fe3O4@Ag SERS sensor. Chem Commun 50:347–349
Gan ZB, Zhao AW, Zhang MF et al (2013) Fabrication and magnetic-induced aggregation of Fe3O4-noble metal composites for superior SERS performances. J Nanopart Res 15:15662–15666
Esenturk EN, Walker ARH (2013) Gold nanostar @ iron oxide core-shell nanostructures: synthesis, characterization, and demonstrated surface-enhanced Raman scattering properties. J Nanopart Res 15:1364–1374
Wang ZJ, Wu LN, Wang FP, Jiang ZH, Shen BZ (2013) Durian-like multi-functional Fe3O4–Au nanoparticles: synthesis, characterization and selective detection of benzidine. J Mater Chem A 1:9746–9751
Zhu SM, Fan CZ, Wang JQ, He JN, Liang EJ, Chao MJ (2015) Realization of high sensitive SERS substrates with one-pot fabrication of Ag–Fe3O4 nanocomposites. J Colloid Interface Sci 438:116–121
Liu B, Bai C, Zhao D et al (2016) Novel ferroferric oxide/polystyrene/silver core–shell magnetic nanocomposite microspheres as regenerable substrates for surface-enhanced Raman scattering. Appl Surf Sci 364:628–635
Shen M, Chen SQ, Jia WP, Fan GD, Jin YX, Liang HD (2016) Facile synthesis of Ag@Fe3O4@C–Au core-shell microspheres for surface-enhanced Raman scattering. Gold Bull 49:103–109
Caro C, Sayagues MJ, Franco V, Conde A, Zaderenko P, Gámez F (2016) A hybrid silver-magnetite detector based on surface enhanced Raman scattering for differentiating organic compounds. Sensor Actuat B-Chem 228:124–133
Du JJ, Jing CY (2011) Preparation of thiol modified Fe3O4@Ag magnetic SERS probe for PAHs detection and identification. J Phys Chem C 115:17829–17835
Hu YX, Sun YG (2012) Stable magnetic hot spots for simultaneous concentration and ultrasensitive surface-enhanced Raman scattering detection of solution analytes. J Phys Chem C 116:13329–13335
Shen JH, Zhu YH, Yang XL, Zong J, Li CZ (2013) Multifunctional Fe3O4@Ag/SiO2/Au core–shell microspheres as a novel SERS-activity label via long-range plasmon coupling. Langmuir 29:690–695
Liu HL, Yang LB, Liu JH (2016) Three-dimensional SERS hot spots for chemical sensing: towards developing a practical analyzer. Trac-Trend Anal Chem 80:364–372
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21665011 and 21705063) and Natural Science Foundation of Jiangxi Province (20161BAB203088).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, H., Xu, F. & Wang, L. A review of the preparation and application of magnetic nanoparticles for surface-enhanced Raman scattering. J Mater Sci 53, 8677–8698 (2018). https://doi.org/10.1007/s10853-018-2095-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2095-9