Abstract
The complex microstructures developed during post-welding heat-treatment in the vicinity of the fusion line between a ferritic and austenitic steel were examined in the case of submerged arc welded 18MND5/309L dissimilar joints. Quantitative measurements of the carbon distribution in the as-welded and post-weld heat-treated conditions were performed by both wavelength dispersive spectrometry and secondary ion mass spectrometry. The extent of carbon diffusion was confirmed by hardness profiles performed by nanoindentation. On the low-alloy ferritic side, decarburization resulted in cementite dissolution allowing the evolution of the bainitic structure toward a large-grained ferritic region. In the weld metal, the carbon content reached unusually high levels and an intense precipitation of chromium-rich carbides was observed in both the interfacial martensitic layer and the austenitic weld metal. The evolution of the precipitation as a function of the distance from the interface was analyzed in terms of crystallography, chemistry, volume fractions, and size distributions. Automated crystal orientation mapping in a transmission electron microscope allowed identification of the precipitates extracted on carbon replicas from both the martensitic and austenitic matrices. A 3D reconstruction of the carbides population in the martensitic layer was performed by serial cutting with a focused ion beam: M 7 C 3 and M 23 C 6 were found to coexist in the two carburized regions, but displayed different sizes, compositions, and morphologies, depending on their location with respect to the fusion line. This evolution in terms of precipitation was analyzed taking into account the local microstructure and composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dissimilar metal welds (DMW) provide a paradigm for coupled long-range diffusion and local precipitation occurring during post-weld heat-treatment (PWHT) in a chemically heterogeneous material. In addition to their industrial importance, they provide an interesting challenge to understand microstructure evolution kinetics. The most widely encountered DMWs are those between two ferritic steels with different chromium contents [1, 2] and those between a low-alloy ferritic steel and an austenitic stainless steel [3, 4]. Even in the case of similar carbon content in both steels, carbon is found to diffuse from the low-alloyed side to the high-alloyed side. A case of uphill diffusion is even reported [5] as in the well-known Darken’s experiment [6]. In all cases the driving force for carbon diffusion is the carbon activity gradient between both steels, which cannot be described by carbon concentration only. This diffusion often results in significant microstructural changes around the fusion line: carbides dissolve on the low-alloy side and create a soft decarburized zone, whereas the high-alloy part is hardened by carburization and carbides precipitation. Several characterization tools can be combined to investigate carbon migration and the resulting local microstructural transformations. Optical microscopy (OM) and scanning electron microscopy (SEM) along with microhardness allow the characterization of the size of the affected regions for different alloys combinations [7, 8]. Electron probe microanalysis (EPMA) and SIMS provide the carbon profile across the fusion line [9–11], and TEM coupled with energy dispersive spectrometry (EDS) gives access to the precipitation sequence based on crystallography and chemistry of the carbides [1, 12]. Chromium and molybdenum-rich carbides are generally found to precipitate on the high-alloyed side. The microstructures on both sides of the interface highly depend on the type of steels and the tempering temperature. They greatly influence the amount of carbon redistribution through the interface due to the higher diffusivity of carbon into ferritic matrix than in austenite. In the case of ferritic-austenitic welds at the temperature of PWHT, the carbon-depleted zone always spans a greater extent than the carbon-enriched part [5, 13]. When low-alloy steel is welded using nickel-base filler metals, a reduction of the size of both the carburized and decarburized regions is observed [7, 14]. This is due to the reduced diffusivity of carbon in the Ni matrix.
As carbon is a crucial element in steel metallurgy and its distribution into an alloy has important consequences on the mechanical properties, significant efforts have been devoted to the challenge of its quantification in the recent years. Even if they remain delicate (especially because of the risk of contamination) several methods such as electron energy loss spectroscopy in a TEM [15, 16], SIMS nano-analysis [17], field emission electron probe microanalysis [18, 19], and atom probe tomography (APT) [20, 21] have been successful in measuring carbon at a local scale (in matrix phases as well as in precipitates). In the case of perfectly flat interfaces as in coatings and diffusion bondings, quantitative carbon profiles are often obtained by glow discharge optical spectroscopy (GDOS) [22, 23]. Except for APT, carbon quantification is based on the use of standards with controlled microstructure and known carbon content. Concerning simulations, several attempts were made to reproduce the carbon profile across the weld, the most relevant being the ones based on the CALPHAD approach [1, 5, 9, 24, 25]. Long-range diffusion in solid solutions under a gradient of chemical potential was coupled with local equilibrium at each space node to predict the local evolution of the volume fraction of precipitates.
In the case of ferritic/austenitic steel fusion welds a layer of martensite characterized by a high hardness and a lathy microstructure is often reported to form at the interface [26, 27]. This martensitic layer is due to low Cr and Ni contents near the fusion boundary in the weld pool and the transformation of this filler-deficient zone into martensite during the subsequent cooling of the weld. Concerning the grain structure, two types of grain boundaries (GB) are frequently reported in dissimilar steel welds [28]: Type-I boundaries separate neighboring solidification grains and are perpendicular to the fusion line, whereas Type-II are parallel to the fusion line and may result from a change in primary solidification mode [29].
The goal of this study is to investigate carbon diffusion occurring during PWHT of welds in the case of welds produced by submerged arc welding and exhibiting a specific three-phase configuration: low-alloyed ferritic base metal/martensitic layer/high-alloyed austenitic weld metal. In terms of initial composition, carbon chemical potential and carbon diffusivity at 610 °C, martensite (considered here as a body-centered cubic—BCC matrix enriched in Cr and Ni, as measured by wavelength dispersive spectrometry (WDS)) displays intermediate properties between those of the base metal and the stainless steel weld, as shown in Fig. 1. As suggested by the chemical potential plot (Fig. 1b) carbon is expected to diffuse from the ferritic base metal into the martensite and austenite of the weld metal. Hence, the objectives of this work were (1) to accurately measure the carbon content on both sides of the fusion line in the as-welded and heat-treated conditions; (2) to quantitatively investigate the microstructural changes occurring in the same region, and especially to characterize the precipitates in terms of crystallography, chemistry, morphology, volume fraction, and size distribution; and (3) to understand how the presence of a martensitic layer influences the extent of carbon diffusion.
Initial state of the weld (as-welded condition) in terms of a Cr and Ni contents measured by WDS and C content estimated by dilution. b Chemical potential of carbon at 610 °C calculated by MatCalc [40] with a local equilibrium hypothesis and the mc-fe.tdb thermodynamic database. c Carbon diffusion coefficient at 610 °C calculated by MatCalc [40] and the mc-fe.ddb kinetic database
Materials and experimental procedures
Base materials, welding process, and heat-treatment
The dissimilar steel welds were obtained by cladding an 18MND5 low-alloy steel plate with two layers of stainless steel filler metals: successively 309L and 308L. The chemical compositions of the alloys of interest are listed in Table 1: Cr, Fe, Mn, Mo, Ni, and Si contents were determined by X-ray fluorescence (XRF) on Thermo-ARL9800, whereas combustion on LECO CS230 was used for carbon. The welding parameters used for each layer are summarized in Table 2. In comparison to 308L, 309L stainless steel is enriched in Cr and Ni in order to compensate dilution and provide two layers of equivalent compositions after welding. In fact, part of the base metal is melted during the deposition of the first layer and it is mixed with 309L in the weld pool reducing the final content in allowing elements, particularly Cr and Ni. For this study, we made use of an 18MND5 plate with dimensions 1200 × 630 × 90 mm3. The strip filler metal (in 309L or 308L) was 60 mm wide and 0.5 mm thick.
Due to solid-state phase transformations in the base metal and differences in the thermal expansion coefficients of the joined materials, residual stresses appeared upon cooling on both sides of the fusion line. To reduce them and temper the microstructure, the following heat-treatment was performed: heating from room temperature up to 610 °C with a rate of 30 °C/h; holding at this temperature for 8 h; then cooling down to 300 °C with a rate of 25 °C/h and finally air cooling to room temperature.
Carbon content measurements
As surface contamination is the major problem in carbon concentration measurements, a particular attention was paid to the samples preparation: they were mechanically polished just before their introduction into the analysis chamber using alumina suspension for the final stage. WDS measurements were performed with a Ni/C multilayer monochromator (2d = 9.5 nm) on a CAMECA SX50 electron microprobe equipped with the SAMx software. The following conditions were used: accelerating voltage of 16 kV, counting time of 40 s, and beam current of 900 nA which allowed sufficient counting statistics in the case of low carbon levels (<0.02 wt%) and one measurement every 4 µm without any contamination. A liquid nitrogen cooling trap together with a low-pressure oxygen jet onto the specimen allowed reducing the carbon contamination from the cracking of residual organic molecules by the electron beam. Quantitative analysis required the use of the calibration curve method described in ISO standard 16592 [30] which postulates a linear relationship between the intensity of the C Kα peak and the carbon content in the probed volume (in wt%). The samples used as references were a piece of electrolytic iron and various Fe–C solid solutions obtained by quenching from the high temperature austenitic domain [31] with carbon contents varying from 6.5 × 10−4 (measured by combustion) to 0.99 wt%. An example of calibration curve is provided in Fig. 2a.
Secondary ion mass spectrometry (SIMS) measurements were carried out on the same samples. This technique has the advantage to be less sensitive to surface contamination since the samples can be cleaned (sputter cleaning) inside the chamber before analysis. SIMS experiments were carried out on a modified CAMECA IMS-6f instrument using a Cs + primary bombardment (impact energy of 5.5 keV) to enhance C − emission. The pressure in the chamber was around 10−8 mbar during the whole experiment. In the image mode, the focused Cs + primary ion beam (diameter smaller than 1 µm, intensity of 200 pA) was scanned across an area of (400 × 400) µm2 to acquire images with a size of 256 × 256 pixels. The mass resolution ∆M/M was 300 and the time necessary to acquire one image around 100 s. This approach was only qualitative and allowed obtaining images of the carbon distribution across the weld. For quantitative measurements of carbon, in-depth profiles were performed. The focused Cs + beam (current of 7 nA) was scanned across a zone of (50 × 50) µm2 and the C − secondary ions were collected from a circular area (diameter of 8 µm) with one measurement every 40 µm. With the same reference samples as those used for WDS, a calibration line was established (Fig. 2b) that allowed quantitative measurements of the carbon concentration across the weld interface.
Nanoindentation
Nanoindentation was chosen as an indirect method to probe the local carbon content in the different regions around the interface. The continuous stiffness method [28, 29] (superimposed oscillation of 2 nm at 45 Hz) allows monitoring the elastic modulus and the hardness as a function of the indentation contact depth. In fact, carbon enrichment leads to an increase of the local hardness, whereas carbon depletion is usually accompanied by a local softening. Nanoindentation experiments were performed using the MTS XP machine equipped with a Berkovich three-sided diamond pyramid as indenter. Profiles were performed on transverse sections of the weld with a minimum distance between two consecutive locations of 4 µm (indentation depth of 200 nm). For all measurements, a steady state corresponding to a hardness plateau was reached after 150 nm of indenter penetration. Local hardness was taken as the mean value between 180 and 190 nm.
Precipitates identification
Specimens for TEM observations were first ion-etched with a Gatan PECS (Precision Etching and Coating System) [32] and then sputter-coated with a 25-nm-thick layer of carbon in the same apparatus. The carbon film was scored with a scalpel into 1 mm squares located at the region of interest and the sample was immersed for 1 h in a solution of 5 % bromine in methyl alcohol. This led to the dissolution of the matrix (ferrite, tempered martensiticFootnote 1 or austenite) and allowed extracting the precipitates embedded in the carbon films floating on the solution. The replicas were deposited on TEM mesh grids and examined on a FEI Tecnai F20 TEM operating at 200 kV and equipped with an orientation-phase mapping unit NANOMEGAS (ASTAR model) [33, 34, 35]. Selected areas were scanned by the electron beam with a step size of 4 nm. For each pixel, the diffraction pattern was recorded with an acquisition speed of 34 frames per second. A 10 µm C2 (condenser diaphragm) aperture and a camera length of 100 mm were used. Phase identification was then performed off-line by a template matching algorithm [36].
Carbides quantification
The size (3D), morphology, and distribution of the precipitates inside the martensitic layer of the post-weld heat-treated sample have been investigated by focused ion beam (FIB) tomography. By means of a SEM–FIB Zeiss NVISION40 Crossbeam® with a 54° angle between the electron and ion beams, slices were cut every 10 nm with a Ga + beam current of 700 pA and an accelerating voltage of 30 kV. A layer of carbon with a thickness of 1.5 µm was deposited on the top of the volume of interest to reduce curtaining during milling due to microstructural heterogeneities. The sample was tilted at 54° in order to mill a perfect planar surface. A secondary electrons in-lens detector operated under a tension of 5 kV was used for imaging at a working distance of 5.1 mm. The image field of 20 × 15 µm2 with a resolution of 2048 × 1536 pixels combined with the 10 nm step in depth allowed a voxel size of 10 nm3. 3D reconstruction and visualization was performed by means of Fiji® and Avizo Fire 6.2® softwares. With the conditions chosen for imaging, the carbides and the martensitic laths appeared in dark and bright gray, respectively (Fig. 13). When performing statistics on the carbides population, all particles with a volume smaller than 8000 nm3 were considered as noise and eliminated. In the area where the serial sectioning was performed, the martensitic layer had a thickness of 35 µm and five stacks of about 1 µm in thickness (100 images) were analyzed at different locations in order to capture the changes affecting the carbides population. This method could not be applied to the carbides in the austenitic matrix as their size is too small (R < 20 nm): for this population, the analysis was limited to bright field (BF) and dark field (DF) images acquired on a Jeol 3010-LaB 6 transmission electron microscope on thin foils prepared by FIB in planes either parallel or perpendicular to the fusion line.
Results and discussion
Carbon profile across the fusion line
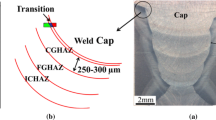
As can be seen on Fig. 3a, the initial microstructure of the weld can be divided into three zones: the base metal 18MND5 (French grade equivalent to A533 gr. B) with a bainitic microstructure (ferrite laths and Fe 3 C carbides), then an intermediate martensitic layer whose width varies between 5 and 70 µm depending on the location along the fusion line, and finally the austenitic weld metal. In contact with the martensite and on the weld metal side, the microstructure is fully austenitic, residual δ-ferrite appearing only after approximately 100 µm from the fusion line. When subjected to the heat-treatment at 610 °C this microstructure is dramatically modified as a result of carbon diffusion from the base metal toward the weld metal. As shown on Fig. 3b, decarburization of the base metal leads to the formation of a zone with large ferrite grains which spans over 200 µm. These grains have a rounded shape typical of abnormal grain growth which can be explained by the simultaneous carbides dissolution and GB migration. On the weld side, carbon diffuses to both the interfacial martensitic layer (that encounters tempering during holding at 610 °C) and the purely austenitic zone (dark layers of Fig. 3b). The dark aspect of the carburized regions results from carbides precipitation.
Whatever the width of the martensitic band, the carburization into austenite spreads over about 60 µm.
Quantitative measurements of the evolution of the carbon content were performed by both WDS and SIMS. A very good agreement between the results of these two techniques was obtained for the post-weld heat-treated sample (Fig. 4b). Starting from the fusion line toward the base metal, a zone almost entirely depleted in carbon (w(C) < 0.015 wt%) was characterized over a distance of 200 µm corresponding to the region where large ferrite grains are observed. Then the carbon content progressively increases to reach the nominal carbon content of the low-alloy steel at about 500 µm from the fusion line (w(C) = 0.20 wt%). Between 200 and 500 µm from the fusion line on the base metal side, there exists a region with intermediate carbon levels. Due to reduced carbon content in this region, Fe3C carbides have begun to dissolve and the bainitic laths to coalesce. The progressive evolution of the microstructure within 18MND5 heat-affected zone is visible on the SEM micrographs of Fig. 5 taken at different distances from the fusion line. On the weld metal side, two maxima of the C content are detected: one into the martensite with a maximum carbon content of 1.3 wt% and another one into the austenite with a maximum C level of 0.7 wt%. The carburized zone width in the austenite is around 60 µm. Both carbon measurements agree well with the extent of the phase transformations observed in the heat-treated microstructure (see Fig. 3b). Images of the C − intensity acquired by SIMS on a post-weld heat-treated sample are displayed on Fig. 6. Although not quantitative they allow mapping the distribution of carbon through the weld and confirm the extent of both carburized and decarburized regions. The two techniques used here appear to be complementary. WDS allows a better spatial resolution that is particularly appropriate to capture variations occurring over a limited space scale, as it is the case in the as-welded condition and for the carbon enrichment of martensite and austenite in the heat-treated sample. The size of the probed volume (<1 µmFootnote 2)3 is also responsible for the small singularities detected in the base metal as it is sensitive to cementite precipitates (around 200 nm in size). WDS also has the advantage of allowing a simultaneous quantification of the substitutional elements, which is not the case in SIMS where Cr and Fe are better measured as positive ions. However, SIMS is able to detect very low carbon contents (such as 0.004 wt% in the present case) thanks to its high sensitivity and strong limitation of surface contamination. Since the quantitative approach with SIMS could only be applied in the in-depth profiling mode with steps of 40 µm, this method appeared to be well suited for variations occurring over larger distances, as it is the case for the decarburization of the base metal in the heat-treated sample. The resulting profile in this zone is quite smooth because the area of analysis (8 µm in diameter) allows measuring a global composition and is not influenced by the two-phase microstructure (ferrite + carbides) of the base metal.
Carbon intensity maps obtained by SIMS on the heat-treated state, white areas being carbon-rich and black ones carbon-poor. The dendrites on the weld metal side appear in white as carbides form by δ-ferrite decomposition during the heat-treatment. The sputter cleaning leads to blurred images mainly on the 18MND5 steel side
For the as-welded sample (Fig. 4b), the agreement between SIMS and WDS is not as good as that for the post-weld heat-treated sample. The variations of the carbon content are expected to happen only over short distances, which makes the quantification by SIMS profiling less relevant with the analytical conditions used (average C content measured on a surface of 8 µm in diameter). The WDS profile revealed that some carbon diffusion already occurs during the welding of the two stainless steel layers (309L over 18MND5 and then 308L above) since the carbon levels observed in the weld are higher than the ones expected by simple dilution. Two small peaks (w(C) < 0.3 wt%) in austenite and martensite together with a decrease of the C content over 200 µm in the 18MND5 steel are visible.
As a complement to carbon measurements, nanoindentation profiles were performed with a spatial resolution similar to the one achieved by WDS. From a macroscopic point of view, a decrease in hardness is observed after heat-treatment on the low-alloy side due to the tempering of the bainitic microstructure and the carbon depletion within the first 500 µm of the heat-affected zone close to the fusion line (Fig. 7). As mentioned previously in the case of carbon quantification by WDS, the size of the nanoindentation probe results in some dispersion on the low-alloyed side (between 2.4 and 3.5 GPa in the heat-treated 18MND5 steel) caused by the heterogeneity of the microstructure. The reduction in hardness in the decarburized region with large ferrite grains is limited but the scatter is highly reduced (variations between 2.37 and 2.70 GPa) as expected for an homogeneous microstructure. Concerning the martensitic layer at the fusion line, its hardness shows almost no significant evolution after heat-treatment, the carbon enrichment being probably counterbalanced by the tempering of the microstructure. However, the local strength of the purely austenitic zone is deeply modified and increases over 60 µm with a maximum around 8 GPa at the interface with the martensite. This huge rise of the local hardness can be related to carbon intake, either in supersaturation into the FCC matrix or as a dispersion of small precipitates.
It is also worth noticing that diffusion during the 8 h at 610 °C is limited to carbon: all the substitutional elements were found not to diffuse over significant distances. The profiles across the weld after PWHT were identical to those after welding that are displayed in Fig. 1a. This could have been anticipated by looking at the diffusion coefficients of Cr and Ni at 610 °C (see Table 3). They are several orders of magnitude lower than the one of carbon in both BCC and FCC matrices.
Nature and composition of precipitates
In the tempered martensitic layer
FIB thin foils extracted perpendicular to the fusion line from both the as-welded and post-weld heat-treated samples revealed almost no precipitation in the as-welded condition, whereas an intense precipitation of large carbides (size up to 300 nm—Fig. 8a) was observed after heat-treatment.
Two types of carbides were identified after 8 h at 610 °C: M 23 C 6 and M 7 C 3 (M stands for Cr, Fe, Mn) which are typically found in Cr-rich steels [37]. M 23 C 6 carbides were detected as a cubic structure (Fm-3m space group) with a cell parameter of 10.68 Å whereas M 7 C 3 were identified with an hexagonal cell (P63mc space group) with a and c parameters equal to 14.01 and 4.53 Å respectively [38]. Long streaks along the ≪110≫ and ≪1\( \bar{1} \)0≫ directions on the selected area electron diffraction (SAED) patterns from the M 7 C 3 carbides were frequently observed. As mentioned in the literature [39], these anisotropic spots are due to stacking faults in planes perpendicular to the basal plane.
The systematic crystallographic mapping performed with Astar system on carbon extraction replicas allowed capturing the evolution in nature of the carbides throughout the martensitic layer: mainly M 7 C 3 were found next to the low-alloy side (Fig. 9a), whereas both M 7 C 3 and M 23 C 6 were present at the extremity adjacent to the austenitic region (Fig. 9b). This change in the carbides population can be related to the evolution of the carbon content from 1.2 to 0.7 wt% (Fig. 4). Indeed a decrease in the carbon concentration destabilizes the M 7 C 3 precipitates (high C/M ratio) leading to the formation of M 23 C 6 (lower C/M ratio). It is shown on the plot of Fig. 10a from the volume fractions of precipitates in the martensite (BCC matrix) calculated by minimization of the total Gibbs free energy (local equilibrium approach) with the MatCalc software [40] that both Cr-rich carbides can coexist within a carbon content range from 0.68 to 1.35 wt%.
Carbides identification in the martensitic layer, M7C3 shown in red, M23C6 in blue. a Next to the interface with the base metal, b next to the interface with the austenitic weld metal. c Optical micrograph of the heat-treated interface with the localization of observations a and b (Color figure online)
Precipitates volume fractions at 610 °C calculated as a function of the global carbon content (MatCalc software [40], mc-fe.tdb database [41]) a For the tempered martensitic layer: w(Cr) = 11 %; w(Ni) = 6 %; w(Mn) = 1.5 %; w(Si) = 0.5 %; w(Mo) = 0.3 %, b for the austenitic weld metal: w(Cr) = 18 %; w(Ni) = 9 %; w(Mn) = 1.2 %; w(Si) = 0.8 %; w(Mo) = 0.2 %
Carbon extraction replicas allowed measuring the content in substitutional elements by EDX in a TEM without the contribution of the X-ray signal from the matrix. The results which are averages over 20 precipitates in the same zone are displayed in Table 4 and show a significant incorporation of iron into the carbides. This can be explained by the limited chromium content (less than 11 wt% in martensite) together with its low diffusion coefficient at 610 °C (D CrCr = 1.6 × 10−19m2/s in the BCC matrixFootnote 3.). However, neither M3C nor M2.4C carbides were found, even in the region close to the base metal where the Cr content is the lowest. The minimum Cr content in this region was measured to be around 1 wt% which seemed already sufficient to favor the formation of Cr-rich precipitates.
In the fully austenitic region
Numerous carbides were extracted from the first micrometers (less than 10 µm) of the austenite adjacent to the martensitic layer and identified by ACOM as shown in Fig. 11: only small M 23 C 6 carbides (less than 50 nm in size) were observed within the grains but large carbides identified either as M 23 C 6 or M 7 C 3 were found aligned along the GB. Both Type-I (perpendicular to the fusion line) and Type-II (parallel to the fusion line) GB were decorated by coarse carbides. The nature, size, and orientation of carbides were not affected by the type of grain boundary. Referring to the plot of Fig. 10, a carbon content higher than 1.1 wt% is necessary for the formation of M 7 C 3 in this zone. The size and nature of these GB particles underline the role played by the GB as shortcuts for carbon diffusion. However, the effect of the GBs on the global carbon diffusion in the austenite is limited in the present case as the grains are large and elongated (100 µm wide). In fact, enhanced carbon diffusion from the GBs to the grain interior and associated precipitation spans over less than 3 µm as can be seen on SEM image of Fig. 12. The fact that some large carbides on the GBs are partly indexed as M 23 C 6 and partly as M 7 C 3 seems to indicate that there could be a direct transformation from one carbide (with the lower C/M ratio) to the other (with a higher C/M ratio) when the local carbon content increases by long-range diffusion. As can be seen in Table 4, carbides in austenite incorporate significant quantities of iron, nickel, and manganese in their structure.
Evolution of carbides population around the interface
In the martensitic layer
Serial sectioning with FIB was applied inside martensite in the post-weld heat-treated sample as it allows 3D reconstruction over large areas with sufficient magnification to image medium-size particles (voxel size of 10 nm3). Larger particles are mainly found on the lath boundaries, whereas small carbides are rather visible in their interior (Fig. 13a). This preferred location is in agreement with both heterogeneous nucleation which is energetically favored by suppression of disordered regions and easy growth by enhanced diffusion of elements along the subgrain boundaries. This leads to fairly elongated shape for the carbides that are better represented as cylinders and plates than spheres (see Fig. 13b).
Going from the fusion line across the martensitic layer (35 µm wide in this case), one observes an evolution in the carbides population: the mean size of the precipitates decreases (Fig. 14a, b), whereas their volume fraction and their density increase (Fig. 14c, d). At first glance, the quasi-constant volume fraction of carbides for the three last series (from 16 to 28 µm) might be surprising as it is associated with a continuous decrease of the global carbon content. Nevertheless, it should be noticed that the nature of the precipitates also changes from M 7 C 3 to M 23 C 6. For each slice (corresponding to different locations inside the martensitic band), we divided the particles into three classes according to their size: the percentage of large particles (radius more than 50 nm) decreases with the distance from the base metal, whereas the small carbides (radius less than 25 nm) become more and more numerous (see Fig. 15). This evolution of precipitates through the martensitic layer is quite unusual. In the case of the nitriding of stainless steel, large precipitates are found in the interior of the sample, whereas a high density of small particles is present at the surface [42]. In the present case, the decrease of the precipitates size within the martensitic layer might be related to the driving force for precipitates nucleation which increases when the Cr content increases.
Characteristic parameters of the carbides population in the tempered martensitic band as a function of the distance from the fusion line, as obtained from FIB tomography. a Volume fraction. b Number of particles. c Mean dimension with the assumption of cubic precipitates (volume V = L 3). d Mean radius with the assumption of spherical precipitates (\( V = \frac{4}{3}\Pi R^{3} \)). e Localization of the stacks inside the martensitic layer
In the fully austenitic region
As the carbon replicas systematically broke at short distances from the martensite, certainly due to the high quantity of particles accumulated in this zone, the carbides extraction had only been efficient in the first micrometers of austenite (<10 µm) next to the martensitic layer. Thus, FIB thin foils removed both perpendicular and parallel to the fusion line were employed to investigate the evolution of the carbides population in the austenite. In agreement with the observations on replicas (see Fig. 11), numerous M 23 C 6 carbides with a size of about 60 nm were found in the near surroundings of the martensite/austenite interface on the FCC side. Cube-on-cube orientation relationship between these carbides and the austenitic matrix was observed: ≪001≫ M23C6 // ≪001≫ FCC (see indexation of the diffraction patterns in Fig. 15c and i). As can be seen on Fig. 15h some of the particles display a cubic shape and seem to be aligned along preferred directions.
At intermediate distances from the fusion line (see Fig. 16e, at 15 µm from the fusion line), two distinct populations are visible. Large cubic carbides seem concentrated along some characteristic features that could be dislocations, and in between what looks like dislocations cells decorated by cubic precipitates (diameter of the cells around 1 µm), a large amount of very small clusters can be seen (radius less than 5 nm). The large precipitates are not coherent with the matrix (see indexation of the diffraction pattern in Fig. 16f), which is in agreement with a nucleation on defects and a larger size. The small ones are numerous and may result from homogeneous nucleation. Either there exists a high driving force for nucleation due to a large carbon intake into the austenitic matrix during the temperature plateau at 610 °C or they precipitate during the subsequent cooling (which lasts several hours between 610 °C and room temperature) with quasi no diffusion and so a limited growth of the particles.
M23C6 carbides in carburized austenite. a Diffraction pattern taken at 5 µm from the interface with martensite. b DF image resulting from the area selection displayed on a. c Indexation of the diffraction pattern ([102] zone axis) with open circles for the dots from the matrix (m) and filled circles for the ones from the precipitates (p). d Diffraction pattern taken at 15 µm from the interface with martensite. e DF image resulting from the area selection displayed on d showing a second population of very fine precipitates. f Indexation of the diffraction pattern ([215] zone axis for the matrix and [213] for the precipitates). g Diffraction pattern taken at 35 µm from the interface with martensite. h DF image resulting from the area selection displayed on e showing the alignment of the precipitates. i Indexation of the diffraction pattern ([214] zone axis)
Further away from the fusion line (see Fig. 16h, at 35 µm from the fusion line), the carbides systematically have a cubic morphology and are aligned along particular crystallographic directions. This can be compared to the growth of γ′ precipitates in Ni–Al alloys. When precipitates nucleate coherently with the matrix phase, differences in lattice parameters create elastic distortions in their neighborhood. Sphere to cube shape transition together with regular spatial arrangement along the least dense directions of the FCC matrix ((100) less rigid than (110) and (111)) have been shown to reduce these coherency stresses [40, 41]. Hence, it corresponds to an energetically favored configuration. Moreover, such a high density of carbides combined with their regular spatial organization could be responsible for a significant strengthening of the austenitic matrix (visible on Fig. 7).
Summary and conclusions
The following main conclusions can be drawn based on the results obtained from this study:
-
1.
From the point of view of the experimental methods, the combination of WDS, SIMS, and nanoindentation has allowed measuring the evolution of the carbon distribution through the ferritic/martensitic/austenitic regions near the fusion line of a dissimilar steel weld. The same reference samples were used for both SIMS and WDS, with the quantitative results being in good agreement, especially for the post-weld heat-treated sample. The development of a quantitative method for carbon measurements with SIMS has allowed detecting contents as low as 0.004 wt% in the decarburized layer. WDS and nanoindentation were well adapted to capture fine and very local variations, whereas SIMS was preferred for quantifying compositional changes occurring over larger scales. Concerning precipitation, ACOM revealed to be a powerful tool to systematically identify a large number of carbides based on their crystallography. Serial cutting with FIB accompanied with 3D reconstruction was efficient to follow the evolution of the main particle features in the case of the medium-size precipitates present in the martensite.
-
2.
Carbon diffusion from the low-alloy to the Cr-rich side was found to create large microstructural heterogeneities over short distances. The decarburization of the base metal resulted in the formation of a narrow soft region made of large ferrite grains in contact with a hardened region (martensite + carburized austenite). The evolution of the local hardness in the surroundings of the interface gave a first insight into the local mechanical heterogeneity that exists in such welds. The decarburization of the base metal led to the formation of a 200 µm-wide region that is the softer than all the other parts of the assembly. During mechanical loading of the assembly, localization of plastic deformation and a state of triaxial stress are expected to occur in this confined and soft layer surrounded by hard regions. However, the complete dissolution of cementite carbides during PWHT leads to the presence of few nucleation sites for ductile failure to initiate and thus limited damage development.
-
3.
The interfacial martensitic layer present between the low-alloy steel and the austenitic stainless steel was found to behave as a sink for carbon, in accordance with the huge carbon chemical potential difference that exists initially with the 18MND5 base metal. During the heat-treatment, a significant quantity of carbon passes through this intermediate zone. Even if most of it precipitates (mainly with Cr atoms) inside the martensitic layer, a non-negligible part probably remains in solid solution and is available to diffuse toward the austenite driven by the high chemical potential gradient across the interface. This dual behavior can be related to the value of the diffusion coefficient for carbon in this zone: not only it is higher in BCC matrix than in FCC (Note that D CC (400 °C, BCC) ≈ 25 × D CC (610 °C, FCC)), but it can also be enhanced along lattice defects such as subgrain boundaries and dislocations.
-
4.
After the PWHT, the carbon concentration in the purely austenitic zone has reached very high values (up to 0.8 wt%), which are completely unusual for stainless steels. Precipitation of coherent M 23 C 6 cubic carbides together with a dense population of very small clusters (radius <5 nm) led to a high level of local strengthening.
Extensive quantitative data have been produced by this thorough experimental study. They provide a strong basis for comparison in the future development of thermo-kinetics models aiming at coupling long-range diffusion and precipitation in dissimilar steel welds.
Notes
In what follows, we will continue designating as martensite the intermediate layer with a lathy microstructure, even if it has been tempered during the welding of the second cladding layer and the subsequent post-weld heat-treatment.
Estimated by Monte-Carlo simulations for a 16 kV electron beam on an iron sample.
Value extracted from the mc-fe.ddb database of the MatCalc software [41]
References
Sopoušek J, Foret R, Jan V (2004) Simulation of dissimilar weld joints of steel P91. Sci Technol Weld Join 9(1):59–64
Race JM, Bhadeshia H (1993) Carbon migration across dissimilar steel welds. Int Trends Weld Sci Technol 1–5
Gómez X, Echeberría J (2000) Microstructure and mechanical properties of low alloy steel T11–austenitic stainless steel 347H bimetallic tubes. Mater Sci Technol 16(2):187–193
Silva CC, Miranda HC, de Sant’Ana HB, Farias JP (2013) Austenitic and ferritic stainless steel dissimilar weld metal evaluation for the applications as-coating in the petroleum processing equipment. Mater Des 47:1–8
Foret R, Zlamal B, Sopousek J (2006) Structural stability of dissimilar weld between two Cr-Mo-V steels. Weld J 85:211s–217s
Darken LS (1949) Diffusion of carbon in austenite with a discontinuity in composition. Trans AIME 180(430–438):53
Christoffel RJ, Curran RM (1956) Carbon migration in welded joints at elevated temperatures. Weld J 35:457s–465s
Race JM, Bhadeshia HKDH (1992) Precipitation sequences during carburisation of Cr–Mo steel. Mater Sci Technol 8(10):875–882
Kozeschnik E, Pölt P, Brett S, Buchmayr B (2002) Dissimilar 2·25Cr/9Cr and 2Cr/0·5CrMoV steel welds: part 1: characterisation of weld zone and numerical simulation. Sci Technol Weld Join 7(2):63–68
Huang ML, Wang DL (1998) Carbon migration in 5Cr-0.5 Mo/21Cr-12Ni dissimilar metal welds. Metall Mater Trans A 29(12):3037–3046
Albert SK, Gill TPS, Tyagi AK, Mannan SL, Kulkarni SD, Rodriguez P (1997) Soft zone formation in dissimilar welds between two Cr-Mo steels. Weld J Weld Res Suppl 76(3):135–142
Kozeschnik E, Warbichler P, Letofsky-Papst I, Brett S, Buchmayr B (2002) Dissimilar 2·25Cr/9Cr and 2Cr/0·5CrMoV steel welds: part 2: identification of precipitates. Sci Technol Weld Join 7(2):69–76
Lundin CD (1982) Dissimilar metal welds-transition joints literature review. Weld J 61(2):58–63
Gauzzi F, Missori S (1988) Microstructural transformations in austenitic-ferritic transition joints. J Mater Sci 23(3):782–789. doi:10.1007/BF01153967
He K, Brown A, Brydson R, Edmonds DV (2006) Analytical electron microscope study of the dissolution of the Fe3C iron carbide phase (cementite) during a graphitisation anneal of carbon steel. J Mater Sci 41(16):5235–5241. doi:10.1007/s10853-006-0588-4
Scott CP, Drillet J (2007) A study of the carbon distribution in retained austenite. Scr Mater 56(6):489–492
Valle N, Drillet J, Bouaziz O, Migeon H-N (2006) Study of the carbon distribution in multi-phase steels using the NanoSIMS 50. Appl Surf Sci 252(19):7051–7053
Laigo J, Christien F, Le Gall R, Tancret F, Furtado J (2008) SEM, EDS, EPMA-WDS and EBSD characterization of carbides in HP type heat resistant alloys. Mater Charact 59(11):1580–1586
Lan L, Qiu C, Zhao D, Gao X, Du L (2012) Analysis of martensite–austenite constituent and its effect on toughness in submerged arc welded joint of low carbon bainitic steel. J Mater Sci 47(11):4732–4742. doi:10.1007/s10853-012-6346-x
Li YJ, Choi P, Borchers C, Chen YZ, Goto S, Raabe D, Kirchheim R (2011) Atom probe tomography characterization of heavily cold drawn pearlitic steel wire. Ultramicroscopy 111(6):628–632
Garcia-Mateo C, Caballero FG, Miller MK, Jimenez JA (2011) On measurement of carbon content in retained austenite in a nanostructured bainitic steel. J Mater Sci 47(2):1004–1010. doi:10.1007/s10853-011-5880-2
Seidel F, Stock H-R, Mayr P (1997) Glow discharge optical spectroscopy depth profiles of ion implanted steel, titanium and titanium nitride coatings. Thin Solid Films 308:425–429
Mändl S, Fritzsche B, Manova D, Hirsch D, Neumann H, Richter E, Rauschenbach B (2005) Wear reduction in AISI 630 martensitic stainless steel after energetic nitrogen ion implantation. Surf Coat Technol 195(2):258–263
Helander T, Ågren J (1997) Computer simulation of multicomponent diffusion in joints of dissimilar steels. Metall Mater Trans A 28(2):303–308
Larsson H, Engström A (2006) A homogenization approach to diffusion simulations applied to α + γ Fe–Cr–Ni diffusion couples. Acta Mater 54(9):2431–2439
Pan C, Wang R, Gui J, Shi Y (1990) Direct TEM observation of microstructures of the austenitic/carbon steels welded joint. J Mater Sci 25(7):3281–3285. doi:10.1007/BF00587687
Ishida T (1991) Formation of stainless steel layer on mild steel by welding arc cladding. J Mater Sci 26(23):6431–6435. doi:10.1007/BF02387825
Nelson TW, Lippold JC, Mills MJ (2000) Nature and evolution of the fusion boundary in ferritic-austenitic dissimilar metal welds. Part 2-On-cooling transformations. Weld Res 79:267s–277s
Wu Y, Patchett BM (1992) Formation of crack-susceptible structures of weld overlay of corrosion resistant alloys. Mater Perform Sulphur Energy 283–295
Iso 16592 Analyse par microfaisceaux—analyse par microsonde électronique (microsonde de Castaing)—lignes directrices pour le dosage du carbone dans les aciers par la droite d’étalonnage
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7(6):1564–1583
Gatan ion beam etching in Material Characterization, Final report Isolde Gräf, TU Darmstadt in cooperation with Gatan GmbH, Münich
Rauch EF, Duft A (2005) Orientation maps derived from TEM diffraction patterns collected with an external CCD camera. Mater Sci Forum 495–497:197–202
Rauch EF, Véron M, Nicolopoulos S, Bultreys D (2012) Orientation and phase mapping in TEM microscopy (EBSD-TEM like): applications to materials science. Solid State Phenom 186:13–15
Oliver WC, Pharr GM (2004) Measurement of hardness and elastic modulus by instrumented indentation: advances in understanding and refinements to methodology. J Mater Res 19(01):3–20
Moeck P, Rouvimov S, Rauch EF, Veron M, Kirmse H, Häusler I, Neumann W, Bultreys D, Maniette Y, Nicolopoulos S (2011) High spatial resolution semi-automatic crystallite orientation and phase mapping of nanocrystals in transmission electron microscopes. Cryst Res Technol 46(6):589–606
Shtansky DV, Nakai K, Ohmori Y (2000) Decomposition of martensite by discontinuous-like precipitation reaction in an Fe–17Cr–0.5C alloy. Acta Mater 48(4):969–983
Berkane R, Gachon JC, Charles J, Hertz J (1987) A thermodynamic study of the chromium-carbon system. CALPHAD 11(4):375–382
Shtansky DV, Nakai K, Ohmori Y (1999) Crystallography and interface boundary structure of pearlite with M 7 C 3 carbide lamellae. Acta Mater 47(4):1105–1115
Kozeschnik E, Buchmayr B (1999) MATCALC—a simulation tool for multicomponent thermodynamics, diffusion and phase transformations. In Fifth international seminar on the numerical analysis of weldability. pp. 349–361
Van Landeghem HP, Gouné M, Redjaïmia A (2012) Nitride precipitation in compositionally heterogeneous alloys: nucleation, growth and coarsening during nitriding. J Cryst Growth 341(1):53–60
Villars P, Cenzual K (2010) Pearson’s crystal data: crystal structure database for inorganic compounds. ASM International, Materials Park
Acknowledgement
This study was sponsored by both EDF and AREVA through a PhD Grant (FM). In addition, the authors wish to thank M. Verdier (SIMaP) for nanoindentation scans, M. Véron (SIMaP) for her assistance in operating Astar software, P. Perrenot (SIMaP) for his help in the FIB data analysis, B. El Adib (CRP-GL) for the valued contribution to SIMS measurements, and E. Kozeschnik (TU Wien) for enabling thermodynamics calculations with the MatCalc software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by EDF R&D and AREVA. F. Mas has received a research Grant from AREVA. The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mas, F., Tassin, C., Valle, N. et al. Metallurgical characterization of coupled carbon diffusion and precipitation in dissimilar steel welds. J Mater Sci 51, 4864–4879 (2016). https://doi.org/10.1007/s10853-016-9792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-9792-z