Abstract
Evolution of \(\omega \) phase during heating of metastable \(\beta \) titanium alloy Ti–15Mo was investigated in situ during heating by electrical resistance measurements and accompanied by transmission electron microscopy. Different heating rates were employed aiming to determine kinetics of occurring phase transformations. Sharp change of temperature dependence of electrical resistance caused by complete dissolution of \(\omega \) phase was observed at 560 \(^{\circ }\hbox {C}\) independently of heating rate. Majority of \(\omega _{\mathrm{iso}}\) particles revert back to \(\beta \) phase at 560 \(^{\circ }\hbox {C}\); therefore, they are not direct precursors of \(\alpha \) precipitation during continuous heating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metastable \(\beta \) titanium alloys are used in a wide range of components for the aerospace and chemical industry and as alternative structural materials for the automotive sector due to their high specific strength and excellent fatigue and corrosion resistance [1]. Moreover, due to biocompatibility and partial or total superelasticity [2], \(\beta \)-Ti alloys are also considered for biomedical applications [3]. The mechanical properties of these alloys are mainly determined by the distribution and the size of metastable \(\omega \) particles or stable \(\alpha \) precipitates in \(\beta \) matrix [4, 5]. The formation of the precipitates, their morphology, volume fraction, the size and the distribution are influenced by phase transformations occurring during heat treatment [6, 7]. Therefore, a thorough understanding of these processes is necessary to optimize the mechanical properties of the material through thermomechanical treatment.
The phase transformations which are expected to occur during heat treatment of the studied metastable \(\beta \) titanium alloy Ti–15Mo are \(\beta \leftrightarrow \omega \), \(\omega \rightarrow \alpha \) and \(\beta \leftrightarrow \alpha \) [1]. In this paper \(\beta \leftrightarrow \omega \) transition will be mainly addressed.
The \(\omega \) phase has received extensive attention since its discovery in 1954 [8] mainly due to \(\omega \) particles possibly serving as preferential nucleation sites for \(\alpha \) precipitates. Fine and uniformly dispersed \(\alpha \) precipitates in \(\beta \) matrix result in excellent mechanical properties of the material [9, 10]. The \(\omega \) particles also affect some physical properties such as ductility, superconductivity, etc. Two stages of \(\omega \) phase are distinguished according to the conditions of their formation [11]. The mechanism of \(\omega \) phase formation was proposed in [12]. So-called athermal \(\omega \) (\(\omega _{\mathrm{ath}}\)) forms by a diffusionless, displacive transformation upon quenching from temperatures above \(\beta \)-transus. \(\omega _{\mathrm{ath}}\) particles are extremely fine (\(\sim \)1–4 nm in length), their number density is extremely large [13] and they are ordered in a cubic array along \(\langle 100\rangle \) directions in \(\beta \) matrix [14]. The experiments showed that even extremely fast quenching rates were insufficient to suppress \(\beta \rightarrow \omega _{\mathrm{ath}}\) transformation [15]. Moreover, the reversibility of \(\beta \leftrightarrow \omega _{\mathrm{ath}}\) transition between \(-171\) and +5 \(^{\circ }\hbox {C}\) was reported in [16]. Results presented in our previous work [17] extend this regime up to +100 \(^{\circ }\hbox {C}\), which is consistent with [18]. Subsequent heating of the Ti–15Mo alloy to higher temperatures would result in the formation of \(\omega _{\mathrm{iso}}\) which is irreversible in nature [12].
\(\omega \) phase particles further evolve and grow during ageing in temperature range of 200–500 \(^{\circ }\hbox {C}\), depending primarily on the alloy composition [19]. This process is accompanied by the rejection of \(\beta \) stabilizing elements from \(\omega \) phase causing a decrease of lattice parameters of both \(\omega \) and \(\beta \) phases [20]; thus, the reaction is diffusion controlled and irreversible. Such grown \(\omega \) phase particles are called isothermal \(\omega \) (\(\omega _{\mathrm{iso}}\)) and can develop to the size of tens of nanometres.
Ageing of \(\omega _{\mathrm{iso}}+\beta \) at higher temperatures results in \(\alpha \) phase precipitation [21]. Different mechanisms have been proposed describing the role of \(\omega \) particles in the nucleation of \(\alpha \) precipitates [22,23,24]. For systems where the critical misfit of the interface is exceeded [22], \(\alpha \) phase should preferentially precipitate at \(\omega /\beta \) interfaces. The \(\alpha \) phase could also precipitate at a certain distance from \(\omega /\beta \) interface [23] or within the core of \(\omega \) precipitates [25].
Materials and methods
The metastable \(\beta \)-Ti alloy (Ti–15Mo) used in this study contains 15 wt% (8.1 at.%) of molybdenum and negligible amounts of impurity elements (less than 0.5 of O, 0.1 of C and Fe, 0.05 of Ni and 0.015 of H in wt. %). The material was solution treated (ST) at 900 \(^{\circ }\hbox {C}\) for 4 hours in a quartz tube filled with high-purity Ar and quenched in water. This condition corresponds to the ST initial state for the investigation.
In order to investigate the microstructure evolution of the material in more detail, additional two conditions of material were prepared. For each condition, a bulk specimen was sealed in a quartz tube filled with Ar and heated up to the temperature of 550 or 580 \(^{\circ }\hbox {C}\) using the heating rate of 5 \(^{\circ }\hbox {C}\)/min followed by immediate water quenching.
ST initial material and additional two pre-annealed conditions were studied both by in situ electrical resistance measurement and ex-situ (post-mortem) TEM observations. In the following text, these two conditions are referred to as pre-annealed conditions/samples.
Electrical resistance measurements
The electrical resistance measurement is widely used method for the detection of phase transformations in titanium alloys [26,27,28,29,30,31,32,33] due to its high sensitivity to the formation and the development of \(\omega \) phase. However, the resistance measurement is an indirect method and should be accompanied by electron microscopy observations in order to interpret correctly the processes detected by electrical resistance measurements.
The in situ electrical resistance measurements during heating were performed using a precise in-house built apparatus. The voltage was measured simultaneously with commutating direct electric current using the nanovoltmeter Keithley 2182 and the SourceMeter Keithley 2400 devices, respectively. The relative error of electrical resistance is lower than 10\(^{-4}\) of the measured value. Two experimental values are acquired per second [34]. The material was cut into S-shaped specimens, based on simplification of M-shaped sample introduced in [34]. The outer dimensions of the specimens were about \(10\times 15\times 0.6\) mm. These samples were placed in a specially designed furnace which allows precise heating of the sample in a protective argon atmosphere. The evolution of electrical resistance during heating was measured from room temperature to 850 \(^{\circ }\hbox {C}\), but the results are presented only up to 700 \(^{\circ }\hbox {C}\). Above 700 \(^{\circ }\hbox {C}\), the sample surface undergoes oxidation from the remaining oxygen in the Ar atmosphere. The results of resistance measurement above 700 \(^{\circ }\hbox {C}\) are therefore not reliable and not reproducible.
The kinetics of occurring phase transformations were determined at heating rates of 1, 5 and 50 \(^{\circ }\hbox {C}\)/min.
Transmission electron microscopy
Based on results of resistance measurements, selected microstructure conditions were characterized by transmission electron microscopy (TEM).
Thin foils for TEM were prepared by mechanical grinding with fine emery papers to the thickness of 150 mm followed by electropolishing (Tenupol-5, Struers) with a solution containing HClO\(_4\), butanol and methanol at the voltage of 20 V and the temperature of \(-20\,^{\circ }\hbox {C}\). The final thinning step was the ion milling using PIPS Gatan operating at 4 kV. TEM observations were performed on JEOL 2000FX and JEOL 2200FS microscopes at 200 kV.
Results and discussion
The influence of heating rate on phase transitions measured by in situ electrical resistance
The temperature dependence of electrical resistance of the ST sample is shown in Fig. 1. Three heating rates were employed (1, 5 and 50 \(^{\circ }\hbox {C}\)/min) with the aim to determine the kinetics of occurring phase transformations. The effect of heating rate on \(\omega \) phase evolution in some metastable \(\beta \) alloys was shown in previous studies [35,36,37,38].
The absolute resistivity value of the material cannot be determined with sufficient accuracy due to a complex shape of the sample required for the measurement. The efficient length and efficient cross section of the conductor cannot be estimated with sufficient accuracy to calculate the resistivity. Therefore, only relative changes of resistance during heating provide meaningful information about undergoing processes in material. Relative resistance is calculated by dividing the measured resistance evolution of the sample by some reference resistance measured for the same sample, typically the resistance of the sample measured at room temperature, i.e. \(R/R_{\mathrm{RT}}\). Comparison of relative resistance evolution of two different samples is substantiated only if the two reference values correspond to the same microstructural condition and phase composition (and, of course, the same temperature). At room temperature the initial condition of all three specimens is obviously identical (this is the case of measurement depicted in Fig. 1—a single initial condition is subjected to heating with different heating rate), the resistance at room temperature measured for each sample would be a valid reference value in this case.
On the other hand, as described in previous section, pre-annealed samples were prepared and then subjected to resistance measurement. Obviously, at room temperature, the phase composition of each of the pre-annealed samples is different from the initial ST condition. Therefore, another selection of reference value must be considered. A natural choice of such reference value would be resistance of each sample at any temperature above \(\beta \)-transus (\(T>730\) \(^{\circ }\hbox {C}\)) as the material consists of pure \(\beta \) phase only. However, this choice is would be misleading due to enhanced oxidation of specimens and contacts in the furnace at elevated temperatures [39, 40], which influences the measured resistance.
The best compromise is therefore to use the resistance measured at 560 \(^{\circ }\hbox {C}\) (\(R_{560}\)) as the reference value for each sample. This paper shows that \(\omega \) phase dissolves at 560 \(^{\circ }\hbox {C}\) and the volume fraction of precipitated \(\alpha \) is very low in all studied samples. The phase composition at 560 \(^{\circ }\hbox {C}\) is the most comparable in all three studied samples and \(R_{560}\) is therefore used as the reference value in all resistance measurements described further in Sects. 3.1 and 3.3.
Note that the particular temperature of a change in the monotonicity of a resistance curve does not correspond to the onset of a new phase transformation; it rather relates to the temperature at which the new process (electron scattering mechanism) prevails over the previous one. This differs from differential scanning calorimetry curve, in which each peak corresponds exactly to the temperature of the onset and/or the end of a particular phase transformation.
In Fig. 1, the initial decrease of electrical resistance during heating from room temperature to 225 \(^{\circ }\hbox {C}\) (for the heating rate of 5 \(^{\circ }\hbox {C}\)/min, solid line in Fig. 1) is attributed to the reversible dissolution of \(\omega _{\mathrm{ath}}\) particles [17, 26, 33].
The increase of resistance between 225 and 365 \(^{\circ }\hbox {C}\) is caused by the formation of \(\omega _{\mathrm{iso}}\) phase as reported in [33], where the authors investigated the resistance evolution of a similar titanium alloy (Ti–6.8Mo–4.5Fe–1.5A1).
The decrease of resistance between 365 and 560 \(^{\circ }\hbox {C}\) is, according to [35, 41], related to progressive vanishing of \(\omega _{\mathrm{iso}}\) through \(\omega \rightarrow \alpha \) phase transformation. \(\omega _{\mathrm{iso}}\) should serve as the precursor phase for the heterogenous nucleation of \(\alpha \) phase in this temperature range [25, 42].
The comparison of different heating rates in Fig. 1 shows shifts of the first local minimum and maximum of resistance curves to higher temperatures with increasing heating rate. For heating rates of 1, 5 and 50 \(^{\circ }\hbox {C}\)/min, the first minima are at 190, 225 and 300 \(^{\circ }\hbox {C}\), respectively, while the respective maxima are at 335, 365, 435 \(^{\circ }\hbox {C}\). These shifts indicate that diffusion controlled processes, which affect the electrical resistance, occur in the material. On the other hand, the abrupt change in the monotonicity at 560 \(^{\circ }\hbox {C}\) does not depend on the heating rate. The authors in [35] claim that this sharp minimum in the relative resistance corresponds to the return towards equilibrium in \(\alpha +\beta \) domain. However, all \(\alpha \) phase related transformations should be also a diffusion controlled processes and therefore the independence on the heating rate is rather surprising. In order to understand the underlying processes another set of experiments was proposed whose results will be described in the following sections.
During further heating (\(T>560\) \(^{\circ }\hbox {C}\)), the resistance increases and the concentration of \(\alpha \) phase first increases to reach an equilibrium and than close to \(\beta \)-transus decreases again. Above \(\beta \)-transus (about 730 \(^{\circ }\hbox {C}\)) \(\alpha \) phase dissolves completely.
Microstructure observations of ST and pre-annealed material
The most striking feature of the resistance measurements presented in Fig. 1 is that all curves exhibit the same sudden change at 560 \(^{\circ }\hbox {C}\) irrespective of heating rate. To examine this fact, a set of pre-annealed samples was prepared as described in Materials and methods" section.
TEM observations of ST specimen and samples quenched from 550 and 580 \(^{\circ }\hbox {C}\) were performed to gain more insight into undergoing phase transformation at 560 \(^{\circ }\hbox {C}\). The microstructures of these specimens are presented in Figs. 2, 3 and 4.
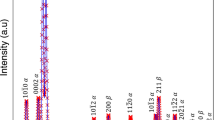
The diffraction pattern of the ST sample is shown in Fig. 2a. The strong spots arise from \(\beta \) matrix. The less intense reflections correspond to two different crystallographic orientations of \(\omega \) phase [43]. The dark field (DF) image, Fig. 2b, shows one crystallographic family of \(\omega _{\mathrm{ath}}\) particles, which formed during quenching. Their longitudinal dimension is of the order of nanometres, which is consistent with [44] where the authors showed by HRTEM that ST Ti–15Mo consists of \(\beta \) phase and small ellipsoidal-shaped \(\omega _{\mathrm{ath}}\) particles (<1.5 nm in length).
Microstructure of the specimen quenched from 550 \(^{\circ }\hbox {C}\). a Electron diffraction pattern with a [110]\(_\beta \) zone axis, showing the presence of \(\beta \) matrix and \(\omega \) particles. The extra reflections marked by the black arrows are the result of double diffraction. b Superimposed DF TEM image, obtained using the reflection spots marked by red and green circles in a representing two families of \(\omega \) particles
Figure 3a, b show selected area diffraction patterns (SAEDs) and a superimposed DF image of two (out of four) crystallographic families of \(\omega _{\mathrm{iso}}\) particles present in the sample quenched from 550 \(^{\circ }\hbox {C}\), respectively. From Fig. 3a, it is obvious that the material consists of \(\beta \) matrix and \(\omega \) phase particles. No \(\alpha \) phase was found in grain interior. However, some \(\alpha \) precipitates were found at grain boundaries (not shown in this paper). The \(\omega _{\mathrm{iso}}\) particles shown in Fig. 3b (two crystallographic families) are uniformly dispersed in the pre-annealed material, have ellipsoidal shape and their longitudinal dimension is about 50 nm. The length of these \(\omega _{\mathrm{iso}}\) particles is therefore approximately 30 times bigger than of \(\omega _{\mathrm{ath}}\) in the ST sample (cf. Fig. 2b or [44]). The \(\omega _{\mathrm{iso}}\) particles observed in Fig. 3b are also chemically stabilized, which was proven by our previous SEM observations using chemical contrast of back-scattered electrons [45].
Microstructure of the specimen quenched from 580 \(^{\circ }\hbox {C}\). a Electron diffraction pattern with a [110]\(_\beta \) zone axis, showing the presence of \(\beta \) matrix, \(\omega \) particles and probably \(\alpha \) precipitates with weak point of double diffraction (in blue circles). b DF obtained using the reflection spot marked red circle in a representing one family of \(\omega \) particles. c Computed diffraction pattern showing Pitsch–Schrader orientation relationship between \(\alpha \) and \(\beta \) phases
SAED pattern of the sample quenched from 580 \(^{\circ }\hbox {C}\) (Fig. 4a) contains besides strong reflections from \(\beta \) phase matrix and reflections from \(\omega \) phase other weak spots marked with blue circles. Most probably, these weak spots originate from small newly developed \(\alpha \) precipitates. Additional weak spots in blue circles result from the double diffraction. Simulated diffraction pattern on Fig. 4c shows reflections of all three phases with Pitsch–Schrader orientation relationship between \(\alpha \) and \(\beta \) phases (\(\left( 0001\right) _\alpha \parallel \left( 110\right) _\beta \) and \(\left[ 11\overline{2}0\right] _\alpha \parallel \left[ 100\right] _\beta \)) already observed in Ti–Mo alloys in [46]. The DF image in Fig. 4b was obtained using a reflection spot of only one family of \(\omega \) phase particles. It can be observed that the microstructure of this sample is very similar to the ST material (cf. Figs. 2b and 4b). Therefore, it is assumed that the observed \(\omega \) particles are \(\omega _{\mathrm{ath}}\) particles formed after quenching. Apparently lower number of \(\omega _{\mathrm{ath}}\) particles at Fig. 4b compared to Fig. 2b is caused by different thickness of both specimens.
From the comparison of the microstructures of the material in all three conditions suggest that coarse \(\omega _{\mathrm{iso}}\) particles, which are present in the sample quenched from 550 \(^{\circ }\hbox {C}\), completely dissolve during heating between 550 and 580 \(^{\circ }\hbox {C}\). This dissolution manifests itself most likely as the steep change in the resistance curve at 560 \(^{\circ }\hbox {C}\) with no (or very small) dependence on the heating rate (cf. Fig. 1). Small \(\omega \) particles found in the sample quenched from 580 \(^{\circ }\hbox {C}\) probably form during quenching. The results indicate that the vast majority of \(\omega _{\mathrm{iso}}\) particles revert back to \(\beta \) phase at 560 \(^{\circ }\hbox {C}\) and consequently they are not direct precursors of \(\alpha \) precipitation. However, possible compositional pockets with low Mo content left in \(\beta \) matrix in places of former presence of \(\omega _{\mathrm{iso}}\) particles could affect \(\alpha \) phase precipitation. Consequently, there could be an indirect influence of \(\omega \) particles in \(\alpha \) phase precipitation, which is consistent with previous reports [10, 47].
The absence of \(\alpha \) phase in the sample quenched from 550 \(^{\circ }\hbox {C}\) proves that \(\alpha \) phase does not precipitate during heating between 365 an 560 \(^{\circ }\hbox {C}\) with heating rate of 5 \(^{\circ }\hbox {C}\)/min. This fact does not rule out the possibility that \(\alpha \) phase precipitates in this temperature range during isothermal annealing. However, the decrease of electrical resistance in this temperature range cannot be attributed to precipitation of \(\alpha \) particles.
Resistance evolution of ST and pre-annealed material
In order to investigate the behaviour of the material quenched from 550 and 580 \(^{\circ }\hbox {C}\) in more detail, the electrical resistance during heating of both specimens employing the heating rate of 5 \(^{\circ }\hbox {C}\)/min was compared with the resistance evolution of the ST condition in Fig. 5. Note that the normalization \(R/R_{560}\) was necessary to allow the comparison of measurements of samples with different initial conditions. Unlike Fig. 1 where both normalization values \(R_{\mathrm{RT}}\) and \(R_{560}\) are correct, in this case only the latter value \(R_{560}\) may be used as only at this temperature the microstructure of all three specimens is the same.
The resistance curves of individual pre-annealed conditions in Fig. 5 differ. At low temperatures, the resistance evolution of the sample quenched from 550 \(^{\circ }\hbox {C}\) considerably differs from both other curves (the ST specimen and the sample quenched from 580 \(^{\circ }\hbox {C}\)). A much smaller initial decrease attributed to \(\omega _{\mathrm{ath}}\) dissolution can be observed. It may be explained by much lower volume fraction of \(\omega _{\mathrm{ath}}\) in the sample quenched from 550 \(^{\circ }\hbox {C}\) than in the other two. In this specimen, large \(\omega _{\mathrm{iso}}\) particles remain; therefore, the formation of \(\omega _{\mathrm{ath}}\) during quenching is suppressed. On the other hand, the resistance of the sample quenched from 580 \(^{\circ }\hbox {C}\) is very similar to the ST specimen. It indicates the similar microstructure consisting of \(\beta \) and \(\omega _{\mathrm{ath}}\) phases in both samples. Slight difference of resistance between the ST material and the sample quenched from 580 \(^{\circ }\hbox {C}\) at room temperature might be caused by a small volume fraction of \(\alpha \) phase in the sample quenched from 580 \(^{\circ }\hbox {C}\).
Note, that the minimum at 560 \(^{\circ }\hbox {C}\) was not affected by the previous heating run for any sample.
The results from electrical resistance measurements combined with microstructure observations (of all three conditions) indicate that grown \(\omega \) particles in the sample quenched from 550 \(^{\circ }\hbox {C}\) hinder the formation of \(\omega _{\mathrm{ath}}\) during quenching. After heating up to 580 \(^{\circ }\hbox {C}\), \(\omega _{\mathrm{iso}}\) particles are dissolved and consequently \(\omega _{\mathrm{ath}}\) can form during quenching. The presence of \(\omega _{\mathrm{ath}}\) particles and their subsequent dissolution during heating results in the initial decrease of the resistance in a similar way as observed in the ST material (cf. Fig. 5).
The abrupt change in the temperature dependence of the electrical resistance, which is in Ti–15Mo alloy caused by a disappearance of \(\omega _{\mathrm{iso}}\), was also observed in another ST metastable \(\beta \)-Ti alloys (Timetal LCB—Ti–6.8Mo–4.5Fe–1.5Al [41] and Ti–6Mo–5Ta–4Fe [35] in wt%). The sudden disappearance of \(\omega \) phase during heating of Ti-5553 (Ti–5Al–5Mo–5V–3Cr) alloy between the room temperature and 600 \(^{\circ }\hbox {C}\) without serving as \(\alpha \) phase precursor was reported in [9, 10]. As a consequence, the electrical resistance measurements allow to precisely identify the exact temperature of \(\omega \) dissolution.
Conclusions
In situ electrical resistance measurements and TEM SAED provided insight into phase transformations in Ti–15Mo alloy during heating. The most attention was given to \(\omega \leftrightarrow \beta \) transitions. The following conclusions may be drawn from our experimental observations:
-
\(\omega \) phase completely dissolves during heating of Ti–15Mo at about 560 \(^{\circ }\hbox {C}\).
-
This dissolution is independent of the heating rate.
-
Coarse \(\omega _{\mathrm{iso}}\) particles hinder the formation of \(\omega _{\mathrm{ath}}\) during quenching.
-
The vast majority of \(\omega _{\mathrm{iso}}\) particles revert back to \(\beta \) phase at 560 \(^{\circ }\hbox {C}\) and consequently they are not direct precursors of \(\alpha \) precipitation during continuous heating. Moreover, there could be an indirect influence of \(\omega \) particles in \(\alpha \) phase precipitation through compositional pockets with low Mo content.
-
Similar abrupt change of resistance, which is in Ti–15Mo alloy caused by the dissolution of \(\omega \) phase has been also observed in Timetal LCB alloy, which suggests that the abrupt dissolution of \(\omega \) phase during heating may occur in many other \(\beta \) Ti alloys (e.g. Timetal LCB and 21S, Ti–6Mo–5Ta–4Fe, Ti-5553, TCFA).
-
Electrical resistance measurements can precisely reveal the temperature of complete dissolution of \(\omega _{\mathrm{iso}}\) particles.
References
Lütjering G, Williams JC (2007) Titanium. Engineering materials, processes. Springer, Berlin, pp 1–39. doi:10.1007/978-3-540-73036-1
Maeshima T, Nishida M (2004) Shape memory and mechanical properties of biomedical Ti–Sc–Mo alloys. Mater Trans 45(4):1101–1105. doi:10.2320/matertrans.45.1101
Duraccio D, Mussano F, Faga MG (2015) Biomaterials for dental implants: current and future trends. J Mater Sci 50(14):4779–4812. doi:10.1007/s10853-015-9056-3
Duerig TW, Williams JC (1984) In: Boyer RR, Rosenberg HW (eds) Beta-Titanium Alloys in the 1980’s. The Metallurgical Society of AIME, pp 19–69
Wang CH, Yang CD, Liu M, Li X, Hu PF, Russell AM, Cao GH (2016) Martensitic microstructures and mechanical properties of as-quenched metastable \(\beta \)-type Ti–Mo alloys. J Mater Sci 51(14):6886–6896. doi:10.1007/s10853-016-9976-6
Duerig TW, Albrecht J, Richter D, Fischer P (1982) Formation and reversion of stress induced martensite in Ti–10V–2Fe–3Al. Acta Metall 30(12):2161–2172. doi:10.1016/0001-6160(82)90137-7
Tang X, Ahmed T, Rack HJ (2000) Phase transformations in Ti–Nb–Ta and Ti–Nb–Ta–Zr alloys. J Mater Sci 35(7):1805–1811. doi:10.1023/A:1004792922155
Frost PD, Parris WM, Hirsch LL, Doig JR, Schwartz CM (1954) Isothermal transformation of titanium–chromium alloys. Trans Am Soc Met 46:231
Zheng Y, Williams RE, Sosa JM, Alam T, Wang Y, Banerjee R, Fraser HL (2016) The indirect influence of the \(\omega \) phase on the degree of refinement of distributions of the \(\alpha \) phase in metastable \(\beta \)-titanium alloys. Acta Mater 103:165–173. doi:10.1016/j.actamat.2015.09.053
Zheng Y, Williams RE, Sosa JM, Wang Y, Banerjee R, Fraser HL (2016) The role of the \(\omega \) phase on the non-classical precipitation of the \(\alpha \) phase in metastable \(\beta \)-titanium alloys. Scripta Mater 111:81–84. doi:10.1016/j.scriptamat.2015.08.019
Sikka SK, Vohra YK, Chidambaram R (1982) Omega phase in materials. Prog Mater Sci 27(3):245. doi:10.1016/0079-6425(82)90002-0
Hickman BS (1969) The formation of omega phase in titanium and zirconium alloys: a review. J Mater Sci 4(6):554
Banerjee S, Mukhopadhyay P (2007) Phase transformations: examples from titanium and zirconium alloys. No. 12 in Pergamon materials seriesv. Elsevier, Pergamon
Šmilauerová J, Harcuba P, Stráský J, Stráská J, Janeček M, Pospíšil J, Kužel R, Brunátová T, Holý V, Ilavský J (2014) Ordered array of \(\omega \) particles in \(\beta \)-Ti matrix studied by small-angle X-ray scattering. Acta Mater 81:71–82. doi:10.1016/j.actamat.2014.06.042
Bagaryatskiy A, Yu I, Nosova G (1962) Phys Met Metallogr 13(3):92
De Fontaine D, Paton NE, Williams JC (1971) The omega phase transformation in titanium alloys as an example of displacement controlled reactions. Acta Metall 19(11):1153. doi:10.1016/0001-6160(71)90047-2
Zháňal P, Harcuba P, Šmilauerová J, Stráský J, Janeček M, Smola B, Hájek M (2015) Phase transformations in Ti–15Mo investigated by in situ electrical resistance. Acta Phys Pol A 128(4):779. doi:10.12693/APhysPolA.128.779
Nejezchlebová J, Janovská M, Seiner H, Sedlák P, Landa M, Šmilauerová J, Stráský J, Harcuba P, Janeček M (2016) The effect of athermal and isothermal \(\omega \) phase particles on elasticity of \(\beta \)-Ti single crystals. Acta Mater 110:185–191. doi:10.1016/j.actamat.2016.03.033
Froes F, Staff AI (2015) Titanium: physical metallurgy processing and applications. ASM International, New York
Šmilauerová J, Harcuba P, Pospíšil J, Matěj Z, Holý V (2013) Growth of \(\omega \) inclusions in Ti alloys: an X-ray diffraction study. Acta Mater 61(17):6635–6645. doi:10.1016/j.actamat.2013.07.059
Ohmori Y, Ogo T, Nakai K, Kobayashi S (2001) Effects of \(\omega \)-phase precipitation on \(\beta \rightarrow \alpha \), \(\alpha \)” transformations in a metastable \(\beta \) titanium alloy. Mater Sci Eng A 312(1):182. doi:10.1016/S0921-5093(00)01891-8
Williams JC, Blackburn MJ (1969) The influence of Misfit on the morphology and stability of the omega phase in titanium–transition metal alloys. Trans Met Soc AIME 245:2352–2355
Nag S, Banerjee R, Srinivasan R, Hwang JY, Harper M, Fraser HL (2009) \(\omega \)-Assisted nucleation and growth of \(\alpha \) precipitates in the Ti–5Al–5Mo–5V–3Cr–0.5Fe \(\beta \) titanium alloy. Acta Mater 57(7):2136. doi:10.1016/j.actamat.2009.01.007
Li T, Kent D, Sha G, Stephenson LT, Ceguerra AV, Ringer SP, Dargusch MS, Cairney JM (2016) New insights into the phase transformations to isothermal \(\omega \) and \(\omega \)-assisted \(\alpha \) in near \(\beta \)-Ti alloys. Acta Mater 106:353–366. doi:10.1016/j.actamat.2015.12.046
Prima F, Vermaut P, Texier G, Ansel D, Gloriant T (2006) Evidence of \(\alpha \)-nanophase heterogeneous nucleation from \(\omega \) particles in a \(\beta \)-metastable Ti-based alloy by high-resolution electron microscopy. Scripta Mater 54(4):645. doi:10.1016/j.scriptamat.2005.10.024
Ho JC, Collings EW (1972) Anomalous electrical resistivity in titanium–molybdenum alloys. Phys Rev B 6(10):3727
Luhman TS, Taggart R, Polonis DH (1968) A resistance anomaly in beta stabilized Ti–Cr alloys. Scr Metall 2(3):169. doi:10.1016/0036-9748(68)90219-6
Hake RR, Leslie DH, Berlincourt TG (1961) Electrical resistivity, Hall effect and superconductivity of some b.c.c. titanium–molybdenum alloys. J Phys Chem Solids 20(3):177. doi:10.1016/0022-3697(61)90002-6
Komatsu SY, Ikeda M, Sugimoto T, Kamei K, Maesaki O, Kojima MA (1996) Aging behaviour of Ti15Mo5Zr and Ti15Mo5Zr3Al alloy up to 573 K. Mater Sci Eng A 213(1—-2):61–65. doi:10.1016/0921-5093(96)10228-8
Sun F, Prima F, Gloriant T (2010) High-strength nanostructured Ti–12Mo alloy from ductile metastable beta state precursor. Mater Sci Eng A 527(16–17):4262–4269. doi:10.1016/j.msea.2010.03.044
Hill MA, Polonis DH (1987) Influence of beta phase decomposition on the temperature coefficient of resistivity of titanium alloys. J Mater Sci 22(6):2181–2184. doi:10.1007/BF01132957
Yoshida S, Tsuya Y (1956) The temperature dependence of the electrical resistivity of the \(\beta \)-phase titanium–molybdenum alloys. J Phys Soc Jpn 11(11):1206. doi:10.1143/JPSJ.11.1206
Prima F, Debuigne J, Boliveau M, Ansel D (2000) Control of omega phase volume fraction precipitated in a beta titanium alloy: development of an experimental method. J Mater Sci Lett 19(24):2219
Hájek M, Veselý J, Cieslar M (2007) Precision of electrical resistivity measurements. Mater Sci Eng A 462(1–2):339–342. doi:10.1016/j.msea.2006.01.175
Gloriant T, Texier G, Sun F, Thibon I, Prima F, Soubeyroux J (2008) Characterization of nanophase precipitation in a metastable \(\beta \) titanium-based alloy by electrical resistivity, dilatometry and neutron diffraction. Scripta Mater 58(4):271. doi:10.1016/j.scriptamat.2007.10.007
Settefrati A, Aeby-Gautier E, Dehmas M, Geandier G, Appolaire B, Audion S, Delfosse J (2011) Precipitation in a near beta titanium alloy on ageing: influence of heating rate and chemical composition of the beta-metastable phase. Solid State Phenom 172–174:760–765. doi:10.4028/www.scientific.net/SSP.172-174.760
Boeckels H (2012) Phase transformations in titanium–molybdenum–oxygen. Ph.D. thesis, Clemson University
Barriobero-Vila P, Requena G, Warchomicka F, Stark A, Schell N, Buslaps T (2015) Phase transformation kinetics during continuous heating of a \(\beta \)-quenched Ti–10V–2Fe–3Al alloy. J Mater Sci 50(3):1412–1426. doi:10.1007/s10853-014-8701-6
Donachie M (1988) Titanium: a technical guide. AMS International, New York
Gemelli E, Camargo NHA (2007) Oxidation kinetics of commercially pure titanium. Matéria (Rio de Janeiro) 12(3):525–531. doi:10.1590/S1517-70762007000300014
Prima F, Vermaut P, Thibon I, Ansel D, Debuigne J, Gloriant T (2002) Nanostructured metastable \(\beta \)-titanium based alloy. J Metastab Nanocrystal Mater 13:307. doi:10.4028/www.scientific.net/JMNM.13.307
Takemoto Y, Hida M, Sakakibara A (1993) Mechanism of \(\omega \rightarrow \alpha \) transformation in \(\beta \)-Ti alloy. J Jpn Inst Met 57(3):261–267
Devaraj A, Nag S, Srinivasan R, Williams R, Banerjee S, Banerjee R, Fraser H (2012) Experimental evidence of concurrent compositional and structural instabilities leading to \(\omega \) precipitation in titanium–molybdenum alloys. Acta Mater 60(2):596. doi:10.1016/j.actamat.2011.10.008
Schryvers D, Tanner L (1990) High resolution electron microscopy observations of athermal omega phase in Ti–Mo alloys. Mater Sci Forum 56–58:329. doi:10.4028/www.scientific.net/MSF.56-58.329
Zháňal P, Harcuba P, Janeček M, Smilauerová J, Veselý J, Smola B, Zimina M (2016) In: Venkatesh V, Pilchak AL, Allison JE, Ankem S, Boyer R, Christodoulou J, Fraser HL, Imam MA, Kosaka Y, Rack HJ, Chatterjee A, Woodfield A (eds) Proceedings of the 13th world conference on titanium, TMS (The Minerals, Metals & Materials Society), pp 431–436
Furuhara T, Makino T, Idei Y, Ishigaki H, Takada A, Maki T (1998) Morphology and crystallography of \(\alpha \) precipitates in \(\beta \) Ti–Mo binary alloys. Mater Trans JIM 39(1):31–39. doi:10.2320/matertrans1989.39.31
Zheng Y, Williams REA, Wang D, Shi R, Nag S, Kami P, Sosa JM, Banerjee R, Wang Y, Fraser HL (2016) Role of \(\omega \) phase in the formation of extremely refined intragranular \(\alpha \) precipitates in metastable \(\beta \)-titanium alloys. Acta Mater 103:850–858. doi:10.1016/j.actamat.2015.11.020
Acknowledgements
This work was financially supported by the Czech Science Foundation under the Project 16-12598S. Partial support by the project “Nanomaterials centre for advanced applications”, Project No. CZ.02.1.01/0.0/0.0/15 003/0000485, financed by ERDF is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zháňal, P., Harcuba, P., Hájek, M. et al. Evolution of ω phase during heating of metastable β titanium alloy Ti–15Mo. J Mater Sci 53, 837–845 (2018). https://doi.org/10.1007/s10853-017-1519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1519-2