Abstract
The brand-new magnetic–fluorescent bifunctional heterogeneous nanofiber yarns have been successfully prepared by using a conjugate electrospinning method for the first time. The heterogeneous nanofiber yarns consist of [Fe3O4/polyacrylonitrile (PAN)] magnetic nanofibers and [Eu(BA)3phen/PAN] fluorescent nanofibers, which benefit for separating Fe3O4 nanoparticles (NPs) from Eu(BA)3phen complexes effectively. The morphology and properties of as-prepared samples have been studied in detail by X-ray diffractometer, scanning electron microscope, energy-dispersive spectrometer, vibrating sample magnetometer and fluorescence spectrophotometer. The results reveal that the prepared heterogeneous nanofiber yarns have large aspect ratio and uniform diameter, and the nanofibers in the yarns exhibit high orientation. The magnetism of heterogeneous nanofiber yarns can be adjusted by modulating the contents of Fe3O4 NPs. It is satisfactorily found that the fluorescence intensity of heterogeneous nanofiber yarns is much higher than that of counterpart [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns under the same compositions and contents. The new heterogeneous nanofiber yarns have the potential applications in nanodevices, fluorescent labeling, etc., due to the superior magnetic–fluorescent bifunctional properties. Furthermore, the design idea and preparation technique also provide a simple but effective method for the preparation of other bifunctional or multifunctional nanofiber yarns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrospinning has been identified as a straightforward and economical way to manufacture continuous polymer nanofibers with the diameter ranging from micron to nanometer, which becomes a common method used by many researchers in recent years [1,2,3,4,5]. Universally, electrospun nanofibers have the potential of being applied in many fields such as electrode materials, catalysts, sensor materials, biomaterials and tissue engineering scaffolds due to their great specific surface, high aspect ratio and radius of curvature, strong ability to penetrate with other materials and nano-/micron-sized pores [6,7,8,9,10]. Most of the electrospun fibers, however, are prepared in the form of non-woven fiber mats with randomly oriented nanofibers, which are attributed to the bending instability of the high charged jet [11]. The fiber mats possess the disordered structure and low mechanical properties, which severely restrict the application of nanofibers [12, 13]. In order to overcome these shortcomings and open up new fields of applications, the non-woven fiber mats have shifted from random orientation to orderly arrangement of nanofiber yarns, which has become a hot spot of research. Up to now, nanofiber yarns with highly aligned structure have been reported by some researchers. Liu et al. [14] fabricated high orientated PPy (polypyrrole)-coated PAN nanofiber yarns with excellent sensing properties via in situ chemical polymerization and electrospinning technique. Wang et al. prepared poly(caprolactone) (PCL)/silk fibroin (SF)/polyaniline (PANI) aligned nanofiber yarns by using a modified dry–wet electrospinning way. The aligned nanofiber yarns have good biocompatibility and ability to guide the cell alignment, elongation and differentiation [15]. Chang et al. [16] reported a method to prepare twisted electrospun microfiber/nanofiber yarns by using a high-speed rotating electrospinning equipment. Wu et al. [17] successfully manufactured uniaxially aligned nanofiber yarns (UANY) by electrospinning method, and the UANY possessed high cell survivability and displayed abilities to add the cell proliferation and adhesion.
In addition to the oriented nanofiber yarns, functionalized nanomaterial is one of the hot spots of research in recent years. Since single functional nanomaterials are limited in practical applications, multifunctional nanomaterials have drawn the much attention of researchers [18]. Among the multifunctional nanomaterials, magnetic–fluorescent bifunctional nanomaterials have become one of the most commonly used materials in medical imaging, drug delivery, medical diagnostics, biosensors, nanodevices, etc. [19, 20]. Generally, the rare earth (RE) complexes have excellent fluorescent properties as a result of the f–f electron transition in RE ions, which have applications in luminescence, laser materials, solar energy conversion materials, etc. [21,22,23]. Fe3O4 nanoparticles (NPs) have prospective applications in various areas such as magnetic-controlled switches due to the unique superparamagnetism and high permeability [24]. It is known that the fluorescent intensity of RE complexes will strongly reduce if Fe3O4 NPs or other magnetic materials like Fe2O3 are directly mixed with the RE complexes for the preparation of magnetic–fluorescent bifunctional nanomaterials [25]. In order to obtain the strong fluorescent properties, the magnetic materials have to be separated from the RE complexes effectively to avoid immediate contact. So far, some types of magnetic–fluorescent bifunctional nanomaterials have been reported such as [Fe3O4/polymethyl methacrylate(PMMA)]//[Tb(BA)3phen)/PMMA] coaxial nanobelts [26], [Fe3O4/PMMA]//[Tb(BA)3phen)/PMMA] Janus nanobelts [27] and [Fe3O4/polyvinylpyrrolidone(PVP)]//[(Dy(BA)3phen + Eu(BA)3phen)/PVP] Janus nanofibers [28], which provide benefits for many applied areas such as cell separation, magnetic resonance imaging, biological imaging, drug targeting. PAN is one of the most major high-polymer materials in electrospinning due to its low price, good thermal and chemical stability, antioxidation and high mechanical properties, as well as the prepared nanofibers with stable physical and chemical properties [29,30,31], and thus, it was selected as the fiber matrix in this work.
Inspired by some of the above literature, herein, we designed and fabricated [Fe3O4/PAN]//[Eu(BA)3phen/PAN] nanofiber yarns with bifunctionality of magnetism and fluorescence by using a conjugate electrospinning technique. The [Fe3O4/PAN]//[Eu(BA)3phen/PAN] nanofiber yarns are composed of two different nanofibers, namely twisted [Fe3O4/PAN] nanofibers and [Eu(BA)3phen/PAN] nanofibers. We named this kind of nanofiber yarns as heterogeneous nanofiber yarns. In order to highlight the excellent performance of heterogeneous nanofiber yarns, [Fe3O4/Eu(BA)3phen/PAN] nanofiber yarns were also prepared via using the same method, which was denoted as homogeneous nanofiber yarns. The homogeneous nanofiber yarns consist of only one kind of nanofibers, that is, twisted [Fe3O4/Eu(BA)3phen/PAN] nanofibers. Finally, the prepared nanofiber yarns were systematically characterized by using various modern testing technologies. So far, there are no reports on synthesizing of nanofiber yarns which possess bifunctionality of magnetism and fluorescence. What is more, the synthetic conception in this work can be extended to prepare other multifunctional nanofiber yarns.
Experimental sections
Chemical reagents
Eu2O3 (99.99%), concentrated nitric acid (HNO3) (A.R.), 1,10-phenanthroline (phen) (A.R.), benzoic acid (BA) (A.R.), FeSO4·7H2O (A.R.), ammonia (NH3·H2O) (A.R.), FeCl3·6H2O (A.R.), oleic acid (OA) (A.R.), polyethylene glycol (PEG, M w ≈ 20000) (A.R.), NH4NO3 (A.R.), polyacrylonitrile (PAN) (A.R.), N, N-dimethylformamide (DMF) (A.R.) and anhydrous ethanol (A.R.).
Syntheses of OA-modified Fe3O4 NPs and Eu(BA)3phen complexes
OA-modified Fe3O4 NPs and Eu(BA)3phen complexes were used as raw materials for preparing spinning solutions. The former material was synthesized by co-precipitation method, and the specific preparation process was described in the literature [32]. The morphology of prepared Fe3O4 NPs is spherical, and average particle size is ca. 10 nm. The latter material was prepared based on the typical method described in the literature [33]. There are two reasons for the preparation of Fe3O4 NPs coated with OA: one is to prevent the oxidation of Fe3O4 NPs directly exposed to the air, and the other is to improve the dispersibility in spinning solution and to avoid agglomerating.
Fabrication of [Fe3O4/PAN]//[Eu(BA)3phen/PAN] magnetic–fluorescent bifunctional heterogeneous nanofiber yarns by conjugate electrospinning
For preparing the first electrospinning solution for fabricating [Eu(BA)3phen/PAN] nanofibers, Eu(BA)3phen complexes were added into DMF (5.0000 g) and then PAN (0.5000 g) was introduced under magnetic stirring at 60 °C for 3 h. The obtained uniform solution was named as solution A. For fabricating another spinning solution used for preparing [Fe3O4/PAN] nanofibers, Fe3O4 NPs were dispersed into DMF (9.0000 g) under ultrasonication for 25 min and then PAN (0.9000 g) was added into the suspension with mechanical agitation at 60 °C for 3 h. The prepared solution was denoted as solution B. The actual components of solutions A and B are shown in Tables 1 and 2, respectively.
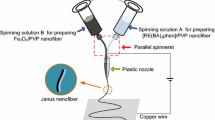
The electrospinning system for fabricating nanofiber yarns is shown in Fig. 1a. The solutions A and B were loaded into two plastic syringes equipped with spinnerets, which were, respectively, connected to positive and negative high-voltage direct current (DC) power supplies by using stainless steel needles. The two syringes were fixed symmetrically on both sides of the grounded copper funnel with t¼he maximum diameter of 9 cm. Applied voltage was controlled to about ± 15 kV, and the distance from the side of copper funnel to the tip of spinneret was 10 cm. The collecting device was placed below the copper funnel, which consisted of an adjustable speed motor and a metal rod. The distance between the lower edge of funnel and collecting device was 18 cm. PAN-based continuous nanofiber yarns were prepared at room temperature of 20 ± 5 °C, and relative humidity was 20 ± 5%.
After the power supplies were turned on, the electrostatic field was formed between positive and negative spinnerets. The spinning solutions A and B were ejected under the action of electrostatic field, and then, nanofibers were formed by pulling and solidifying with opposite charge. The nanofibers produced by solutions A and B were bonded and charge-neutralized after they were encountered. A grounding metal wire was placed near the central area under the copper funnel; then, a stable conical assemblage composed of two kinds of nanofibers was gradually formed between the funnel edges and the end point of metal wire. The twisted nanofiber yarns were successfully prepared by rotating the funnel in a revolving speed of 100 r min−1, as depicted in Fig. 1b. Finally, the yarns were drawn to the metal rod of collecting device by the guidance of the metal wire, and the collection rate was 0.03 m min−1. For comparison, two plastic syringes contained 2 mL of spinning solution during every electrospinning process, respectively. The electrospinning process was stopped until the solutions were synchronously and completely consumed.
Fabrication of the [Fe3O4/Eu(BA)3phen/PAN] magnetic–fluorescent bifunctional homogeneous nanofiber yarns by conjugate electrospinning
For comparison, [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns were also fabricated as a contrast sample. The preparing process is as follows: spinning solutions A4 and B2 were mixed together (the ratio of the volume was 1:1) in a conical flask under mechanical agitation for 2 h until the solutions were mixed uniformly, and then, electrospinning was performed under the same conditions.
Fabrication of the [Eu(BA)3phen/PAN] fluorescent nanofiber yarns by conjugate electrospinning under different rotating speeds of copper funnel
To investigate the effect of twisting degree on the fluorescence intensity of nanofiber yarns, a series of [Eu(BA)3phen/PAN] fluorescent nanofiber yarns prepared under different funnel rotating speeds were fabricated. The preparing process is as follows: two plastic syringes were, respectively, loaded with 2 mL of spinning solution A4, and then, electrospinning process was carried out under the same experimental conditions.
Characterization methods
The as-prepared Fe3O4 NPs, magnetic–fluorescent bifunctional heterogeneous and homogeneous nanofiber yarns were analyzed by using a X-ray power diffractometer (XRD), which was made by the company of Bruker with the model of D8 FOCUS, using Cu Kα radiation (λ = 0.15418 nm) and a Ni filter. The current was kept at 20 mA, and the operation voltage was controlled at 40 kV, respectively. The morphology of the nanofiber yarns was observed by scanning electron microscope (SEM), which is equipped with energy-dispersive X-ray spectrometer (EDS). The properties of fluorescence were studied by using an F-7000 fluorescence spectrophotometer made by Hitachi, using Xe lamp as excitation source, 700 V of voltage and 240 nm min−1 of scanning speed were supplied, and the wavelength range was 200–700 nm. Then, the magnetic performance of Fe3O4 NPs and nanofiber yarns was investigated by using vibrating sample magnetometer (VSM), which was purchased from Quantum Design Inc. with the type of MPMS SQUID XL.
Results and discussion
Phase analyses
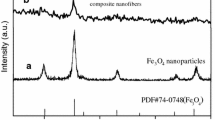
XRD analyses, shown in Fig. 2, reveal the phase compositions of as-prepared Fe3O4 NPs, [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns (fabricated by SA4//SB2) and [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns. The Fe3O4 NPs are conformed to cubic phase of Fe3O4 (PDF#89-0691), and there are no characteristics peaks such as basic ferrous oxide (FeO(OH)) and ferric oxide (Fe2O3). XRD patterns of the heterogeneous nanofiber yarns and homogeneous nanofiber yarns reveal that these two kinds of nanofiber yarns contain Fe3O4 NPs. The weak diffraction peak between 20° and 25° in the patterns demonstrates the existence of amorphous PAN.
Morphology and structure
The SEM images at low magnification and EDS spectra of [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns and [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns are demonstrated in Fig. 3. The SEM images at high magnification and histograms of diameter distribution of the nanofibers in the yarns are also indicated in Fig. 3. An obvious twisted structure of the yarns can be clearly seen from SEM images at low magnification, and the twist angles for heterogeneous nanofiber yarns and homogeneous nanofiber yarns, respectively, are 32° and 30° which are very close, as shown in Fig. 3a, e. The corresponding EDS spectra shown in Fig. 3b, f reveal that heterogeneous nanofiber yarns and homogeneous nanofiber yarns consisted of elements C, N, O, Fe, Eu, Pt. The peaks of element Pt in the spectra were originated from the conductive film covered on the surface of yarns for SEM observation. The nanofibers in the yarns exhibit high degree of alignment, as shown in the SEM images at high magnification (Fig. 3c, g). As shown in Fig. 3c, some aggregations of Fe3O4 NPs can be seen on the surface of some part of the nanofibers, and on the contrary, the other nanofibers have smooth surface, which can be attributed to the fact that Fe3O4 NPs are only distributed in the [Fe3O4/PAN] nanofibers but not in [Eu(BA)3phen/PAN] nanofibers in the heterogeneous nanofiber yarns. As for the homogeneous nanofiber yarns, the Fe3O4 NPs are dispersed in every single [Fe3O4/Eu(BA)3phen/PAN] nanofiber, as revealed in Fig. 3g. Figure 3d, h demonstrates the histograms of the diameter distribution of the nanofibers in heterogeneous nanofiber yarns and homogeneous nanofiber yarns, respectively. The average diameters of heterogeneous nanofiber yarns and their internal nanofibers are 242 μm and 553 ± 2 nm, and those of the homogeneous nanofiber yarns and their internal nanofibers are 233 μm and 418 ± 1 nm, respectively.
SEM images at low magnification (a, e), EDS spectra (b, f), SEM images at high magnification (c, g), the histograms of the diameter distribution (d, h) of the nanofibers in [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns (a–d) and [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns (e–h)
The digital photographs of conical nanofibers assemblage formed during the experiment process and final yarns are shown in Fig. 4a–f. As shown in Fig. 4a, d, the conical nanofibers assemblage and yarns are made up of [Eu(BA)3phen/PAN] nanofibers, and thus, they exhibit the color of white owing to without colorful substances in the samples. As shown in Fig. 4b, e, the conical nanofibers assemblage and yarns are composed of two different nanofibers which are [Fe3O4/PAN] nanofibers and [Eu(BA)3phen/PAN] nanofibers, respectively. The conical nanofibers assemblage and yarns revealed in Fig. 4c, f are comprised of [Fe3O4/Eu(BA)3phen/PAN] nanofibers. From Fig. 4a–c, as well as Fig. 4d–f, one can clearly see that the color of the samples becomes darker, which is due to the existence of dark-colored Fe3O4 NPs. Figure 4g represents that the [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns have large aspect ratio, and Fig. 4h shows the red light emitted from the heterogeneous nanofiber yarns under 281-nm UV illumination in darkness.
Digital photographs of conical nanofibers assemblage formed during the experimental process and final [Eu(BA)3phen/PAN] fluorescent nanofiber yarns (a, d), [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns (b, e) and [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns (c, f); digital photographs of [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns (g) and the emission light of the heterogeneous nanofiber yarns under 281-nm excitation in darkness (h)
Magnetism
The typical hysteresis loops of as-prepared Fe3O4 NPs, [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns and [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns with different mass ratios of Fe3O4 NPs are shown in Fig. 5, and their saturation magnetizations are revealed in Table 3. It is known to us all that the saturation magnetization of the magnetic material is determined by the content of the magnetic substance doped in it [34]. It is shown in Table 3 that the saturation magnetization of the prepared Fe3O4 NPs reaches to 74.27 emu g−1, which is relatively lower than its bulk value of 92 emu g−1 [35]. This phenomenon comes from the nanosized effect: the surface layer has a number of defects for the superfine magnetic particles, and thus, it barely contributes to the magnetization. In addition, with the increasing contents of Fe3O4 NPs in heterogeneous nanofiber yarns, the saturation magnetization of the yarns increases. The saturation magnetization values enhance from 10.10 to 28.77 emu g−1, implying that the heterogeneous nanofiber yarns possess tunable magnetism. [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns have close magnetic property to [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns owing to the fact that both of them have the same theoretical Fe3O4 NPs masses. Compared with Fe3O4 NPs, the loss of saturation magnetization of the yarns is due to the existence of the nonmagnetic materials such as PAN and Eu(BA)3phen complexes.
Fluorescent performance
In order to seek out the optimal percentage of Eu(BA)3phen complexes to PAN, a series of [Eu(BA)3phen/PAN] fluorescent nanofibers were prepared by using a traditional electrospinning method; the mass percentages of Eu(BA)3phen complexes to PAN were set to 1, 5, 10, 15 and 20%, respectively. Figure 6a, b, respectively, demonstrates the excitation and emission spectra of the above nanofibers. According to the excitation spectra in Fig. 6a, a wide excitation band from 200 to 500 nm of samples is observed when monitoring wavelength is 616 nm. The π → π* electronic transition of ligands at 281 nm can be identified as well. As illustrated in Fig. 6b, the strong characteristic emission peaks of Eu3+ are observed at 580, 593, 616 nm under 281-nm excitation, which are, respectively, ascribed to 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2 energy-level transitions. 5D0 → 7F2 transition at 616 nm (red light) is the primary emission peak. It also can be found that the fluorescent intensity of nanofibers is gradually increased and then decreased with addition of more Eu(BA)3phen complexes. When the fluorescent intensity reaches the highest value, the percentage of Eu(BA)3phen complexes to PAN is 15%. When the percentage of Eu(BA)3phen complexes to PAN is over 15% in the polymer matrix, the complexes will be clustered, resulting in the decreased distance and aggravated non-radiation transition among the Eu3+ ions, which leads to the decreased fluorescent intensity. Therefore, 15% of Eu(BA)3phen complexes to PAN was selected for fabricating magnetic–fluorescent bifunctional heterogeneous and homogeneous nanofiber yarns in this study.
As shown in Fig. 6c (inset), d (inset), the rotating speed of funnel has no obvious effect on the excitation and emission intensity of [Eu(BA)3phen/PAN] nanofiber yarns. The above analysis result means that the fluorescence intensity of the yarns is hardly affected by the degree of twist.
To study the influence of the mass ratios of Fe3O4 NPs to PAN on the fluorescent property of nanofiber yarns, a series of [Fe3O4/PAN]//[Eu(BA)3phen/PAN] magnetic–fluorescent bifunctional heterogeneous nanofiber yarns were fabricated by using spinning solutions SB1//SA4, SB2//SA4, SB3//SA4 and SB4//SA4. Figure 7 displays the excitation and emission spectra of the above yarns. It is shown in Fig. 7 that the intensity of each emission peak decreases gradually with the increase in the amounts of Fe3O4 NPs incorporated into the yarns. This can be explained by the stronger absorption of light by adding more dark-colored Fe3O4 NPs. The intensities of the excitation and emission peaks of the heterogeneous nanofiber yarns are decreased with the increase in Fe3O4 NPs contents, which are shown in the insets in Fig. 7a, b. It has been known that the absorption of Fe3O4 NPs in the ultraviolet light (the wavelength is < 400 nm) is much easier than in visible light (the wavelength ranges from 400 to 760 nm) [36]. Thus, Fe3O4 NPs can absorb the excitation light (281 nm) and emission light (580, 593 and 616 nm) of the heterogeneous nanofiber yarns; furthermore, the more Fe3O4 NPs introduced in the yarns, the darker color of the samples and the stronger absorption of light by Fe3O4 NPs, as shown in Fig. 8, leading to lower excitation and emission peaks intensities.
As illustrated in Fig. 9, the intensities of excitation and emission peaks of [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns (fabricated by SB2//SA4) are much stronger (about 25 times) than those of [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns under the same compositions and contents. The heavy loss in fluorescent excitation and emission intensity is from the strong absorption of the dark-colored Fe3O4 NPs when Fe3O4 NPs and Eu(BA)3phen complexes are directly mixed together, and the heterogeneous nanofiber yarns can effectively avoid this phenomenon because Fe3O4 NPs and Eu(BA)3phen complexes are thoroughly separated, which greatly broaden practical applications. The schematic diagram of [Fe3O4/PAN]//[Eu(BA)3phen/PAN] heterogeneous nanofiber yarns and [Fe3O4/Eu(BA)3phen/PAN] homogeneous nanofiber yarns in Fig. 10 shows the detailed structures of nanofiber yarns. As shown in the left part of Fig. 10, heterogeneous nanofiber yarns are made up of two different nanofibers, the light-colored [Eu(BA)3phen/PAN] nanofibers and dark-colored [Fe3O4/PAN] nanofibers, respectively. This special structure effectively separates Eu(BA)3phen complexes from Fe3O4 NPs so that the exciting light and emitting light in [Eu(BA)3phen/PAN] nanofibers will be hardly affected by the dark-colored Fe3O4 NPs. The homogeneous nanofiber yarns, demonstrated in the right part of Fig. 10, only contain one kind of nanofibers. Eu(BA)3phen complexes and Fe3O4 NPs are randomly dispersed in the [Fe3O4/Eu(BA)3phen/PAN] nanofibers in homogeneous nanofiber yarns. Hence, the exciting light must pass through many Fe3O4 NPs to approach and excite the Eu(BA)3phen complexes, and part of exciting light is absorbed by Fe3O4 NPs in this process, so the exciting light is weakened to a great extent before it reaches the Eu(BA)3phen complexes. Similarly, the emitting light emitted by the Eu(BA)3phen complexes also must pass through the Fe3O4 NPs and is absorbed by them. Therefore, both the exciting light and emitting light are severely weakened. According to the above analysis, heterogeneous nanofiber yarns possess superior performances to the homogeneous nanofiber yarns.
Conclusions
In summary, [Fe3O4/PAN]//[Eu(BA)3phen/PAN] magnetic–fluorescent bifunctional heterogeneous nanofiber yarns have been successfully fabricated by using a conjugate electrospinning method. The average diameter of the heterogeneous nanofiber yarns and their internal nanofibers is 242 μm and 553 ± 2 nm, respectively. The heterogeneous nanofiber yarns consist of two kinds of nanofibers which are, respectively, [Fe3O4/PAN] nanofibers and [Eu(BA)3phen/PAN] nanofibers. The heterogeneous nanofiber yarns have obviously twisted structure, and the nanofibers in the yarns exhibit the highly alignment and orientation. Compared with [Fe3O4/Eu(BA)3phen/PAN] magnetic–fluorescent bifunctional homogeneous nanofiber yarns, the heterogeneous nanofiber yarns have the same saturation magnetization and enhanced fluorescence owing to their peculiar structure, realizing the effective separation of Eu(BA)3phen complexes from Fe3O4 NPs. In addition, the magnetism of heterogeneous nanofiber yarns can be modulated by changing the amounts of the Fe3O4 NPs. Our new findings establish a novel strategy to achieve the separation of magnetic nanoparticles from fluorescent materials besides existing methods. Furthermore, the design conception and preparation method can also be used to fabricate other multifunctional nanofiber yarns, such as fluorescent–electrical, electrical–magnetic and fluorescent–electrical–magnetic bi- or trifunctional nanofiber yarns.
References
Gao S, Guo WM, Chen MX, Yuan ZG, Wang MG, Zhang Y, Liu SY, Xi TF, Guo QY (2017) Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J Mater Chem B 5:2273–2285
Lu N, Shao CL, Li XH, Miao FJ, Wang KX, Liu YC (2017) A facile fabrication of nitrogen-doped electrospun In2O3 nanofibers with improved visible-light photocatalytic activity. Appl Surf Sci 391:668–676
Lv N, Zhang JL, Li GM, Wang X, Ni JZ (2017) Novel strategy for facile synthesis of C-shaped CeO2 nanotubes with enhanced catalytic properties. J Phys Chem C 121:11926–11931
Geng YT, Zhang P, Wang QT, Liu YX, Pan K (2017) Novel PAN/PVP Janus ultrafine fiber membrane and its application for biphasic drug release. J Mater Chem B 5:5390–5396
Ma HQ, Chen GK, Zhang JN, Liu Y, Nie J, Ma GP (2017) Facile fabrication of core-shell polyelectrolyte complexes nanofibers based on electric field induced phase separation. Polymer 110:80–86
Zhu TH, Yu K, Bhutto MA, Guo XR, Shen W, Wang J, Chen WM, El-Hamshary H, Al-Deyab SS, Mo XM (2017) Synthesis of RGD-peptide modified poly(ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering. Chem Eng J 315:177–190
Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A (2014) Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater 10:11–25
Bhardwaj N, Kundu SC (2010) Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28:325–347
Sill TJ, von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006
Li HM, Zhang QG, Guo NN, Zhu AM, Liu QL (2015) Ultrafine polystyrene nanofibers and its application in nanofibrous membranes. Chem Eng J 264:329–335
Wu SH, Qin XH (2013) Uniaxially aligned polyacrylonitrile nanofiber yarns prepared by a novel modified electrospinning method. Mater Lett 106:204–207
Tian L, Yan T, Pan ZJ (2015) Fabrication of continuous electrospun nanofiber yarns with direct 3D processability by plying and twisting. J Mater Sci 50:7137–7148. doi:10.1007/s10853-015-9270-z
Afifi AM, Nakano S, Yamane H, Kimura Y (2010) Electrospinning of continuous aligning yarns with a ‘funnel’ target. Macromol Mater Eng 295:660–665
Liu PH, Wu SH, Zhang Y, Zhang HG, Qin XH (2016) A fast response ammonia sensor based on coaxial PPy-PAN nanofiber yarn. Nanomaterials 6:121
Wang L, Wu YB, Guo BL, Ma PX (2015) Nanofiber yarn/hydrogel core-shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano 9:9167–9179
Chang GQ, Li AK, Xu XQ, Wang XL, Xue G (2016) Twisted polymer microfiber/nanofiber yarns prepared via direct fabrication. Ind Eng Chem Res 55:7048–7051
Wu SH, Duan B, Liu PP, Zhang CD, Qin XH, Butcher JT (2016) Fabrication of aligned nanofiber polymer yarn networks for anisotropic soft tissue scaffolds. ACS Appl Mater Interfaces 8:16950–16960
Chen HY, Colvin DC, Qi B, Moore T, He J, Mefford OT, Alexis F, Gore JC, Anker JN (2012) Magnetic and optical properties of multifunctional core-shell radioluminescence nanoparticles. J Mater Chem 22:12802–12809
Zeng QB, Guo QN, Yuan YP, Yang YQ, Zhang B, Ren LL, Zhang XX, Luo Q, Liu ML, Bouchard LS, Zhou X (2017) Mitochondria targeted and intracellular biothiol triggered hyperpolarized 129Xe magnetofluorescent biosensor. Anal Chem 89:2288–2295
Xi X, Wang JX, Dong XT, Ma QL, Yu WS, Liu GX (2014) Flexible Janus nanofiber: a new tactics to realize tunable and enhanced magnetic-luminescent bifunction. Chem Eng J 254:259–267
Li K, Zhang Y, Li XJ, Shang MM, Lian HZ, Lin J (2015) Host-sensitized luminescence in LaNbO4:Ln3+ (Ln3+=Eu3+/Tb3+/Dy3+) with different emission colors. Phys Chem Chem Phys 17:4283–4292
Tian Y, Wei Y, Zhao Y, Quan ZW, Li GG, Lin J (2016) Photoluminescence tuning of Ca5(PO4)3Cl:Ce3+/Eu2+, Tb3+/Mn2+ phosphors: structure refinement, site occupancy, energy transfer and thermal stability. J Mater Chem C 4:1281–1294
Liang SS, Shang MM, Lian HZ, Li K, Zhang Y, Lin J (2017) An efficient rare-earth free deep red emitting phosphor for improving the color rendering of white light-emitting diodes. J Mater Chem C 5:2927–2935
Li T, Liu H, Wu Z, Liu Y, Guo Z, Zhang H (2016) Seeded preparation of ultrathin FeS2 nanosheets from Fe3O4 nanoparticles. Nanoscale 8:11792–11796
Xu XY, Yan B (2015) Eu(III)-functionalized MIL-124 as fluorescent probe for highly selectively sensing ions and organic small molecules especially for Fe(III) and Fe(II). ACS Appl Mater Interfaces 7:721–729
Ma QL, Wang JX, Dong XT, Yu WS, Liu GX (2014) Fabrication of magnetic-fluorescent bifunctional flexible coaxial nanobelts by electrospinning using a modified coaxial spinneret. ChemPlusChem 79:290–297
Ma QL, Yu WS, Dong XT, Wang JX, Liu GX (2014) Janus nanobelts: fabrication, structure and enhanced magnetic-fluorescent bifunctional performance. Nanoscale 6:2945–2952
Zhou XJ, Ma QL, Dong XT, Wang JX, Yu WS, Liu GX (2015) Flexible Janus nanofibers: a feasible route to realize simultaneously tuned magnetism and enhanced color-tunable luminescence bifunctionality. RSC Adv 5:35948–35957
Miao FJ, Shao CL, Li XH, Lu N, Wang KX, Zhang X, Liu YC (2015) Flexible solid-state supercapacitors based on freestanding electrodes of electrospun polyacrylonitrile@polyaniline core-shell nanofibers. Electrochim Acta 176:293–300
Zhao R, Li X, Sun B, Ji H, Wang C (2017) Diethylenetriamine-assisted synthesis of amino-rich hydrothermal carbon-coated electrospun polyacrylonitrile fiber adsorbents for the removal of Cr(VI) and 2,4-dichlorophenoxyacetic acid. J Colloid Interface Sci 487:297–309
Niu QJ, Guo JX, Chen BL, Nie J, Guo XD, Ma GP (2017) Bimetal-organic frameworks/polymer core-shell nanofibers derived heteroatom-doped carbon materials as electrocatalysts for oxygen reduction reaction. Carbon 114:250–260
Ma QL, Wang JX, Dong XT, Yu WS, Liu GX (2015) Flexible Janus nanoribbons array: a new strategy to achieve excellent electrically conductive anisotropy, magnetism, and photoluminescence. Adv Funct Mater 25:2436–2443
Liu YW, Ma QL, Dong XT, Yu WS, Wang JX, Liu GX (2015) A novel strategy to directly fabricate flexible hollow nanofibers with tunable luminescence-electricity-magnetism trifunctionality using one-pot electrospinning. Phys Chem Chem Phys 17:22977–22984
Wang ZJ, Ma QL, Dong XT, Li D, Xi X, Yu WS, Wang JX, Liu GX (2016) Novel electrospun dual-layered composite nanofibrous membrane endowed with electricity-magnetism bifunctionality at one layer and photoluminescence at the other layer. ACS Appl Mater Interfaces 8:26226–26234
Han DH, Wang JP, Luo HL (1994) Crystallite size effect on saturation magnetization of fine ferrimagnetic particles. J Magn Magn Mater 136:176–182
Tian J, Ma QL, Dong XT, Yu WS, Yang M, Yang Y, Wang JX, Liu GX (2016) Flexible Janus nanoribbons to help obtain simultaneous color-tunable enhanced photoluminescence, magnetism and electrical conduction trifunctionality. RSC Adv 6:36180–36191
Funding
This study was funded by National Natural Science Foundation of China (51573023, 50972020), Natural Science Foundation of Jilin Province of China (20170101101JC), Industrial Technology Research and Development Project of Jilin Province Development and Reform Commission (2017C051), Science and Technology Research Planning Project of the Education Department of Jilin Province during the 13th Five-Year Plan Period (JJKH20170608KJ), Youth Foundation of Changchun University of Science and Technology (No. XQNJJ-2016-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fan, L., Ma, Q., Tian, J. et al. Conjugate electrospinning-fabricated nanofiber yarns simultaneously endowed with bifunctionality of magnetism and enhanced fluorescence. J Mater Sci 53, 2290–2302 (2018). https://doi.org/10.1007/s10853-017-1661-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1661-x