Abstract
3D hierarchical flower-like TiO2 nanospheres with rutile phase were synthesized via a facile hydrothermal method without further calcination. The morphology, crystalline phase and pore structure of the products were characterized by scanning electron microscope, transmission electron microscope, X-ray diffraction, Raman and nitrogen adsorption–desorption test. The results show that the hierarchical flower-like TiO2 nanospheres are assembled from well-ordered nanorods. It is found that the nanostructure of TiO2 nanospheres varies with the elevation of reaction temperatures, indicating a critical effect on the formation of hierarchical TiO2 nanospheres with a large proportion of (110) facets. The obtained TiO2 nanospheres can be used as photocatalyst, and the photocatalytic activity was evaluated by photodegrading a complex dye solution containing rhodamine B and methylene blue. It turned out that the TiO2 photocatalysts prepared at 160 and 190 °C showed the highest photocatalytic activity under ultraviolet light irradiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the pioneering work about photosplitting of water on TiO2 electrode reported by Honda and Fujushima [1], photocatalysts have become the ideal solution to eliminate dye pollutants. Among the various semiconductor metal oxides, TiO2 has been always regarded as one of the most promising photocatalysts due to its high chemical stability and activity, non-toxicity, corrosion resistance and low cost [2,3,4]. But, there is still a problem that TiO2 can only catalyze simple dye. In order to expand photodegrading scale, this material needs to be doped and modified [5,6,7,8]. To the best of our knowledge, there is a little report on photodegrading a complex dye solution. As a result, the development of photocatalyst that can degrade complex dyes simultaneously with high efficiency has received great attention.
As is well known, the photocatalytic performance of TiO2 strongly depends on its morphology, structure, size, crystal phase and surface property [9, 10]. Up to now, many kinds of TiO2 morphologies have been synthesized, such as nanoparticles, naonorods, nanosheets [11,12,13] and so on. Among various morphologies, hierarchical 3D architecture has attracted extensive attention because they may exhibit interesting properties due to the large numbers of active sites, unique multidimensional morphology and the combination of micro–nanoscales [14,15,16,17,18,19]. Therefore, 3D architecture self-assembled by 1D nanostructure is very essential, and there are few reports on this kind of nanocomposite for photocatalysts.
To date, the majority of studies of TiO2 photocatalysts have focused on anatase phase. There seems to be a general agreement that the anatase crystal structure exhibits higher photocatalytic activities than rutile [20,21,22], but through the change in the structure and surface area, the rutile phase photocatalysts can also works well. Some researchers have pointed out that the poor catalytic activity of rutile structured powders may be related with the low surface area and the poorly controlled morphology [23], rutile TiO2 can also be prepared with high specific surface area [24,25,26]. There are also some reports mentioned that the rutile crystalline have the same photoactivity as anatase, or even higher [27,28,29], which may be a comprehensive result of multifactors, such as specific surface area, pore size distribution, crystal size and so on. More importantly, the Fermi level in rutile is lower than that of anatase by about 0.1 eV, indicating that rutile TiO2 should be more suitable for practical application. However, studies of hierarchical TiO2 nanostructures with rutile phase applied in photocatalysts are far from satisfactory [30, 31].
In this research, we fabricate a novel self-assembly 3D hierarchical flower-like rutile TiO2 successfully through hydrothermal method without further calcination, and the formation mechanism is also speculated. The photocatalytic performance of the hierarchical TiO2 nanospheres is investigated by photodegrading a complex dye solution containing methylene blue (MB) and rhodamine B (RhB) simultaneously [32, 33]. Moreover, the photocatalysis kinetics of the rutile TiO2 nanospheres is also evaluated.
Experimental
Reagents and materials
All chemicals used in this study were analytical-grade reagents. Titanium (IV) butoxide (Ti(OC4H9)4, TBOT), hydrochloric acid (HCl, 37 wt%), nitric acid (HNO3), sulfuric acid (H2SO4), phosphoric acid (H3PO4) and glacial acetic acid (CH3COOH) were purchased from Shanghai Chemical Co. China. Methylene blue (MB) and rhodamine B (RhB) were purchased from Shanghai Chemical Co. China, which were used as the target dye for photodegradation experiments. Deionized water was used for all experiments.
Preparation of 3D hierarchical flower-like TiO2 photocatalysts
In a typical procedure, 15 ml of hydrochloric acid (HCl, 37 wt%) and 15 ml of deionized water were mixed in equal volume with a total volume of 30.0 ml. Then, 3.0 ml of TBOT was dissolved in the solution by magnetic stirring. After stirring for 1 h, the homogeneous solution was transferred to a 45-ml Teflon-lined stainless steel autoclave followed by heating at different temperatures from 160 to 190 °C for 3 h. Finally, the synthesized products were thoroughly washed with deionized water and dried at 80 °C in air for 12 h. The samples synthesized at 160, 170, 180, 190 °C were marked as S160, S170, S180, and S190, respectively.
Photocatalytic activity measurements
The photodegradation reactions were carried out under atmospheric condition using a 300-W mercury lamp as the irradiation source at ambient temperature. The irradiation distance between lamp and sample was 10 cm. Hundred milligrams of TiO2 photocatalysts was added into a cylindrical glass vessel containing 100 ml of complex solution with a concentration of 10 mg/l RhB and 10 mg/l MB, respectively. Before exposing the samples to the light source, the solution was agitated thoroughly with the catalyst slide in the dark for 30 min to reach the adsorption equilibrium of the dye on the catalyst. At intervals, the photodegradation reaction was ceased and the solution was centrifuged. The residual dye concentration in the supernatant was measured by a UV–Vis spectrometer (Lambda 850) at maximum absorption wavelength. Parallel degradation reactions under same conditions were conducted for varied time intervals. The intensity of the absorption band peak was recorded against time.
Characterization methods
The morphologies and nanostructures of the samples were investigated by scanning electron microscopy (SEM) using Hitachi S-4800. Transmission electron micrographs (TEM) were taken with JEM-2010F of JEOL electron microscope. X-ray diffraction (XRD) patterns were measured using a D/max-2600PC with Cu Kα radiation (λ = 1.5406 Å) operated at 40 kV and 150 mA at a scan rate of 5.0°/min. Qualitative analysis was performed with the X’pert High-Score software and the JCPDSPDF-2 database. The average crystallite size was calculated from the Scherrer equation, using the X-ray diffraction peak at 2θ = 27.4° (rutile). Fourier-transformed Raman spectroscopic measurements in the ultraviolet light were taken on a Renishaw inVia Reflex Raman Spectrometer. Nitrogen adsorption–desorption isotherms were measured with Micromeritics ASAP 2010 nitrogen adsorption apparatus. All the samples were degassed at 473 K prior to the measurements. The optical absorption spectra were recorded on a UV–Vis spectrophotometer (Agilent 8453) at room temperature. The Brunauer–Emmett–Teller (BET) method was used for surface area calculation, and the pore size distribution (pore diameter and pore volume of the samples) was determined by the Barrett–Joyner–Halenda (BJH) method.
Results and discussion
Morphology observation
The SEM morphologies of the as-obtained TiO2 nanoparticles synthesized at different reaction temperatures are shown in Fig. 1. When the temperature was 160 °C, it is found the sample exhibits flower-like nanoclusters with big gaps (Fig. 1a), which are core-centered and radiate outward, and the diameter of each nanocluster is about 4–6 µm. The highly magnified image clearly displays that the samples are composed of numerous well-ordered nanorods with a diameter of about 20 nm (Fig. 1b). The nanorod with tetragonal crystal structure has the trend to aggregate and form a cluster. When the temperature increased to 170 °C (Fig. 1c, d), the intervals narrowed down. The compact architecture has a diameter of 5 µm and a uniform distribution. The highly magnified image demonstrates clearly that these nanorods contain bunch of aligned nanoparticles. Figure 1e shows the morphology of sample fabricated at 180 °C, the gap vanished and formed TiO2 microspheres with a diameter of 7.5 µm, and the highly magnified image shows that TiO2 microspheres are composed of uniform distribution nanorods containing bunch of aligned nanoparticles (Fig. 1f). As the temperature continued increasing to 190 °C (Fig. 1g), TiO2 microspheres with novel hollow urchin nanostructures with 1 mm diameter appeared. However, there was a sunken area at the outer surface of the ball, which promotes the expansion in the specific area and the number of active sites. The 3D dendritic hollow urchin structure may be formed due to selected surface etching of Ti species in strong acid medium at relatively higher temperature.
Figure 2a, d shows representative TEM images of S160 and S190, indicating the formation of hierarchical structured TiO2 composed of nanorods (Fig. 2b, e). High-resolution TEM (HRTEM) analysis was employed to determine the crystal facets. There is no lattice distortion, and all atoms are well aligned in the lattice of the sample S160. The lattice spacing of (110) atomic plane is around 0.32 nm, corresponding to rutile (110) facet. Correspondingly, the digital diffraction pattern (Fig. 2c, inset) displays clear diffraction spots instead of diffraction rings, suggesting a single-crystal-like nature within the whole nanostructure. Moreover, two sets of clear lattice fringe are present in sample S190, indicating their well crystalline structure.
Structural analysis
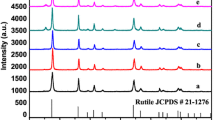
XRD patterns of the prepared samples demonstrate that the diffraction peaks of samples synthesized at 160, 170 and 180 °C are indexed to pure rutile phase. The main peaks located at approximately 27.5°, 36.1°, 41.3°, 44.1°, 54.4°, 56.7°, 62.8°, 64.1°, 69.1° are observed, which can be readily indexed as the (110), (101), (111), (211), (210), (220), (002), (310), (301) lattice planes of rutile. But when the reaction temperature is 190 °C, small amounts of brookite phase are observed, indicating the formation of biphasial rutile/brookite composites. It is speculated that the formation of rutile phase is partly related with the high acidity. When H+ concentration is higher, it can attack the atom bridge between Ti atoms, break the oxolation bonds and form new species. The new formed species are unstable and lead to structure rearrangement, forming the rutile phase with corner-shared bonding [34].
The crystalline size of the formed TiO2 was further calculated based on the strongest peak of the XRD patterns via the Scherrer equation (shown in Fig. 3):
where λ is the X-ray wavelength; β is the full width at the half maximum (FWHM) intensity of the diffraction peak; θ is the incident angle; and k is a constant equal to 0.89.
The crystalline dimensions of all the samples are presented in Table 1, which is in good agreement with the TEM observation. Meanwhile, we can calculate that the crystalline sizes of S160, S170 and S180 are similar, but that of S190 is bigger than that of others.
Crystallographic structure of the as-prepared samples judged by Raman spectroscopy shown in Fig. 4 displays that the Raman bands at 609.9, 445, 143.4 and 233.2 cm−1 are attributed to the A 1g , E g , B 1g , and a second-order phonon mode of rutile phase of TiO2 [35], respectively. No other bands are observed besides the characteristic bands of rutile TiO2 in the Raman spectrum of S160, S170 and S180. However, new Raman peaks at 323.9 and 366.4 cm−1 ascribed to B 1g and B 2g modes are observed (Fig. 2d), which is characteristic of brookite TiO2. The results mentioned are well consistent with the above XRD result.
Growth mechanism
In order to find out the role of HCl to the formation of TiO2 nanostructure, a series of contrast experiments were conducted. As seen in Fig S1 (referred to the supplement data), when HCl was changed to other kinds of acid, such as HNO3, H2SO4, H3PO4 and CH3COOH, distinct bulk structures were achieved even under the same amount of H+. Therefore, the formation of nanorods can be ascribed to the Cl− from HCl, which could affect the coordination structure of the growth unit. It is supposed that Cl− can be selectively adsorbed on the (110) facet of rutile TiO2 during TBOT hydrolysis, the growth in (110) direction is suppressed, and thus, the TiO2 particles grow along with the (001) direction [27]. Besides, XRD patterns of Fig S2 in the supplement data displayed that pure anatase or rutile/anatase mixed crystal structure emerged when the acid was HNO3, H2SO4 or H3PO4, indicating that the anion can affect not only the subunit, but also the crystal structure. However, rutile phase still can be obtained along with the increase in the concentration of the HCl (Fig S2e). Therefore, it can be deduced that the coexistence of high acidity and Cl− prefers to form pure rutile phase, as analyzed before. As for CH3COOH, faintly acid property leads to the poor crystallinity.
Meanwhile, the influence of the concentration of HCl on the morphology was also studied. When the ratio of HCl/H2O is about 1/2 (v/v), compact balls were formed at both 160 and 190 °C (Fig S3a and 3c), because the lower concentration of H+ cannot hinder the hydrolysis rate of TBOT, and the crystallization nucleus grew too fast to be controlled. Though the products are still composed of nanorods, they NRs stacked too compact to obtain a high specific surface area. When the ratio of HCl/H2O increased to 2/1 (v:v) (Fig S3b and 3d), the TiO2 nanorods aggregated together and distributed disorderly with no complex nanostructure. Above all, we can conclude that the concentration of HCl is a vital factor to obtain the varied nanostructures.
Furthermore, the mechanism for the formation of hierarchical flower-like TiO2 nanospheres with rutile phase can be described as follows (Fig. 5). The hydrolysis takes place at lower temperature than 160 °C. Before the temperature reaches 160 °C, the hydrolysis can be completed and structure already exists in similar morphology than that of the final form. Above 160 °C, larger crystallites were obtained due to the crystal growth or recrystallization. At a lower temperature, the amount of crystal nuclei is relatively scarce, leading to form little nanorods. With the temperature elevated, crystal growth effect become more obvious, the size of the subunits decreased and XRD results clearly reveal that the average crystallite size of subunits gradually increased. From 160 to 190 °C, the number of nanorods increases and they can fill up the gaps between clusters. Gradually, the hierarchical flower-like nanospheres are obtained.
BET analysis
The nitrogen adsorption–desorption analysis suggests the porous hierarchy of the TiO2 materials. All the samples show representative type-IIb(non-reversible type II) curves with H3 hysteresis loops (Fig. 6). These isotherms are characteristic of aggregated powders with slit-shaped pores, and similar isotherms with H3 hysteresis loop were obtained in studies previously [36, 37]. Samples of S160, S170, S180 show capillary condensation steps at a little lower relative pressure of 0.4–0.5, suggesting a narrow pore size distribution, which can be confirmed by the pore size distribution inset the isotherm curves. However, the capillary condensation of sample S190 postpones to a higher relative pressure of 0.7–0.8, distinctively different from the other three samples. The corresponding pore size distribution curve exhibits a wider pore size distribution, and it has two peak pore diameters of about 3 and 35 nm, while the pore size of other three samples is all around 2.6 nm. It is universally acknowledged that the type H3 hysteresis loop is associated with slit-like pores formed by the aggregations of the plate-like particles. Besides, the isotherms show high adsorption at high relative pressure (P/P 0) range (approaching 1.0), indicating the formation of large mesopores and macropores, as analyzed above.
The BET surface area, total pore volume and average pore size of the materials are listed in Table 2. It is found that all the samples have relatively low specific surface areas, calculated by the Brunauer–Emmett–Teller (BET) method. The existence of large mesopores of S190 gives rise to a higher surface area.
Photocatalytic activity
Photocatalytic performance of the hierarchical TiO2 synthesized at various temperatures was tested via the degradation of a complex dye solution containing 10 mg/l RhB and 10 mg/l MB under ultraviolet irradiation. As shown in Fig. 7, the photocatalytic activities of the catalysts showed stronger degradation ability for RhB than for MB. It was found that the S160 and S190 presented the highest photocatalytic activity. As to S160, nearly 35% RhB and 27% MB were degraded after degradation of 10 min. Furthermore, 90% RhB and 73% MB were absorbed after 60 min of irradiation. The S190 exhibited almost equal catalytic activity compared to the S160, but it showed a better ability to eliminate MB and the opposite performance to RhB than the S160, with 80% MB and 84% RhB absorbed within 60 min. It can be concluded that the photocatalytic performance follows neither the order of the crystallite size nor the order of specific surface area. The result lies in the synergistic effect of various coexisting factors, especially the high harvesting and good transferring properties of 3D hierarchical flower-like TiO2 nanostructure. The high photoactivity of S160 can be ascribed to the existence of exposed (110) facet [19]. Potentially, the rutile (110) facet offers the reduction sites. The excited holes are more likely to stay at Ti–O–Ti bridging sites rather than at the edge-shared oxygen under irradiation, which leads to the weakening of the Ti–O bond and the formation of oxygen defect. It is common sense that the rutile (001) facet involves only edge-shared oxygen atoms and both types of atoms are present in (110) plane. Moreover, titanium atoms on the rutile (110) facet prefer the adsorption of the dissolved oxygen molecules. Therefore, samples containing exposed (110) facet turn out to be more robust catalysts.
BET surface area plays a significant role when the reaction sites were (001) facet. The presence of the sunken hole, large mesopores and macropores increases the BET surface area of S190 and subsequently increases the number of active sites. However, the existence of brookite prevents further improvement of the efficiency. Therefore, S170 which also contained the exposed (110) facets performed much better than S180, to which almost 53% MB and 51% RhB still remained even after 60 min of irradiation, owning to the lower BET surface area.
Conclusion
In summary, hierarchical flower-like TiO2 nanospheres with rutile phase were synthesized successfully via a facile one-step and cost-effective hydrothermal method. The architectures were assembled from highly ordered nanorods and turned into 3D dendrites with the increase in reaction temperature, suggesting that the morphology of rutile TiO2 can be tuned conveniently. Besides, the results show that the high acidity prefers to form rutile phase, and the selective adsorption of Cl− affects the growth direction of the TiO2 nanoparticles. Moreover, the photocatalytic degradation of a complex dye solution containing RhB and MB revealed that S160 can work as efficient as S190 because of the exposed (110) facet though the BET surface area of S160 is much smaller. Above all, the complex structured TiO2 with rutile phase can be tailored easily and it is expected to be promising for the practical use in cleaning up the environment.
References
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Ohno T, Tsubota T, Nishijima K, Miyamoto Z (2004) Degradation of methylene blue on carbonate species-doped TiO2 photocatalysts under visible light. Chem Lett 33:750–751
Ohko Y, Ando I, Niwa C, Tatsuma T (2001) Degradation of bisphenol A in water by TiO2 photocatalyst. Environ Sci Technol 35:2365–2368
Montoya JF, Atitar MF, Bahnemann DW (2014) Comprehensive kinetic and mechanistic analysis of TiO2 photocatalytic reactions according to the direct–indirect model: (ii) experimental validation. J Phys Chem C 118:14276–14290
Zhu YX, Wang YF, Chen Z et al (2015) Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl Catal A Gen 498:159–166
Soltani RDC, Jorf S, Ramezani H, Purfadakari S (2016) Ultrasonically induced ZnO–biosilica nanocomposite for degradation of a textile dye in aqueous phase. Ultrason Sonochem 28:69–78
Liu JM, Zhang QC, Yang JC, Ma HY, Tade MO, Wang SB, Liu J (2014) Facile synthesis of carbon-doped mesoporous anatase TiO2 for the enhanced visible-light driven photocatalysis. Chem Commun 50:13971–13974
Hsieh SH, Chen WJ, Wu CT (2015) Pt-TiO2/graphene photocatalysts for degradation of AO7 dye under visible light. Appl Surf Sci 340:9–17
Chen XB, Liu L, Yu PY, Mao SS (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331:746–750
Varghese OK, Paulose M, Grimes CA (2009) Long vertically aligned titania nanotubes on transparent conducting oxide for highly efficient solar cells. Nat Nano Technol 4:592–597
Hamid MG, Saif MHQ, Mahmoud H, Joselito PL, Mohammad A, Idriss MB, Abdullah SA (2016) Laser induced photocurrent and photovoltage transient measurements of dye-sensitized solar cells based on TiO2 nanosheets and TiO2 nanoparticles. Electrochim Acta 212:992–997
Wang F, Ma ZZ, Ban PP, Xu XH (2017) N and S codoped rutile TiO2 nanorods for enhanced visible-light photocatalytic activity. Mater Lett 195:143–146
Yang QQ, Peng P, Xiang ZH (2017) Covalent organic polymer modified TiO2 nanosheets as highly efficient photocatalysts for hydrogen generation. Chem Eng Sci 162:33–40
Lee HU, Lee SC, Lee SM, Lee JW, Kim HJ, Lee J (2013) Improved photocatalytic and antibacterial activities of three-dimensional polycrystalline anatase TiO2 photocatalysts. Appl Catal A Gen 467:394–399
Xu F, Zhang XY, Wu Y, Wu DP, Gao ZY, Jiang K (2013) Facile synthesis of TiO2 hierarchical microspheres assembled by ultrathin nanosheets for dye-sensitized solar cells. J Alloys Compd 574:227–232
Bai HW, Liu ZY, Lee SS, Sun DD (2012) The effect of fabrication method of hierarchical 3D TiO2 nanorod spheres on photocatalytic pollutants degradation. Appl Catal A Gen 447:193–199
Tao YG, Xu YQ, Pan J, Gu H, Qin CY, Zhou P (2012) Glycine assisted synthesis of flower-like TiO2 hierarchical spheres and its application in photocatalysis. Mater Sci Eng B 177:1664–1671
Liao JY, Lei BX, Kuang DB, Su CY (2011) Tri-functional hierarchical TiO2 spheres consisting of anatase nanorods and nanoparticles for high efficiency dye-sensitized solar cells. Energy Environ Sci 4:4079–4085
Fang S, Jing S, Lian G (2011) Template-free synthesis of hierarchical TiO2 structures and their application in dye-sensitized solar cells. ACS Appl Mater Interfaces 3:2148–2153
Wang XL, He HL, Chen Y, Zhao JQ, Zhang XY (2012) Anatase TiO2 hollow microspheres with exposed 001 facets: facile synthesis and enhanced photocatalysis. Appl Surf Sci 258:5863–5868
Dong YS, Fei XN, Liu ZF, Zhou YZ, Cao LY (2017) Synthesis and photocatalytic redox properties of anatase TiO2 single crystals. Appl Surf Sci 394:386–393
Yadav Hemraj M, Jung-Sik Kim (2016) Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J Alloys Compd 688:123–129
Kobayashi M, Petrykin V, Kakihana M (2009) Hydrothermal synthesis and photocatalytic activity of whisker-like rutile-type titanium dioxide. J Am Ceram Soc 92:S21–S26
Tao T, Chen Y (2013) Direct synthesis of rutile TiO2 nanorods with improved electrochemical lithium ion storage properties. Mater Lett 98:112–115
Truong QD, Hoa HT, Le TS (2017) Rutile TiO2 nanocrystals with exposed 331 facets for enhanced photocatalytic CO2 reduction activity. J Colloid Interface Sci 504:223–229
Han B, Kim SJ, Hwang BM, Kim SB, Park KW (2013) Single-crystalline rutile TiO2 nanowires for improved lithium ion intercalation properties. J Power Sources 222:225–229
Sun B, Zhou GW, Zhang Y, Liu RR, Li TD (2015) Photocatalytic properties of exposed crystal surface-controlled rutile TiO2 nanorod assembled microspheres. Chem Eng J 264:125–133
Yurdakal S, Palmisano G, Loddo V, Augugliaro V, Palmisano L (2008) Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J Am Chem Soc 130:1568–1569
Ohno T, Tsubota T, Toyofuku M, Inaba R (2004) Photocatalytic activity of a TiO2 photocatalyst doped with C4+ and S4+ ions having a rutile phase under visible light. Catal Lett 98:255–258
Lin J, Yu JC, Lo D, Lam SK (1999) Photocatalytic activity of rutile Ti1−x Sn x O2 solid solutions. J Catal 183:368–372
Ramanathan R, Bansal V (2015) Ionic liquid mediated synthesis of nitrogen, carbon and fluorine-codoped rutile TiO2 nanorods for improved UV and visible light photocatalysis. RSC Adv 5:1424–1429
Yan C, Feng DJ, Jiang YJ, An XY, Ye LJ, Guan WS, Bai B (2015) Bio-template route for the facile fabrication of TiO2@bacillus subtilis composite particles and their application for the degradation of rhodamine B. Catal Lett 145:1301–1306
Du SN, Liao ZJ, Qin ZL, Zuo F, Li XH (2015) Polydopamine microparticles as redox mediators for catalytic reduction of methylene blue and rhodamine B. Catal Commun 72:86–90
Yang J, Mei S, Ferreira JMF (2000) Hydrothermal synthesis of nanosized titania powders: influence of peptization and peptizing agents on the crystalline phases and phase transitions. J Am Ceram Soc 83:1361–1368
Cheng HM, Ma JM, Zhao ZG, Qi LM (1995) Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem Mater 7:663–671
Kőrösi L, Prato M, Scarpellini A, Kovács J, Dömötör D, Kovács T, Papp S (2016) H2O2-assisted photocatalysis on flower-like rutile TiO2 nanostructures: rapid dye degradation and inactivation of bacteria. Appl Surf Sci 365:171–179
Kőrösi L, Papp S, Oszkó A, Dékány I (2012) Low-temperature sintering behavior of nanocrystalline indium tin oxide prepared from polymer-containing sols. Mater Res Bull 47:933–940
Acknowledgements
This work was financially supported by the Shanghai Rising-Star Program (B-type) (15QB1402300), the Minhang District Leading Talent Project (No. 201541), the Shanghai Talent Development Project (No. 201531), Science and technology project Supported by Minhang Government Funds (2016MH195), Natural of science Foundation of Shanghai (17ZR1420000).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, Y., Xu, X., Lin, L. et al. 3D hierarchical flower-like rutile TiO2 nanospheres-based versatile photocatalyst. J Mater Sci 53, 385–395 (2018). https://doi.org/10.1007/s10853-017-1498-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1498-3