Abstract
A highly selective electrochemical sensor for lead ions was fabricated using mutiwalled carbon nanotube as the backbone. The binding sites for lead ions were sculpted with lead ion as template and NNMBA-crosslinked polyacrylamide as the solid matrix on MWCNTs (MWCNT-IIP) on lead ion sensing and selectivity. System without lead ions was also synthesized (MWCNT-NIP). To check the role of the MWCNT, ion-imprinted polymer (IIP) and non-imprinted polymer (NIP) without MWCNT were also synthesized. In both systems, the ion-imprinted polymer showed high specificity towards lead ion. The developed materials were characterized using various analytical techniques. The sites left by the lead ions in MWCNT-IIP are highly selective to lead ions and resulted in electrochemical response when a platinum electrode was modified with this nanostructure. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were employed to explore the features of the developed electrochemical sensor towards lead ions. The developed material could sense Pb(II) ions in the presence of other metal ions, and the limit of detection was found to be 2 × 10−2 μM. The sensing system could successfully discriminate Pb(II) ions from different real samples which includes environmental sample such as lake water, mining effluent, food sample and cosmetics. Also the same was exploited for the extraction of Pb(II) ions. The recoveries from various samples using MWCNT-IIP were > 99%. But those of MWCNT-NIP were in the range 62–68%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monitoring of heavy metals at low concentration is important as in the case of human health and environmental protection [1]. The hazardous heavy metals are non-biodegradable and can accumulate in living tissues causing dangerous physiological disorders. The major environmental metal pollutions are the collective effect of toxic heavy metals such as lead, copper, mercury and cadmium [2]. Lead is a leading toxic heavy metal that produces many threats to human health. The highly toxic Pb(II) ion disturbs almost all organs and systems in the body [3]. The main origins of Pb(II) ions are aquatic system, food, painting industry, cosmetics, automobile exhausts, old paints, mining wastes and water from lead pipes. Lead ion poisoning produces constipation, gastrointestinal disorder, central nerve system damage and abdominal pain [4, 5]. It can also affect our kidney function, reproduction system, liver, brain functions and so on. The toxicity symptoms of Pb(II) ions are headache, renal damage, anaemia, dizziness and hallucination [6]. The poisonous Pb(II) ion should be removed from our environment in order to protect human health. Several methods have been reported for metal detection such as chemical precipitation, membrane filtration, ion-exchange, cloud point extraction [7], optical emission spectroscopy [8], liquid–liquid extraction [9] and solid-phase extraction [10]. However, majority of these methods have some disadvantages like expensive instruments [11], usage of toxic solvents, needs of large volume of solvents and lack of selectivity and specificity. But an electrochemical sensor shows high selectivity, inexpensive solvents and with very low detection limits. There are large number of voltammetric methods for the electrochemical sensing of Pb(II) ions. But it shows some limitations, such as low sensitivity, high over potential, electrode contamination and low reproducibility [12]. Different methods have been reported to improve sensitivity and selectivity properties of electrochemical sensors.
Considering all these limitations, we have developed an electrochemical sensor based on molecular imprinting technology on MWCNT. Molecular imprinting technology has some special features that help to improve the selectivity of an electrochemical sensor [13, 14]. Nowadays imprinted polymers considered as selective adsorbents for some exact chemical form of the given element have got much more attention [15]. In addition, they show tremendous applications in sensors, membrane separations of the imprinted toxic metal ions and in solid-phase extractions [16]. MIPs have some unique properties such as chemical stability, low cost, high selectivity and easy to preparation [17,18,19,20,21]. MIPs are normally synthesized by polymerizing a mixture of template, functional monomers and a crosslinker [22]. The specific cavities complementary to the size and shape of the template molecules are created after the removal of template from the polymer. Nowadays, MIPs explore all the areas for large applications such as materials for solid-phase extraction [23], artificial enzymes sensor for detection of drugs [24, 25], proteins [26,27,28,29], peptides [30] and cancer viruses [31]. However, some limitations that MIPs exhibit are poor site availability for templates, slow binding kinetics and low conductivity [32]. These drawbacks will reduce the sensing possibilities of MIPs. In order to alter the situation, the nanomaterial MWCNTs can be an exceptional choice as a supporting material to defeat the troubles that are associated with the use of MIPs. Current studies demonstrated that MWCNTs show tremendous electrocatalytic properties because of their significant mechanical and electronic properties. The exceptional properties of MWCNTs make them to be a striking electrochemical sensor [33, 34]. Because of their nanodimension, high electrical conductivity, good chemical stability, electronic structure and topological imperfection, MWCNTs exhibit excellent electron transfer reactions when it employed as electrode objects [35,36,37]. Therefore, MWCNTs have been successfully applied in the fabrication of electrochemical sensor for a wide range of applications [38,39,40,41,42]. The electrochemical sensors have got great attention during these years, because of their low cost, portability and ease of operation techniques [43].
In the present study, the fabrication of an electrochemical sensor and sorbent for Pb(II) ions using molecular imprinting technology on MWCNTs is effectively contemplated. Pb(II) ion-imprinted polymer layered on MWCNTs was applied as the electrode modifier. To find out the role of MWCNT on the specific rebinding of Pb(II) ions, conventional IIP and NIP without MWCNT were also synthesized from acrylamide and NNMBA. Electrochemical sensing studies using these IIP/NIP-modified platinum electrodes were carried out using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). In both cases, the imprinted system showed high specificity towards Pb(II) ions. Due to the nanosize and easy accessibility of the binding site and covering of the surface of MWCNT with the imprinted layer, the MWCNT-IIP/PE brings innovative possibilities for electrochemical sensing owing to the combined effect of IIPs and functionalized MWCNTs. The effect of pH, scan rate and selectivity of the developed sensor was studied by the use of cyclic voltammetry. The limit of detection of the sensor was analysed using differential pulse voltammetry. The developed system was successfully used for the fast sensing and extractions of Pb(II) ions in various real samples such as lake water, mining effluent, food sample and cosmetics.
Experimental
Materials and apparatus
MWCNT, Nafion solution (5 wt%) and 2, 2′ azobisisobutyronitrile (AIBN) were purchased from Sigma-Aldrich (USA). Allyl alcohol, thionylchloride (SOCl2), chloroform, tetrahydrofuran (THF), 4-dimethylaminopyridine (DMAP), lead nitrate, cadmium chloride, zinc chloride and ferric chloride were obtained from Merck (India). Triethylamine (TEA), acrylamide (AA) and N,N′-methylene-bis-acrylamide were purchased from SRL (India). FTIR studies were done by Perkin-Elmer 400 FTIR spectrometer. The morphological studies were carried out using JEOL-2100 model transmission electron microscope. X-ray diffraction studies by PAN analytic XPERT-PRO. The entire electrochemical studies were done by Biologic SP-200 electrochemical workstation. Atomic absorption spectral studies of metal ion solutions were carried out in a Perkin-Elmer Atomic Absorption Spectrometer (Pinnacle 900H).
Electrochemical sensing of Pb(II) ions
Synthesis of vinyl functionalized MWCNTs
For MWCNT functionalization, crude MWCNTs (0.5 g) were suspended in 60 mL Conc.HNO3 and sonicated for 10 min. The mixture was stirred at 85 °C for 16 h and cooled to room temperature. Filtered through a 0.22-µm polycarbonate film and washed with distilled water until the filtrate became neutral. The solid was dried out using vacuum, and acid-functionalized MWCNTs (MWCNTs-COOH) was obtained.
For acyl functionalization, MWCNTs-COOH (0.4 g) was suspended in the mixture of 10 mL of sulfoxide chloride (SOCl2) and 30 mL chloroform at 60 °C for 24 h under reflux. The solid was washed by anhydrous tetrahydrofuran (THF) for a number of times to take away the excess SOCl2 and dried under vacuum to give MWCNTs-COCl.
For vinyl functionalization of MWCNTs-COCl, 1.16 g of allyl alcohol, 0.244 g of 4-dimethylaminopyridine and 6.06 g of triethylamine were added to 0.2 g of MWCNTs-COCl in anhydrous 30 mL THF. The mixture was stirred at 60 °C for 24 h, then collected by centrifugation and washed with anhydrous THF. After the washing and centrifugation, the solid was dried in vacuum desiccators.
Synthesis of Pb(II) ion-imprinted and non-imprinted polymers
MWCNT-IIP with lead ion as template was synthesized by polymerizing IIP onto the surface of vinyl group fabricated MWCNT. Molar amounts of MWCNT-CH=CH2, Pb(II) ion and NNMBA were taken for the synthesis. Lead and acrylamide were dissolved in distilled water and added to the MWCNT-CH=CH2 and mixed. The crosslinker NNMBA and initiator AIBN were also added. The reaction temperature was increased to 70 °C, and the reaction was permitted to continue for 5 h. The obtained polymer was centrifuged and washed with water to remove unreacted monomer and crosslinker. The polymer was eluted by water for several times to pull out the template Pb(II) ions until no lead ions could be identified by AAS in the eluent. The polymers obtained were dried in the vacuum desiccator for 24 h. For evaluation, MWCNT-NIP without using template lead ion was also prepared in the same way. To decipher the role of MWCNT on the sensing and extraction of the template Pb(II) ions, conventional IIP and NIP without MWCNT were also prepared.
Fabrication of modified platinum electrodes
Before the electrode modification, the platinum electrode was cleaned with 3.0 M nitric acid for 10 min, after washing with water. The platinum electrode was wiped with cotton to clean the surface and dried. The respective polymer paste was prepared by mixing the powder with Nafion solution using a mortar. The paste was taken in a syringe to avoid unwanted contamination and placed it on the electrode and kept it for 30 min for complete drying.
Detection of Pb(II) ion
For the detection of Pb(II) ion, the MWCNT-IIP/PE was dipped in 10 mL of 5 ppm Pb(II) ion solution. The solution pH was preserved as 5.0 using acetate buffer solution (ABS). Provide maximum of 10 min to MWCNT-IIP/PE for the equilibration of Pb(II) ion from the solution. It leads to the assembly of Pb(II) ion in the imprinted cavities of MWCNT-IIP on the surface of platinum electrode. Then, the electrochemical studies like cyclic voltammetry and differential pulse voltammetry were carried out in a potential range of − 400 to + 1200 mV with a scan rate of 100 mV/s.
Sensing of Pb(II) ion by MWCNT-IIP/PE from real samples
For the demonstration of practical application of the developed system, four different real samples were selected which include lake water, mining effluent, food sample and cosmetics. Before the sensing, the samples were centrifuged to remove unwanted interfering substances. MWCNT-IIP/PE was introduced into the samples and equilibrated. The electrochemical measurements were carried out. For comparison, the experiments were repeated with MWCNT-NIP/PE.
Lead ion extraction by imprinted and non-imprinted polymers
Extraction procedure
Definite amount of the respective imprinted and non-imprinted polymer was equilibrated with different concentrations of Pb(II) ion solutions ranging from 1 to 5 ppm. The metal ions bound by the polymer were calculated by the equation,
where C o (ppm) and C e (ppm) are the initial and equilibrium concentrations, V (L) is the volume of Pb(II) ion solution and M (g) is the weight of the adsorbent.
Adsorption isotherm helps to know the theoretical values of adsorption capacity. Langmuir and Freundlich equations are used for this purpose. The Langmuir adsorption is suitable for monolayer adsorption. The equation of Langmuir isotherm model is represented as:
where q e is the amount of adsorbate on the surface of adsorbent, C e is the equilibrium concentration of template solution, q o is the maximum surface density and b is the Langmuir adsorption constant.
The equation of Freundlich isotherm model is represented as:
where K is the Freundlich constant and n is the Freundlich exponent.
Time study
In order to check the adsorption kinetics of Pb(II) ions, a batch equilibrium method was conducted. 0.01 g of MWCNT-IIP/IIP/MWCNT-NIP/NIP was treated with 10 mL of template solution. At regular intervals of time, the sorbent particles were removed from the mixture by centrifugation. The Pb(II) ion in the supernatant was analysed by AAS. The amount of Pb(II) ion at each time interval was calculated with Eq. (1).
The adsorption kinetic parameters were determined using pseudo-second-order rate equation. The equation was represented as:
where K 2 is the rate constant of pseudo-second-order rate equation. Q e is the amount of Pb(II) ion (mg L−1) at equilibrium, and Q t is the amount of Pb(II) ion (mg L−1) at time t (min). From the graph t/Q t versus t, the time needed for the maximum adsorption of template at equilibrium was calculated. Moreover, corresponding rate constant of the reaction was determined.
Adsorbent dosage
To determine the effect of adsorbent dosage of MWCNT-IIP/IIP/MWCNT-NIP/NIP towards Pb(II) ion, different masses of adsorbent ranging from 10 to 50 mg of MWCNT-IIP/IIP/MWCNT-NIP/NIP were added to optimum concentration of Pb(II) ion solution. The Pb(II) ion bound by the polymer was calculated using Eq. (1).
Reusability
The reusability of the polymer was tested for 6 cycles under the same conditions with the same polymer. For this purpose, 0.01 g of the polymer (MWCNT-IIP/IIP/MWCNT-NIP/NIP) was mixed with Pb(II) ion solution having concentration 5 ppm. The mixture was then equilibrated for 4 h. After equilibration, the solution was filtered and the amount of Pb(II) ion bound by the polymer was tested by AAS. Then, the samples were washed and dried for the next cycles.
Selectivity study
The selectivity of the imprinted polymers towards the template Pb(II) ion was carried out by batch equilibrium method. For this, 5 ppm solution of Pb(II), Cd(II), Zn(II) and Fe(II) ion solutions was used. The selectivity parameters were calculated using the following equations.
where Q denotes adsorption capacity (mg L−1), C e is represented as concentration of metal ions at equilibrium (mg L−1). D Pb and D M (mg L−1) notes the distribution ratios of Pb(II) ion and other competitive ions, respectively. α r represents relative selectivity coefficient, αi shows the selectivity factors of imprinted adsorbent, and α n is the selectivity factor of non-imprinted polymers, respectively.
Swelling study
MWCNT-IIP/IIP/MWCNT-NIP/NIP (0.01 g) was permitted to swell in water for 24 h. After equilibration, sorbents were filtered and excess water was rubbed off carefully. The swollen weights of the polymer were noted. The equilibrium water content of the sample was calculated using the equation
Effect of pH
MWCNT-IIP/IIP/MWCNT-NIP/NIP (0.01 g) was equilibrated with 10 mL of Pb(II) ion solution having concentration 5 mg L−1 at different pH. The solution was filtered, and the amount of Pb(II) ion removed by the polymer at each pH was analysed by AAS.
Dispersion test
The dispersibility of MWCNT, MWCNT-CH=CH2 and MWCNT-IIP was checked with water as the solvent. 0.01 g of each sample was dispersed in 30 mL solvent and sonicated for half an hour. The dispersibility of the samples was observed very closely and recorded.
Extraction of Pb(II) ion from real samples
For the analytical extraction of Pb(II) ion, 50 mg/L of MWCNT-IIP/MWCNT-NIP/IIP/NIP was filled in a SPE (solid-phase extraction) glass tube and the respective test solution was percolated at a fixed flow rate with suitable pH. The concentration of lead ions in the eluent was followed by atomic absorption spectroscopy. The experiment was triplicate.
Results and discussion
Characterization techniques
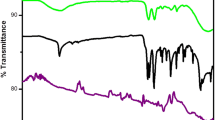
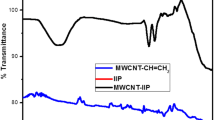
Figure 1a shows the FTIR spectra of MWCNT, MWCNT-CH=CH2 and MWCNT-IIP. Pristine MWCNT has no characteristic peaks in IR spectra confirmed that they do not carry any functional groups. But after vinyl functionalization, spectrum exhibits characteristics peak of C=C at 1630 cm−1. The result suggests that C=C vinyl functionalization is successful. In MWCNT-IIP, a strong broad peak was obtained at 3300 cm−1 due to N–H stretching of the amide group of acrylamide. Moreover, intensity of the C=O peak at 1630 cm−1 was increased due to existence of additional –C≡N bond from acrylamide. In addition, several low-intensity peaks from 500 to 1000 cm−1 range are due to the coupling of acrylamide with vinyl functionalized MWCNTs. All the above results confirm vinyl group incorporation and subsequent coploymerization of it with acrylamide and NNMBA.
X-ray diffractogram shows the crystallinity of a polymer. Figure 1b exhibits XRD spectra of MWCNT, MWCNT-CH=CH2 and MWCNT-IIP. The existence of peak at 2θ = 44°, 65° and 80° of MWCNTs shows the crystalline nature. Moreover, MWCNT-IIP shows some intense peaks in addition to the broad peak of the polymer. The results assure that the imprinted layer was successfully coated over the nanostructure. It can be concluded that the crystalline nature of MWCNT was preserved in MWCNT-IIP.
For the specific morphological studies, HR-TEM was used as shown in Fig. 1c. From the TEM images, it is clear that the pristine MWCNTs show narrow–tubular structures with small diameters. But after functionalization, the diameter of the nanotube increases 3 times than that of pristine MWCNTs. On the other hand, MWCNT-IIP demonstrated tubular structure coated with a rigid homogeneous surface. This proves that the polymer layer was successfully coated on the surface of vinyl functionalized MWCNTs.
The dispersibility of MWCNT, functionalized MWCNT and MWCNT-IIP was checked as illustrated in Fig. 1d. The complete dispersion of all the three samples was done with sonication. The dispersibility was checked after one day. MWCNT completely settled after 24 h. It shows that there is no direct interaction between the MWCNT and solvent taken. But functionalized MWCNT, a slight increase is happened for its dispersibility. On other hand, MWCNT-IIP is very easily dispersed in water. And it did not settled down after one day. So the chemical modification of MWCNTs helps to increase its interaction with aqueous medium and thus supports its dispersibility.
The existence and removal of Pb(II) ion bound and imprinted polymers were confirmed by energy-dispersive X-ray analysis shown in Fig. 1e. A clear, sharp signal is present in Pb(II) ion bound polymers due to the presence of Pb(II) ion. But that peak was disappeared as in the case of Pb(II) ion-imprinted polymers. The results confirm the synthesis of Pb(II) ion-imprinted polymers.
The swelling action of metal ion-imprinted and non-imprinted polymer was investigated. Swelling studies explain the morphological difference between the polymers listed in Table 1. As the investigation results, the maximum swelling was observed for MWCNT-IIP. This is due to the adsorption of water in the pockets created by Pb(II) ion by imprinting. In addition, water molecule was entering into the cavity of nanostructures in the polymer metrics. So the swelling of MWCNT-IIP was too high. Such cavity created by Pb(II) ions is absent in non-imprinted polymer and thus decreases the swelling. The order of swelling was MWCNT-IIP > IIP > MWCNT-NIP > NIP.
Electrochemical sensing of Pb(II) ion
Electrochemical sensing of Pb(II) with cyclic voltammetry
Cyclic voltammetry is an efficient technique for the sensing of various chemical components. Figure 2 represents the measured cyclic voltammetric runs of MWCNT-IIP/PE in acetate buffer solution (ABS) having pH 5.0. As in the case of bare electrode, the cyclic voltammogram shows reduced electrochemical response with acetate buffer. Moreover, the cyclic voltammetric response was apparently enhanced when we introduced MWCNT-IIP/PE. In addition, MWCNT-IIP/PE in buffer with template Pb(II) ion shows an obvious reduction peak around − 100 mV and a less intense oxidation peak around 700 mV assigned to the internal electrochemical interaction of MWCNT-IIP on the surface of platinum electrode. But MWCNT-NIP/PE, IIP/PE and NIP/PE did not show any redox peak, but it shows some changes on the electrochemical response when we compared with bare electrode. So MWCNT-NIP/PE, IIP/PE and NIP/PE did not taken for any further studies because of its poor electrochemical behaviour. The results confirm that the metal ions are entered into the cavities of the imprinted polymer and that could be sensed by graphite electrode with a potential range from − 400 to + 1200 mV.
Effect of Pb(II) ion concentration
The effect of concentration of Pb(II) ion on MWCNT-IIP/PE in ABS was studied with cyclic voltammetry as represented in Fig. 3. The redox peak current increases with Pb(II) ion concentration from 1 to 5 ppm and reaches a maximum at 5 ppm. The concentration effect shows a linear relationship with its redox peak current.
Effect of pH
The power of the pH on Pb(II) ion sensing by MWCNT-IIP/PE was optimized to obtain the most excellent performance. The potential and current of MWCNT-IIP-modified electrode were altered in ABS with different pH, and a maximum redox current was attained at pH 5.0 as shown in Fig. 4. In addition, as the pH changed, electrochemical behaviour of Pb(II) ion on MWCNT-IIP/PE may also varied. When we increased pH above 5.0, the free form of Pb(II) ion in the test solution was changed, which was not analysed by MWCNT-IIP/PE; thus, the redox current decreases. So in summary, solution with pH 5.0 is chosen as most appropriate pH condition for sensing Pb(II) ion.
Effect of scan rate
The redox current increases with scan rate in the range from 10 to 100 mV/s as shown in Fig. 5. In addition, the cathodic peak potential totally shifted towards the positive side when the scan rate increased. The shifting of voltammogram towards the positive side represents electrochemical influence of proposed electrochemical sensor towards Pb(II) ion. Besides, the plot of peak current with square root of scan rate shows a linear relationship with a correlation coefficient of 0.9977. The results confirm that the electrode process was adsorption controlled, and the linear behaviour was reliable with electrochemical response of Pb(II) ion on MWCNT-IIP-modified electrode.
Differential pulse voltammetry
DPV was used for the determination of limit of detection (LOD) of proposed electrochemical sensor. DPV was employed to discuss the sensing abilities of Pb(II) ion using MWCNT-IIP/PE as revealed in Fig. 6. The concentration effect of Pb(II) ion towards MWCNT-IIP-modified electrode was checked in acetate buffer of pH 5.0. With the successive addition of Pb(II) ion, current response of MWCNT-IIP/PE was increased and it exhibited oxidative peak around 650 mV. A linear calibration curve that ranges from 1 to 5 ppm with a detection limit of 2 × 10−2 µM is obtained for Pb(II) ion with a correlation coefficient 0.9987. The results prove that the fabricated MWCNT-IIP-modified electrochemical sensor can detect Pb(II) ion at very small concentrations.
Selectivity study
The selectivity study of MWCNT-IIP-modified electrode was done with different metal ions such as Cd(II), Fe(II) and Zn(II) ions. The MWCNT-IIP-modified electrode showed high sensing towards Pb(II) ion as illustrated in Fig. 7. The Pb(II) ion can easily occupied by the cavities created on the surface of the polymer of MWCNT-IIP/PE, and the cavities match with the size of Pb(II) ion and can be easily captured by the MWCNT-IIP/PE. But the size of other metal ions and cavities are mismatching, and thus, the sensing for ions was too low. The redox peak was created only by the specific Pb(II) ion. The results suggested that the only metal that can be sensed by the modified electrode was Pb(II) ion. The fabricated MWCNT-IIP/PE was highly selective towards the target Pb(II) ion.
Sensing of Pb(II) ion from real samples using modified electrodes
The efficiency of developed system was analysed by detection of Pb(II) ion content in wastewater, food samples and cosmetics. The results in Table 2 mentioned that the developed techniques can be used for direct testing of real samples by a simple procedure. The results indicate the quality of the developed system.
MWCNT-IIP as nanosorbent for the extraction of Pb(II) ions
Optimization steps for adsorption study
Effect of concentration The binding abilities of various Pb(II) ion-imprinted and non-imprinted systems were examined in different concentrations ranging from 1 ppm to 5 ppm. The impact shows that the binding of Pb(II) ion by various system increases with increasing the initial concentration of Pb(II) ion solution as shown in Fig. 8. In addition, the concentration of Pb(II) ion bound to MWCNT-IIP was much higher than that of other polymers due to the incorporation of IIP layer on MWCNTs. This nanostructure increases the effective interaction between MWCNT-IIP and template. But on other side, IIP has no such large number of free active binding sites since the absence of nanostructures, it shows a dominant effect. Thus, adsorption capacity was lower than that of MWCNT-IIP. In case of MWCNT-NIP and NIP, they are not having imprinted sites. So the orders of adsorption capacity of the polymers are MWCNT-IIP > IIP > MWCNT-NIP > NIP.
At the initial stage, adsorption capacity was too high because of the availability of free active binding sites. Then, adsorption reaches a maximum and gets saturated as the template concentration increases. From the figure, it is observed that the maximum adsorption found for Pb(II) ion was at 5 ppm.
Adsorption isotherm illustrates the qualitative knowledge about the nature of the solute–surface interactions and also specific relation between the concentration of adsorbate and amount absorbed on adsorbent surface at constant temperature. The adsorption isotherm of Pb(II) ion is better fitted with Langmuir isotherm model. Langmuir model sounds for monolayer adsorption and homogeneous coverage of adsorbent surface.
As described in figure, a straight line graph with correlation coefficient (R 2) of 0.9980 was obtained for MWCNT-IIP by plotting C e/Q against C e. Freundlich isotherm shows a straight line graph with correlation coefficient of 0.9074 by plotting log Q e against log C e, from the Fig. 9, it is clear that Langmuir isotherm model is better fit than Freundlich isotherm model.
Time study
In adsorption kinetics, rate of adsorption was studied as a function of time. From Fig. 10, it is clear that the rate of adsorption of Pb(II) ion by Pb(II) ion-imprinted and non-imprinted system was investigated. As the time increases, the rate of adsorption was also increases. At the initial stage, the imprinted polymers possess large number of active free binding sites and the adsorption shows a sudden increase within 20 min; then, it reaches an adsorption maximum at 40 min. But non-imprinted polymers do not possess large number of binding sites. So the rate of adsorption was lower than that of imprinted polymers. The maximum adsorption capacity was reached within 40 min, and it reaches equilibrium. In addition, pseudo-second-order rate equation was applied to study the kinetics of Pb(II) ion adsorption on MWCNT-IIP. Adsorption kinetics shows a straight line graph of pseudo-second-order rate equation with a correlation coefficient of 0.9984. The results certified that pseudo-second-order kinetic equation provided a better rate as compared to other kinetic rate equation. The rate constant of pseudo-second-order of MWCNT-IIP is 0.5526 min−1, and for IIP it is 0.0172 min−1.
Effect of pH
Adsorption ability of both imprinted and non-imprinted polymer was examined at different pH ranging from 3 to 5.5 and is represented in Fig. 11. The results pointed that adsorption of Pb(II) ion was strictly affected by pH of the medium. From the graph, it is clear that the adsorption of Pb(II) ion by imprinted and non-imprinted polymer was increased with pH. At the initial stage, rate of adsorption increases with pH and it reaches a maximum at pH value of 5.0, and then it decreases as the pH again increases. The reason may be that the stability of the imprinted system may diminish at high alkaline medium. Thus, pH 5.0 is taken as suitable pH for further adsorption studies.
Factors affecting adsorption rate
Adsorbent dosage
The impact of adsorbent dosage of Pb(II) ion adsorption was investigated by changing the dosage from 10 to 50 mg as shown in Fig. 12. From the experimental data, it was found that the efficiency of metal removal increases with adsorbent dosage. The high competency of adsorption with adsorbent dosage is due to the presence of large number of binding sites. The rate of adsorption higher for MWCNT-IIP may be because of raised surface-to-volume ratio of nanostructures. The adsorption rate of non-imprinted polymer was too low as compared to imprinted polymer because of unavailability of specific binding sites.
Reusability
Metal removal is more economical if the polymer synthesized can be reusable. In order to check the reusability of prepared system, the analysis was repeated for 6 successive cycles as represented in Fig. 13. The result proves that the Pb(II) ion-imprinted system can be applied for several times without conservable decrease in its adsorption efficiency. The experiment demonstrated that after 6 cycles the reusability of the system slightly decreased but not noticeable. This may be due to the destruction of few recognition sites in the polymer network at the time of rewashing; thus, some sites were not fit for template accumulation. So the results confirm the reusability and selectivity efficiency of synthesized nanosorbent.
Effect of binding media
Binding medium plays an important function in polymerization. The binding medium for the synthesis of imprinted polymers is to create the porous structure. And all the polymerization elements must be soluble in the binding medium. The effect of binding medium on Pb(II) ion adsorption was studied with three different solvents as illustrated in Fig. 14. As a result, water was selected as good solvent for the adsorption of Pb(II) ion. Acetone and THF show very low adsorption efficiency towards Pb(II) ion because these solvents are not able to create porosity on polymer surface and that will decrease the adsorption rate. So based on the results, water is selected as suitable binding medium for the further studies.
Selectivity study
Three other metals (Fe(II), Cd(II), Zn(II)) were selected as competitive metal ions to check the selectivity of ion-imprinted and non-imprinted polymers towards Pb(II) ion. The results show that the binding capacity of Pb(II) ion by imprinted polymers was too much higher than that of non-imprinted polymers which show no selectivity towards Pb(II) ions (Fig. 15). The reason behind this observation is that the cavities left by lead ions in MWCNT-IIP were complementary in size and shape to the template Pb(II) ion, but there were no such imprinted sites in the case of MWCNT-NIP/NIP. On the basis of these results, it can be concluded that the synthesized Pb(II) ion-imprinted polymer was highly selective towards the template Pb(II) ion from a mixture of its competitive metal ions. The selectivity factor revealed that the Pb(II) metal ion-imprinted polymer shows high selectivity towards Pb(II) ion in Pb–Cd mixture with high selectivity factor because of the similar chemical properties of Pb(II) and Cd(II) ions (Table 3). But in case of Pb–Fe mixture, the imprinted polymer shows low selectivity factor.
Extraction of Pb(II) ion from real samples
In order to check the applicability of developed systems, all the systems were applied to real samples such environmental samples such as mining effluent, lake water, food sample and cosmetics. The analysis was done with AAS. Table 4 shows the extraction efficiency of the developed systems for further applications.
Comparison of various modified electrode for Pb(II) ion detection
Reported information of Pb(II) ion detection by various modified electrodes was compared with this work (Table 5). The results show the competence of the developed system towards Pb(II) ion detection.
Conclusions
A novel, simple and selective electrochemical sensor-based nanosorbent was fabricated for the sensing and extraction of Pb(II) ions. The two-step process established in the present work includes synthesis of MWCNT-IIP and fabrication of MWCNT-IIP/PE sensor towards the detection of Pb(II) ion. The prepared MWCNT-IIP was successfully characterized by FTIR, TEM and XRD. The developed MWCNT-IIP/PE sensor exhibits very good electrochemical detection for Pb(II) ion in ABS having pH 5.0. The sensor has the ability to detect Pb(II) ion at very low limit of detection. The fabricated sensor was successfully applied to real samples for Pb(II) ion sensing. The synthesized system was successfully utilized for the extraction of Pb(II) ion from real samples using AAS. So it concluded that the fabricated system can be used as a powerful tool for the sensing and extraction of Pb(II) ion for future purpose.
References
Lauriane NTR, Najih R, Chtaini A (2014) Electrochemical sensor of heavy metals based on chelating compounds. Pharm Anal Acta 4172:2153
Roy E, Patra S, Madhuri R, Sharma PK (2014) Simultaneous determination of heavy metals in biological samples by a multiple-template imprinting technique: an electrochemical study. RSC Adv 4:56690
Girija P, Beena M (2014) Sorption of trace amounts of Pb(II) ions on an ion imprinted interpenetrating polymer network based on alginic acid and crosslinked polyacrylamide. J Sep Sci Technol 49:1053
Ernhart CB (1992) A critical review of low-level prenatal lead exposure in the human: 2. Effects on the developing child. Reprod Toxicol 6:9
Ernhart CB (1992) A critical review of low-level prenatal lead exposure in the human: 2. Effects on the developing child. Reprod Toxicol 6:21
Naseem R, Tahir S (2001) Removal of Pb(II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res 35:3982
Chen D, Hu B, Huang C (2009) A four-electrode microconstant direct current resistance detector for ion chromatography applying ion-exchange membrane and porous electrode. Talanta 78:491
Chen C, Niu X, Chai Y, Zhao H, Lan M (2013) Bismuth-based porous screen-printed carbon electrode with enhanced sensitivity for trace heavy metal detection by stripping voltammetry. Sens Actuators B Chem 178:339
Abbasi S, Khodarahmiyan K, Abbasi F (2011) Simultaneous determination of ultra trace amounts of lead and cadmium in food samples by adsorptive stripping voltammetry. Food Chem 128:254
Divrikli U, Kartal A, Soylak M, Elci L (2007) Preconcentration of Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II) ions in environmental samples by membrane filtration prior to their flame atomic absorption spectrometric determinations. J Hazard Mater 145:459
Shirzadmehr A, Rezaei M, Bagheri H, Khoshsafar H (2016) Novel potentiometric sensor for the trace-level determination of Zn2+ based on a new nanographene/ion imprinted polymer composite. Int J Environ Anal Chem 96:929
Xu F, Wang L, Gao M, Jin L, Jin J (2002) Amperometric sensor for glucose and hypoxanthine based on a PdIrO2 modified electrode by a co-crosslinking bienzymic system. Talanta 57:365
Cieplak M, Szwabinska K, Sosnowska M, Chandra BKC, Borowicz P, Noworyta K (2015) Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens Bioelectron 74:960
Li J, Wang X, Duan H, Wang Y, Bu Y, Luo C (2016) Based on magnetic graphene oxide highly sensitive and selective imprinted sensor for determination of sunset yellow. Talanta 147:169
Dakova I, Karadjova I, Georgieva V, Georgiev G (2009) Ion-imprinted polymethacrylic microbeads as new sorbent for preconcentration and speciation of mercury. Talanta 78:523
Shamsipur M, Besharati-Seidani A, Fasihi J, Sharghi H (2010) Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper(II) ions. Talanta 83:674
Lanza F, Sellergren B (2001) The application of molecular imprinting technology to solid phase extraction. Chromatographia 53:599
Andersson LI (2000) Molecular imprinting for drug bioanalysis. J Chromatogr B Biomed Sci Appl 739:163
Do MH, Florea A, Farre C, Bonhomme A, Bessueille F, Vocanson F, Tran-Thi N-T, Jaffrezic-Renault N (2015) Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int J Environ Anal Chem 95:1489
Özkahraman B (2017) Synthesis of ion-imprinted bioadsorbents based on chitosan and its usage in Al(III) removal. J Polym Environ 1–8. https://doi.org/10.1007/s10924-017-0996-3
Wang N, Xiao S-J, Su C-W (2016) Preparation of molecularly imprinted polymer for methylene blue and study on its molecular recognition mechanism. Colloid Polym Sci 294(8):1305–1314
Gholivand MB, Torkashvand M (2011) A novel high selective and sensitive metronidazole voltammetric sensor based on a molecularly imprinted polymer-carbon paste electrode. Talanta 84:905
Jiang X, Zhao C, Jiang N, Zhang H, Liu M (2008) Selective solid-phase extraction using molecular imprinted polymer for the analysis of diethylstilbestrol. Food Chem 108:1061
Horemans F, Alenus J, Bongaers E, Weustenraed A, Thoelen R, Duchateau J, Lutsen L, Vanderzande D, Wagner P, Cleij TJ (2010) MIP-based sensor platforms for the detection of histamine in the nano- and micromolar range in aqueous media. J Sens Actuators B Chem 148:392
Jafari MT, Rezaei B, Zaker B (2009) Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal Chem 81:3585
Bossi A, Piletsky SA, Piletska EV, Righetti PG, Turner APF (2001) Surface-grafted molecularly imprinted polymers for protein recognition. Anal Chem 73:5281
Bossi A, Bonini F, Turner APF, Piletsky SA (2007) Molecularly imprinted polymers for the recognition of proteins: the state of the art. Biosens Bioelectron 22:1131
Bonini F, Piletsky S, Turner APF, Speghini A, Bossi A (2007) Surface imprinted beads for the recognition of human serum albumin. Biosens Bioelectron 22:2322
Bereli N, Andaç M, Baydemir G, Say R, Galaev IY, Denizli A (2008) Protein recognition via ion-coordinated molecularly imprinted supermacroporous cryogels. J Chromatogr A 1190:18
Kempe M, Mosbach K (1995) Separation of amino acids, peptides and proteins on molecularly imprinted stationary phases. J Chromatogr 691:317
Wang Y, Zhang Z, Jain V, Yi J, Mueller S, Sokolov J (2010) Potentiometric sensors based on surface molecular imprinting: detection of cancer biomarkers and viruses. Sens Actuators B Chem 146:381
Rezaei B, Rahmanian O, Ensafi AA (2014) An electrochemical sensor based on multiwall carbon nanotubes and molecular imprinting strategy for warfarin recognition and determination. Sens Actuators B Chem 196:539
Sherigara BS, Kutner W, D’Souza F (2003) Electrocatalytic properties and sensor applications of fullerenes and carbon nanotubes. Electroanalysis 15:753
Xu L, Xu Z (2012) Molecularly imprinted polymer based on multiwalled carbon nanotubes for ribavirin recognition. J Polym Res 19:8
Wang Z, Liang Q, Wang Y, Luo G (2003) Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J Electroanal Chem 540:129
Rivas G, Rubianes M, Rodriguez M, Ferreyra N, Luque G, Pedano M (2007) Carbon nanotubes for electrochemical biosensing. Talanta 74:291
Ensafi AA, Karimi-Maleh H (2010) A voltammetric sensor based on modified multiwall carbon nanotubes for cysteamine determination in the presence of tryptophan using p-aminophenol as a mediator. Int J Electrochem Sci 5:392
Wooten M, Gorski W (2010) Facilitation of NADH electro-oxidation at treated carbon nanotubes. Anal Chem 82:1299
Ardakani MM, Beitollahi H, Ganjipour B, Naeimi H (2010) Electrochemical and catalytic investigations of dopamine and uric acid by modified carbon nanotube paste electrode. Int J Electrochem Sci 5:531
Rezaei B, Majidi N, Ensafi AA, Karimi-Maleh H (2011) Molecularly imprinted-multiwall carbon nanotube paste electrode as a biosensor for voltammetric detection of rutin. Anal Met 3:2510
Rezaei B, Mirahmadi-Zare SZ (2011) Nanoscale manipulation of prednisolone as electroactive configuration using molecularly imprinted-multiwalled carbon nanotube paste electrode. Electroanalysis 23:2724
Zhang Z, Hu Y, Zhang H, Luo L, Yao S (2010) Electrochemical layer-by-layer modified imprinted sensor based on multi-walled carbon nanotubes and sol–gel materials for sensitive determination of thymidine. J Electroanal Chem 644:7
Alizadeh T, Amjadi S (2013) Synthesis of nano-sized Eu3+-imprinted polymer and its application for indirect voltammetric determination of europium. Talanta 106:431
Prabakar SJR, Sakthivel C, Narayanan SS (2011) Hg(II) immobilized MWCNT graphite electrode for the anodic stripping voltammetric determination of lead and cadmium. Talanta 85:290
Li H, Li J, Yang Z, Xu Q, Hou C, Peng J (2011) Simultaneous determination of ultratrace lead and cadmium by square wave stripping voltammetry with in situ depositing bismuth at Nafion-medical stone doped disposable electrode. J Hazard Mater 191:26
Senthilkumar S, Saraswathi R (2009) Electrochemical sensing of cadmium and lead ions at zeolite-modified electrodes: optimization and field measurements. Sens Actuators B Chem 141:65
Molina-Holgado T, Pinilla-Macias JM, Hernández-Hernández L (1995) Voltammetric determination of lead with a chemically modified carbon paste electrode with diphenylthiocarbazone. Anal Chim Acta 309:117
Acknowledgements
Maria Sebastian acknowledges the support from the Kerala State for providing KSCSTE (Kerala State Council for Science, Technology and Environment) research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sebastian, M., Mathew, B. Ion imprinting approach for the fabrication of an electrochemical sensor and sorbent for lead ions in real samples using modified multiwalled carbon nanotubes. J Mater Sci 53, 3557–3572 (2018). https://doi.org/10.1007/s10853-017-1787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1787-x