Abstract
Due to their high theoretical capacities and high energy densities, metal fluorides have attracted significant attention as cathodes for lithium-ion batteries. However, thus far, their low conductivities have limited the performance of these materials. In this work, the Fe(1−x)Co x F3/MWCNT (multi-walled carbon nanotube) nanocomposites (x = 0, 0.02, 0.04 and 0.06) are obtained by an in situ solvothermal method with Co-doping and wrapping of the MWCNTs. The results indicate that Co-doping can adjust the crystal structure, decrease the band gaps and enhance the Li+ diffusion coefficient of FeF3. Additionally, the wrapped network of MWCNTs enhances the conductivity of the composites and improves their electrochemical performances. The Fe0.96Co0.04F3/MWCNT nanocomposites exhibit a high initial discharge capacity of 217.0 mAh g−1 at rate of 0.2 C within the potential range of 2.0–4.5 V, which is much higher than that of the FeF3/MWCNT counterpart (192.1 mAh g−1). The discharge capacities of these two samples remain at 187.9 and 160.7 mAh g−1 even after 50 cycles. Meanwhile, the EIS results reveal that both the Li+ charge transfer resistance (R ct = 31.25 Ω) and Li+ diffusion coefficient (1.40 × 10−11 cm2 s−1) are satisfactory from Co-doping and the in situ wrapping of the MWCNTs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With developments in science and technology, the demand for energy is increasingly growing. Lithium-ion batteries (LIBs) have attracted significant attention because of their low cost, low toxicity and sustainable development [1, 2]. FeF3 has attracted considerable attention as an LIBs cathode due to the advantages of a high theoretical capacity (about 712 mAh g−1 in the voltage range of 1.0–4.5 V and 237 mAh g−1 in the range of 2.0–4.5 V), high energy density (~1950 Wh kg−1) and good thermal stability [3,4,5]. Despite these overwhelming advantages, this material still cannot be applied in industry due to the intrinsically slow ionic diffusion and poor conductivity of the fluoride species [6, 7].
To improve the electrochemical performance of FeF3, many methods have been developed, such as creating composites with conductive agents and expanding the crystal structure [8]. The addition of carbon black [9], carbon nanotubes [10], graphene [11, 12] or V2O5 [13] through mechanical ball milling results in FeF3 composites with better electrochemical performances than pure FeF3. The uniform distribution of conductive agents in the composite will benefit the electronic conductivities. Open structures of FeF3, i.e., hexagonal tungsten bronze-type FeF3·0.33H2O [14, 15], pyrochlore-type FeF3·0.5H2O [10, 16] and metal-doped FeF3, can substantially improve the performance of this material [10, 18, 19]. Structural expansion of fluorides can increase ionic conductivities, which has been proved by researchers [8].
In this study, we utilized a solvothermal method and heat treatment to synthesize high-performance Fe(1−x)Co x F3/MWCNTs (multi-walled carbon nanotubes). Since the CoF3 and FeF3 exhibit similar structures and electrochemical properties as the cathode materials in LIBs, the replacement of one Fe atom by a Co atom may have a better performance [17]. It has been found that a suitable Co-doped can slightly distort the crystal structure and still remain structure stable of FeF3 [18]. Additionally, we researched the performances and electronic structures of Co-doped FeF3 with a ReO3-type structure through XRD phase analysis and first-principles methods [19]. It has also been proved that a suitable Co-doped can decrease the band gaps of FeF3 and effectively improve the conductivity of FeF3 [20]. Moreover, through the combination of wrapping with the MWCNTs and Co-doping, the as-prepared Fe(1−x)Co x F3/MWCNTs showed remarkably improved electrochemical properties. The in situ wrapped MWCNTs not only could homogeneously and intimately contact with Fe(1−x)Co x F3 particles, but also improved the particle size distribution. The physicochemical and electrochemical performances of the Fe(1-x)Co x F3/MWCNT nanocomposites were studied in detail.
Experimental
Synthesis of Fe(1-x)Co x F3/MWCNT nanocomposites
Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0, 0.02, 0.04 and 0.06) were synthesized by a solvothermal method. First, 12.12 g of iron(III) nitrate nonahydrate (Fe(NO3)3 ·9H2O) and cobalt(III) oxide (Co2O3) in molar ratios of 1:0, 0.98:0.01, 0.96:0.02 and 0.94:0.03 were homogeneously dissolved in anhydrous ethanol to obtain solutions. Next, 0.2 mL of Tween 80 and the MWCNTs (Fe(1-x)Co x F3: MWCNTs = 0.99:0.01, 0.97:0.03, 0.95:0.05 by weight) were added to the solutions. After the solutions were stirred for 10 min, 12 mL of HF (40 wt%) was added, and the mixtures were transferred to sealed Teflon containers and ultrasonicated for 5 min to form uniform mixtures. Second, the containers were dried at 120 °C under vacuum for 6 h until precipitate was obtained. Then, the precipitates were washed with anhydrous ethanol several times and dried in an oven at 80 °C for 4 h. Finally, the precipitates were calcined at 240 °C for 3 h in a tube furnace under Ar flow (40 sccm), and after high-energy ball milling for 3 h, the Fe(1-x)Co x F3/MWCNT nanocomposites were obtained.
Materials characterization
The crystal structures of the samples were characterized by X-ray diffraction (XRD) patterns on a Bruker D8 Advance diffractometer using Cu Kα radiation. The morphologies of samples were obtained by scanning electron microscopy (SEM, JEOL JMS-6700F) with energy-dispersive spectroscopy (EDS) and transmission electron microscopy (TEM, JEOL JEM-2010).
The working electrodes were composed of the Fe(1-x)Co x F3/MWCNT nanocomposites and polyvinylidene fluoride (PVDF) as a binder at a weight ratio of 95:5 in N-methylpyrrolidone (NMP, Aldrich). Then, each slurry was evenly coated on aluminum foil. Afterward, the electrodes were dried in a vacuum oven at 85 °C for 4 h. A solution of 1 M LiPF6 dissolved in a mixture of ethylene carbonate-dimethyl carbonate-ethyl methyl carbonate (EC-DMC-EMC; 1:1:1, volume ratio) was used as the electrolyte. Coin-type cells (CR2032) were assembled in an Ar-filled glove box with lithium foil as the negative electrode and Celgard 2400 as a separator. The amounts of carbon and cobalt in the samples were verified by elemental analysis (CHNS E.A.).
Electrochemical measurements
Galvanostatic charge–discharge tests were conducted using a Neware CT3008W instrument over a voltage a range from 2.0 to 4.5 V at 25 °C. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed with an electrochemical workstation (Corrtest CS350). The CV experiments were carried out between 2.0 and 4.5 V at a scan rate of 0.1 mV s−1, and the frequency range of the EIS was 105–10−3 Hz at a potentiostatic signal amplitude of 5 mV.
Results and discussion
Structure and morphology characterizations
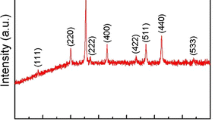
The synthesized samples were characterized by XRD measurements. Figure 1 shows the powder XRD patterns of the Fe(1-x)Co x F3/MWCNT composites (x = 0, 0.02, 0.04 and 0.06), which are in good agreement with those of FeF3 (JCPDS no. 33-0647), in line with previously reported results [9, 18, 21]. It is noticed that the XRD pattern of FeF3 shows the distinct diffraction peaks positioned at 2θ = 24.1°, 48.2° and 54.3°, which correspond to (012), (024) and (116) diffraction peaks of FeF3. Additionally, there are no MWCNT (carbon) signals detected due to its amorphous structure. Table 1 shows the lattice constants of the Fe(1-x)Co x F3/MWCNTs (x = 0, 0.02, 0.04 and 0.06). It is observed that the values of a, b and c become little smaller or bigger, while those of the volume become slightly smaller after Co-doping. It is found that the radius of Fe3+ (0.067 nm) is larger than of Co3+ (0.065 nm), which is possible for Co to occupy the Fe site [19]. As shown by the powder XRD patterns in Fig. 1 (x = 0.02, x = 0.04 and x = 0.06), Co-doping led to only a slight inhomogeneous lattice distortion, but did not significantly influence the FeF3 crystal structure.
The morphologies of the Fe(1-x)Co x F3/MWCNT composites (x = 0, 0.02, 0.04 and 0.06) were characterized by SEM (before and after ball milling). Figure 2a-d shows the SEM images of the FeF3 and Fe(1-x)Co x F3/MWCNT samples (x = 0, 0.02, 0.04 and 0.06) before ball milling. Obviously, the particle sizes with rectangular shapes have average lengths of 8 μm, and the tiny particles attached in the rectangular particles increase with the increasing Co-dopant amount, which can improve the electrolyte permeation and electrochemical performance [18, 22, 23]. Besides, the morphologies of the Fe(1-x)Co x F3/MWCNT nanocomposites, which were obtained by ball milling with different Co contents and a constant MWCNT content, are revealed by the SEM images (Fig. 2e–h).
After ball milling for 3 h, the morphologies of the as-prepared Fe(1-x)Co x F3/MWCNT composites have been changed from rectangular to particles with no visible crystal shape and agglomerating on some level. The particle sizes are reduced to nanoscale (50–200 nm). The MWCNTs are separated into individual carbon nanotubes that reduce the sizes of the interconnected particles, increase the number of nucleation sites and enhance the electronic conductivities [24]. Compared with Fig. 2e–h, the Fe0.96Co0.04F3/MWCNTs (Fig. 2g) exhibit better electrochemical performance due to the minimum amount of agglomeration.
Based on the SEM images in Fig. 3a, EDS elemental mapping was employed to identify the distribution of Fe (Fig. 3b) and Co (Fig. 3c). It is found that Co and Fe are uniformly distributed in the interior space of the Fe0.96Co0.04F3 material. Additionally, EDS (see Fig. 3d) illustrates that the samples contain Fe, Co, F and O (the Cu signal comes from the loading substrate), and no other foreign elements are involved within the whole process.
In order to further accurately observe the morphologies of the Fe0.96Co0.04F3/MWCNT nanocomposites, high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) images are shown in Fig. 4. It can be clearly seen that the crystalline regions of Fe0.96Co0.04F3 and the MWCNTs coexist in the Fe0.96Co0.04F3/MWCNT nanocomposites. In addition, the surfaces of the nanoparticles were fully wrapped by the MWCNT network, which can be beneficial to electron and ion transfer to reduce polarization [25], as shown in Fig. 4a. Figure 4b, c shows the HRTEM of the Fe0.96Co0.04F3/MWCNT nanocomposites. It is noticed that well-crystallized regions and tube-shaped regions coexist in the Fe0.96Co0.04F3/MWCNT nanocomposites. The discrete spots of the SAED pattern of Fe0.96Co0.04F3/MWCNT nanocomposites in Fig. 4d indicate the (012), (024) and (224) planes, and broad ring patterns exist together, which is consistent with XRD results. The SEM and TEM images confirm that the morphology of the Fe0.96Co0.04F3/MWCNTs is consistent with our goals. The Fe0.96Co0.04F3/MWCNT nanocomposites exhibit a high specific surface area, which is beneficial for enhancing charge transfer reactions and the electrode/electrolyte contact area. Meanwhile, the specific contact morphology can reduce the diffusion distance of Li+. Therefore, the samples are expected to display good rate performances and cycling stabilities.
Electrochemical characterization
Electrochemical characterization of the FeF3/MWCNTs
In order to investigate the effect of in situ wrapping MWCNTs, the galvanostatic charge–discharge profiles of the FeF3/MWCNT nanocomposite cathodes were tested within a voltage window from 2.0 to 4.5 V at 0.2 C (1 C = 237 mA g−1; the weight ratio of MWCNTs is ω 1 = 1%, ω 2 = 3% and ω 3 = 5%, respectively), as shown in Fig. 5. The initial discharge capacities of the FeF3/MWCNT electrodes with ω 1 = 1%, ω 2 = 3% and ω 3 = 5% are 179.4, 192.1 and 172.1 mAh g−1, respectively, as shown in Fig. 5a. It is notable that the FeF3/MWCNT electrodes are first discharged before charge. After 50 cycles, the discharge capacities of the FeF3/MWCNT nanocomposites (Fig. 5b) faded to 153.8 160.7 and 146.6 mAh g−1, respectively, which demonstrates that adding ω2 of the MWCNTs effectively improved the specific capacity of FeF3. Moreover, in situ wrapping of the MWCNTs could form conductive networks on the surface of the FeF3 nanoparticles, which is expected to enhance the conductivity of the samples and improve their electrochemical performances [22, 26]. However, excessive MWCNTs will inhibit the initial efficiency due to its large specific surface area. Therefore, we can investigate Co-dopant of FeF3 by selecting the optimal MWCNT amount (ω 2 = 3%).
Electrochemical characterization of Fe(1-x)Co x F3/MWCNTs
The Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0, 0.02, 0.04 and 0.06) were composed of MWCNTs (ω2 = 3%) and Fe(1-x)Co x F3, prepared using the in situ solvothermal method. Figure 6a shows the initial discharge and charge profiles of the Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0, 0.02, 0.04 and 0.06) at a current density of 0.2 C in the voltage range of 2.0–4.5 V. The initial discharge capacities of 192.1, 206.8, 217.0 and 199.1 mAh g−1 were obtained for Co-doping levels of x = 0, 0.02, 0.04 and 0.06, respectively. Additionally, after 50 cycles (Fig. 6b), the discharge capacities of the FeF3/MWCNTs, Fe0.98Co0.02F3/MWCNTs, Fe0.96Co0.04F3/MWCNTs and Fe0.94Co0.06F3/MWCNTs were 160.7, 177.8, 187.9 and 170.5 mAh g−1, respectively. It is clear that the Fe(1-x)Co x F3/MWCNT nanocomposites exhibit sloped reaction plateaus of 2.7 V. Obviously, the Co-doped FeF3/MWCNT nanocomposites show better electrochemical properties than pristine FeF3/MWCNTs. In addition, the Fe0.96Co0.04F3/MWCNT nanocomposite exhibited the highest discharge voltage plateau and the highest specific capacity, which suggests that this composite possesses the smallest electrochemical polarization due to efficient Li+ transport [13, 18].
To further test the cycling performances of the Co-doped composites, the cycling performances of Fe(1-x)Co x F3/MWCNTs at 0.2 and 3 C in the voltage range of 2.0–4.5 V over 50 cycles are shown in Fig. 6c, d. It is notable that the coulombic efficiencies of Co-doped materials are as high as 97% and obviously higher than those of pure FeF3/MWCNTs at 0.2 and 3 C during 50 cycles. As seen from the voltage profiles, the capacity retentions of the Fe0.96Co0.04F3/MWCNT electrode were 92.7% at 0.2 C and 90.1% at 3 C. Similarly, the FeF3/MWCNT electrode displayed capacity retentions of 88.6% at 0.2 C and 79.1% at 3 C; the Fe0.98Co0.02F3/MWCNT electrode displayed 90.2% at 0.2 C and 86.4% at 3 C; the Fe0.94Co0.06F3/MWCNT electrode displayed 89.7% at 0.2 C and 84.1% at 3 C. Thus, the Co-doped samples also showed significantly better cycling stabilities than the undoped sample at 3 C. In addition, the Fe0.96Co0.04F3/MWCNT electrode exhibited the highest capacity retention.
In order to detect the electrochemical performances after Co-doping, CV measurements of the Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0, 0.02, 0.04 and 0.06) were compared, as shown in Fig. 7a. The CV curves of the Fe(1-x)Co x F3/MWCNT cathodes were collected at a scan rate of 0.1 mV s−1 between 2.0 and 4.5 V. The four curves exhibit similar shapes with a pair of reversible reduction and oxidation peaks corresponding to the insertion/extraction of Li+, indicating that these samples have similar electrochemical reaction mechanisms. However, the Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0.02, 0.04 and 0.06) have significantly smaller differences between their cathodic and anodic peaks, with ΔE values of 0.30, 0.24 and 0.32 V, respectively, compared to those of FeF3/MWCNTs (0.43 V). Thus, the FeF3/MWCNT nanocomposite showed th highest polarization. Additionally, Fe0.96Co0.04F3/MWCNTs (ΔE = 0.24 V) showed a higher current and larger area of the CV curve, which are directly related to the achieved capacity. These results demonstrate that Co-doping can improve the electrochemical performance of FeF3/MWCNTs, in complete agreement with the improvements in both the cycling and charge–discharge performances of the Fe0.96Co0.04F3/MWCNT sample shown in Fig. 6.
To assess the rate performance of the Fe(1-x)Co x F3/MWCNT nanocomposites, the rate performance curves shown in Fig. 7b were generated at various current densities from 0.1 to 8 C and finally back to 0.1 C. As the current density is increased, the discharge capacities of the samples decrease. The capacity retentions of the Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0, 0.02, 0.04 and 0.06) were 72.8, 91.8, 94.9 and 88.5%, respectively. Interestingly, the Fe(1-x)Co x F3/MWCNTs (x = 0.02, 0.04 and 0.06) showed significantly enhanced rate capabilities and capacity retentions compared to the FeF3/MWCNTs. Moreover, these results indicate that the Fe0.96Co0.04F3/MWCNT sample exhibits higher structural stability and reversibility than the other samples.
Figure 8a, d shows the EIS curves of the Fe(1-x)Co x F3/MWCNT nanocomposites after the first and 50th cycles at room temperature. Each curve is composed of a semicircle in the high-frequency region and a line in the low-frequency region. An equivalent circuit model was used to analyze the impedance spectra, as shown in Fig. 8c, where R Ω is the electrolyte resistance, R ct is the Li+ charge transfer resistance at the interface and CPE 1 is the constant phase element, which was used to represent the double-layer capacitance and the passivation film capacitance. The slope of the line in the low-frequency region represents the Warburg impedance (W 1), which is attributed to the diffusion of Li+ into the electrode. It is clear that in all the curves the R Ω values (~3 Ω) are almost same due to the same electrolyte. Interestingly, the R ct value of the Fe(1-x)Co x F3/MWCNT electrodes (x = 0.02, 0.04 and 0.06) is smaller than that of FeF3/MWCNTs after the first and 50th cycles, as shown in Table 2. Therefore, the low total resistance of the Co-doped FeF3/MWCNT nanocomposite indicates a higher conductivity and less polarization than of FeF3/MWCNTs which are in favor of the transmission of ions and thus improve cycling and rate performance. In order to calculate the influence of Co-doping on the diffusion of Li+, the following formula was utilized [18]:
In Eq. (1), T is temperature (room temperature in our experiments, 298 K); R is the ideal gas constant (8.314 JK−1 mol−1); n is the number of the electrons per molecule during the electronic transfer reaction; A is the surface area of the electrode; F is the Faraday constant (96500 C mol−1); C is the molar concentration of Li+ in the Fe(1-x)Co x F3 electrode; and σ is the Warburg factor, which has the following relationship with \( Z^{\prime} \) [27]:
Here, ω is the frequency in the low-frequency region, and the slope of line of \( Z^{\prime}\sim\omega^{ - 1/2} \) [28] is obtained as shown in Fig. 8b, e. The parameters of the equivalent circuit obtained by computer simulations are shown in Table 2, which were calculated based on Eqs. (1) and (2). Figure 8b implies the Li+ diffusion coefficients of Fe(1-x)Co x F3/MWCNT electrodes after the first cycle. The calculated Li+ diffusion coefficients of the Fe(1-x)Co x F3/MWCNT electrodes are 8.06 × 10−12, 1.09 × 10−11, 1.72 × 10−11 and 9.73 × 10−12 cm2 s−1, respectively, indicating that the Fe0.96Co0.04F3/MWCNT nanocomposite exhibits the highest Li+ diffusion coefficient. Similarly, Fe0.96Co0.04F3/MWCNT electrode at 50th cycles exhibited the highest Li+ diffusion coefficient (D Li+ = 1.40 × 10−11 cm2 s−1) compared to that of other electrodes according to investigation. Obviously, the Li+ diffusion coefficient was improved after Co-doping, which implies that a larger Li+ diffusion coefficient results in an electrode with a higher capacity during charging and discharging. A less significant increase in impedance during cycling indicates a lower polarization, which results in good cycling behavior. We found that a suitable Co-doping concentration can improve the electrochemical performance of FeF3/MWCNTs. However, the experimental data analysis shows that the crystal growth of Co-dopant also restrains Li+ diffusion and increases surface impedance, which is contradicted to the result of decreasing band gap. These results fully demonstrate the above conclusions of the CV and cycling tests.
Conclusions
In summary, Fe(1-x)Co x F3/MWCNT nanocomposites (x = 0, 0.02, 0.04 and 0.06) were successfully fabricated by an in situ solvothermal method. The MWCNTs (ω2 = 3%) were firmly bonded to the Fe(1-x)Co x F3 materials to provide electron transfer pathways, which were able to enhance the conductivity and reduce the sizes of the interconnected particles of the Fe(1-x)Co x F3 materials. The CV measurement results show that the Co-doped could improve the dynamic behavior. The EIS indicates that the Co-doped greatly enhances the charge transfer resistance of FeF3/MWCNTs. Our analyses demonstrated that Co-doping can adjust the crystal structure, reduce the polarization and improve the Li+ diffusion coefficient of FeF3. However, the overgrowth of Co-dopant will restrain Li+ diffusion and increase surface impedance, which has an opposite effect with the result of decreasing band gap. In order to solve the issue of the microcrystal growth and the band gap reduction, a kind of suitable Co-doping concentration is selected.
Consequently, the Fe0.96Co0.04F3/MWCNT nanocomposite exhibited the highest specific capacity, cycling stability and rate performance. The initial discharge specific capacity of the Fe0.96Co0.04F3/MWCNT nanocomposite reached 217.0 mAh g−1 at 0.2 C. After 50 cycles, the discharge specific capacity was maintained at 187.9 mAh g−1. Meanwhile, the capacity retention remains 89.7% at a rate of 3 C after 50 cycles. The EIS results revealed that both the Li+ charge transfer resistance (R ct = 31.25 Ω) and Li+ diffusion coefficient (1.40 × 10−11 cm2 s−1) were satisfactory from Co-doping and the in situ wrapping of the MWCNTs after the 50th cycle. This study demonstrated that the in situ wrapping of the MWCNTs and Co-doping are effective methods to enhance the electrochemical performance of FeF3. Therefore, the Fe(1-x)Co x F3/MWCNT electrodes are a potential cathode material for LIBs.
References
Bruce PG (2008) Energy storage beyond the horizon: rechargeable lithium batteries. Solid State Ion 179:752–760
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Li H, Balaya P, Maier J (2004) Li-storage via heterogeneous reaction in selected binary metal fluorides and oxides. J Electrochem Soc 151:A1878–A1885
Zhou MJ, Zhao LW, Okada S, Yamaki JI (2011) Thermal characteristics of a FeF3 cathode via conversion reaction in comparison with LiFePO4. J Power Sources 196:8110–8115
Li L, Meng F, Jin S (2012) High-capacity lithium-ion battery conversion cathodes based on iron fluoride nanowires and insights into the conversion mechanism. Nano Lett 12:6030–6037
Jiang J, Li LP, Xu MW, Zhu JH, Ming C (2016) FeF3@thin nickel ammine nitrate matrix: smart configurations and applications as superior cathodes for Li-ion batteries. ACS Appl Mater Interfaces 8:16240–16247
Ma R, Lu Z, Wang C, Wang H, Yang S, Xi L, Chung J (2013) Large-scale fabrication of graphene-wrapped FeF3 nanocrystals as cathode materials for lithium ion batteries. Nanoscale 5:6338–6343
Li CL, Gu L, Tong JW, Tsukimoto S (2011) A mesoporous iron-based fluoride cathode of tunnel structure for rechargeable lithium batteries. Adv Funct Mater 21:1391–1397
Jangwook L, Byoungwoo K (2016) Novel and scalable solid-state synthesis of a nanocrystalline FeF3/C composite and its excellent electrochemical performance. Chem Commun 52:9414–9417
Li CL, Gu L, Tong JW, Maier J (2011) Carbon nanotu be wiring of electrodes for high-rate lithium batteries using an imidazolium-based ionic liquid precursor as dispersant and binder: a case study on iron fluoride nanoparticles. ACS Nano 5:2930–2938
Ma DL, Wang HG, Li Y, Xu D, Yuan S, Huang XL, Zhang XB, Zhang Y (2014) In situ generated FeF3 in homogeneous iron matrix toward high-performance cathode material for sodium-ion batteries. Nano Energy 10:295–304
Chu QX, Xing ZC, Ren XB, Asiri AM, Al-youbi AO, Alamry KA, Sun XP (2013) Reduced graphene oxide decorated with FeF3 nanoparticles: facile synthesis and application as a high capacity cathode material for rechargeable lithium batteries. Electrochim Acta 111:80–85
Wu W, Wang Y, Wang XY, Chen QQ, Wang X, Yang SY, Liu XM (2009) Structure and electrochemical performance of FeF3/V2O5 composite cathode material for lithium-ion battery. J Alloy Compd 486:93–96
Xu XP, Chen S, Shui M, Xu LX, Zheng WD, Shu J, Cheng LL, Feng L, Ren YL (2014) One step solid state synthesis of FeF3·0.33H2O/C nano-composite as cathode material for lithium-ion batteries. Ceram Int 40:3145–3148
Fan LH, Li BJ, Zhang NQ, Sun KN (2015) Carbon nanohorns carried iron fluoride nanocomposite with ultrahigh rate lithium ion storage properties. Sci Rep 5:12154–12162
Li CL, Yin CL, Gu L, Dinnebier RE (2013) An FeF3·0.5H2O polytype: a microporous framework compound with intersecting tunnels for Li and Na batteries. J Am Chem Soc 135:11425–11428
Li H, Richter G, Maier J (2003) Reversible formation and decomposition of LiF clusters using transition metal fluorides as precursors and their application in rechargeable Li batteries. Adv Mater 15:736–739
Bai Y, Zhou XZ, Jia Z, Wu C, Yang LW, Chen MZ, Zhao H, Wu F, Liu G (2015) Understanding the combine effects of microcrystal growth and band gap reduction for Fe(1-x)Ti x F3 nanocomposites as cathode materials for lithium-ion batteries. Nano Energy 17:140–151
Liu L, Zhou M, Yi LH, Guo HP, Tan JL, Shu HB, Yang XK, Yang ZH, Wang XY (2012) Excellent cycle performance of Co-doped FeF3/C nanocomposite cathode material for lithium-ion batteries. J Mater Chem 22:17539–17550
Yang ZH, Pei Y, Wang XY, Liu L, Su X (2012) First principles study on the structural, magnetic and electronic properties of Co-doped FeF3. Comput Theor Chem 1:44–48
Jung H, Shin J, Chae CJK, Lee J Lim (2013) FeF3/ordered mesoporous carbon (OMC) nanocomposites for lithium ion batteries with enhanced electrochemical performance. J Phys Chem C 117:14939–14946
An HF, Wang Y, Wang XY, Zheng LP, Wang XY, Yi LH, Bai L, Zhang XY (2010) Polypyrrole/carbon aerogel composite materials for supercapacitor. J Power Sources 195:6964–6969
Cho J, Kim H, Park B (2004) Comparison of overcharge behavior of AlPO4-coated LiCoO2 and LiNi0.8Co0.1Mn0.1O2 cathode materials in Li-ion cells. J Electrochem Soc 151:A1707–A1711
Wei SY, Wang XY, Zhang R, Hu H, Shen YQ, Liu J (2016) Preparation and performance of spherical FeF2.5·0.5H2O nanoparticles wrapped by MWCNTs as cathode material of lithium ion batteries. RSC Adv 6:97759–97769
Lu Y, Wen ZY, Jin J, Rui K, Wu XW (2014) Hierarchical mesoporous iron-based fluoride with partially hollow structure: facile preparation and high performance as cathode material for rechargeable lithium ion batteries. Phys Chem Chem Phys 16:8556–8562
Fukushima T, Kosaka A, Ishimura Y, Yamamoto T, Takigawa T, Ishii N, Aida T (2003) Molecular ordering of organic molten salts triggered by single-walled carbon nanotube. Science 300:2072–2074
Gao F, Tang ZY (2008) Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim Acta 53:5071–5075
Wang XY, Hao H, Liu JL, Huang T, Yu AS (2011) A novel method for preparation of macroposous lithium nickel manganese oxygen as cathode material for lithium ion batteries. Electrochim Acta 56:4065–4069
Acknowledgements
This work is supported by Science and Technology Plan Foundation of Guangdong (2015A050502046) and Science and Technology Plan Foundation of Guangzhou (201704030031).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Xu, S., Huang, S. et al. In situ synthesis of Fe(1−x)Co x F3/MWCNT nanocomposites with excellent electrochemical performance for lithium-ion batteries. J Mater Sci 53, 2697–2708 (2018). https://doi.org/10.1007/s10853-017-1685-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1685-2