Abstract
This article focuses on the study of the thermal, vibrational, structural, and morphological changes of hydroxyapatite from bovine bone obtained by a three-step process: calcination at two different low heating rates (2.5 and 5 °C/min), at different temperatures ranging from 600 to 1100 °C, and cooled in the air furnace. Differential scanning calorimetric and thermogravimetry showed that for T > 700 °C, no organic compounds were present in the bone matrix. Scanning electron microscopy images showed that the heating rates affect the morphology of the samples. The primary porosity originated by the presence of fat and protein disappears after the coalescence of the poly-hydroxyapatite crystals, and for T > 800 °C, a disorder–order transition (poly-crystal–single crystal) occurs. Full-width at the half-maximum of X-ray diffraction patterns indicated that the heating rate affects the structure of the BIO-Hap. Diffraction peak corresponding to calcium carbonate disappears from X-ray patterns of the samples calcined above 700 °C. The disorder–order (poly-crystal–single crystals) transition occurs for T > 900 °C. Raman experiments showed that for T > 700 °C, no organic phases are present in the samples. Dihydroxylation of hydroxyapatite is present for temperatures up to 800 °C originated Whitlockite. The same thermal conditions during sample calcination process were assured by using a controlled computer system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroxyapatite or bio-hydroxyapatite is usually obtained by the so-called “calcination” method, with different variations such as heating rate and the final calcination temperature. There is a growing demand for bioactive ceramics for medical applications. Autografts are the perfect choice for bone replacement or repair. The disadvantage of autografts (availability and post-operative pain) and allografts (potential infectivity) have made the investigation in biomaterials for medical applications focus on xenografts (Tadic and Epple [1]). Xenografts (animal bone, e.g., bovine) are an alternative as a biomaterial for bone substitution with reserves about the immune response by the receptor. Other alternatives are synthetic materials, but these materials lack osteoinductive properties and have poor mechanical properties [2, 3], as well as, different elemental chemical compositions [4].
Hydroxyapatite (HAp) has been used for orthopedic/dental implants because of its similar chemical composition and structure of the mineral phase of human bone. It can promote sufficient new bone formation for the firm attachment of juxtaposed bone. Vertebral bone and teeth are composed of carbonated HAp [Ca10 (PO4, CO3)6(OH)2], type I collagen, and water that contribute to the properties of bone. Natural HAps contain carbonate groups like human bone and teeth.
In recent years, the use of bio-HAp has increased. Due to this, biomaterial also has others elements that are present in the human HAP. Although ions are found in the bone in small amounts, these ions are very important in biological reactions related with bone metabolism [5]. The main advantages of bio-HAp are lower costs, high availability, and are easier to obtain. Figuereido et al. [2] studied the chemical and structural properties as well as the porosity of human and animal cortical bones calcined at 600, 900, and 1200 °C. It was found that if the calcination temperature increases, the crystalline quality and crystal size increase, while the porosity exhibits an opposite trend. Ooi et al. [6] studied the properties of porous HAp produced by heat treatment (annealing) of bovine bone obtained for temperatures between 400 and 1200 °C. It was found that “as-received” bovine bone contains organic compounds that, upon annealing at temperatures above 600 °C, were removed from the matrices. On the other hand, according to their results, bovine bone annealed between 800 and 1000 °C showed the characteristics of a natural bone with the interconnecting pore network being retained in the structure. However, in both studies, the effects of cooling rate on the structural and microstructural properties of annealed samples were not studied in detail.
Commercial BIO-HAp has been studied by Figuereido et al. [3], fourteen different samples used in dentistry, three of them based on HAp and the others based on calcium carbonate. They found significant differences regarding particle size, crystallinity, porosity, pore size distribution, surface area, and mineral content, which imply that it is necessary to carry out more clinical experiments. On the other hand, Giraldo-Betancur [4] studied physicochemical properties of commercial HAp and made a comparison with Bio-HAp obtained by a defatting and calcination process obtained at 900 °C. They found using TGA that some commercial HAp contains organic materials such as fat and protein, while in the case of BIO-HAp obtained up to 600 °C, fat and collagen were removed. HAp from the National Institute of Standards and Technology (NIST) was used as a reference. By FTIR, they observed that NIST has protein residues. Sofronia et al. [7] studied the thermal and optical behaviors of commercial and natural HAps (bovine) using DSC and TGA: They found that for samples calcined at 800 °C, no organic compounds or carbonates are present, which implies that this temperature is adequate to obtain samples without organic compounds and carbonates. In this case, no information about the thermal profile and calcination sintering time or the cooling process was reported.
The structural changes as a function of the temperature and sintering time have been reported by Kusrini and Sontang [8]. They observed that the XRD profile of BIO-HAp was dependent on the sintering temperatures as there was a substantial increase in peak intensity and a marked decrease in FWHM when the sintering temperature increased up to 800 °C, and then the FWHM decreased steadily as the sintering temperature approached 800 °C. At temperatures higher than 800 °C, the FWHM exhibits an opposite trend. However, the information regarding the thermal profile and sintering time was not reported, and this fact affects the physicochemical properties of the sample.
The vibrational spectroscopy as Raman has been used to determine alteration in the bone composition that takes place during calcination. Penel et al. [9] studied the intravital composition and structure of membranous bone by Raman microspectroscopy. It was found that the composition and structure of all of the biomaterials studied were stable. Recently, Gamsjaeger et al. [10] used Raman spectroscopy to study the bone quality in trabecular bone of children and young adults. This study provided information on mineral/matrix ratio, mineral maturity/crystallinity, relative pyridinoline, collagen cross-link content, relative proteoglycan content, and relative lipid content. Kozielski et al. [11] used Raman microscopy and mapping to determine the content of mineral and organic components as well as the orientation of collagen fibers in spongy human bones.
Different works related to the calcination process have been reported. However, the influence of the cooling rate on the physicochemical properties of BIO-HAp has received some importance. Recently, Ramirez-Gutierrez et al. [12] reported the influence of the cooling rate of bovine BIO-HAp for calcinate sample at 900 °C and heated at 5 °C/min that were subsequently cooled in air, water, liquid nitrogen, and furnace air. They reported that crystallographic characteristic of the BIO-HAp depends on the cooling rate. High crystallinity was obtained for samples obtained at a low cooling rate (furnace air).
The objective of this work was to study the physicochemical changes that take place in BIO-HAp obtained by a hydrothermal and incineration process, at two different heating rates: 2.5 and 5 °C/min, for samples calcined at 700, 800, 900, 1000, and 1100 °C and cooling in furnace air [12]. Thermal changes due to the calcination in bovine bone powder were studied using DSC and TGA. Structural transformation during calcination (700–1100 °C) was studied using X-ray diffraction. Microstructural changes were studied using SEM images, and vibrational analysis was done using Raman spectroscopy.
Materials and methods
Materials
Samples were obtained from cortical bovine bones (2-year old) collected from the local slaughterhouse (folio number SDA-537295-98, 2011) located in Queretaro city, Mexico.
Thermal analysis of bone powder
In order to develop an experimental process to obtain Bio-HAp using fresh bovine bone, it is very important to determine its chemical composition and their thermal behaviors. For this reason, the thermal behaviors of r bone powder were studied using differential scanning calorimetric and thermogravimetric analysis. Bone is formed by organic materials such as protein and fat and inorganic materials formed mainly by HAp and some calcium salts.
Thermal behavior: TG analysis
The thermogravimetric curves as well as their first derivative as a function of temperature were obtained by using TG Q500 equipment (TA Instruments). The sample mass was 12.0 ± 1.0 mg and this was placed in the platinum crucible of thermobalance (TA Instruments, USA). The samples were heated from room temperature to 1100 °C, at a heating rate of 2.5 and 5 °C/min (low cooling rate). The measurements were carried out in a constant N2 flow. The TG data were processed using the Universal Analysis 2000 TA software.
Thermal behavior: DSC analysis
The DSC measurements were performed in a DSC-Q100 TA Instruments calorimeter in modulated mode. Indium metal was used to calibrate the DSC system in relation to temperature and enthalpy. The MDSC curves were performed under nitrogen atmosphere (50 mL/min). The samples were prepared in an aluminum hermetic DSC capsule.
The sample mass was fixed in 6.0 ± 0.1 mg, and an empty capsule was used as a reference. The samples were tested in MDSC ramps from 30 to 1100 °C, at a heating rate of 2.5 and 5 °C/min with 0.796 °C and 60 s of amplitude and period, respectively. The samples were analyzed in duplicate.
Figure 1a shows a characteristic TGA analysis of bone powder without removing water, fat, and protein as a function of the temperature, as well as its first derivative, and Fig. 1b shows the MDSC analysis of the same sample, as well as its first derivate using a ramp at a heating rate of 5 °C/min. As is well known, TGA gives information about the parentage of components, its interaction, and also the structural transformation that takes place as the result of the heating process, commonly referred to as degradation of different phases (organic and inorganic). Taking into account the temperature in which thermal events occur, and the transformation associated with each event, it is possible to determine the calcination temperatures to obtain BIO-HAp.
According to the DSC-TG and DTG curves shown in Fig. 1, the mass loss of 22.65 % of the bone powder sample takes place during the four stages: −2.43 % up to 211 °C due to loss of physisorbed water and to decomposition of a part of the organic matter; −15.46 % in the temperature range 211–645 °C, due to liberation of chemically bonded water, decomposition of the organic matrix and part of carbonates; −3.22 % for temperatures between 645 and 846 °C, attributed both to decarbonization, process that can continue up to 900–1000 °C with different intensities depending on the source of the sample [13] and beginning of the dehydroxylation processes with −1.38 % for dehydroxylation and decomposition for T > 860 °C.
In all the above works, the heating rate, as well as the cooling process, has not been taken into account. In the case of heating, the heat capacity of the sample that is in direct relation to the chemical composition affects the temperature of each one of the thermal events. During calcination, it is clear that there exists a water loss, degradation of fat and protein, organic decomposition, loss of inorganic materials, HAp transformation, and in the case of cooling effects on the physicochemical properties, no information is available.
Three-step process to obtain bio-hydroxyapatite
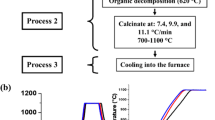
Taking into account the thermal processes that occur during the calcination process, a new process was developed to obtain BIO-HAp, but now controlling the calcination and cooling (sintering times) process in the furnace air. Figure 2a shows the three-step process used to obtain BIO-HAp.
a Block diagram of the three-step process to produce BIO-HAp, for samples cooled in furnace air. b Shows the thermal profile used to obtain the calcined samples at 2.5 and 5 °C/min for the heating and isothermal temperature at 930 °C. b Also shows the cooling rate for each of the studied samples. The inset in this figure corresponds to the SEM image of sample calcined at 600 °C
Slices of bovine bone obtained from the central part of the femur (cortical bone, 2-cm thickness) were used to obtain bone powder; adhering soft tissues were removed manually. After this, the fluids in bone, marrow, and any remaining soft tissue were eliminated by boiling at high pressure (154 °C, 4 atm-Hydrothermal process) using an autoclave in deionized water during 40 min. This process was repeated three times until obtaining bone slices with low organic compounds.
The bone slices were then subjected to vacuum drying at 60 °C for 120 h, in order to denaturalize the protein and facilitate the milling process. Next, dry slices of bone were reduced to small particles 2–5 mm using pressure. Milling process was used to reduce the particle size using a ring powdering system (Vymsa, Peru). Powder bio-HAp was obtained using the US mesh 100 (147 µm) and smaller particles were obtained from the plate. This process is called pretreatment in the flow diagram of Fig. 2a.
The bone powder obtained after using the procedure described above can be considered a hydrothermal cleaning focused in the partial removal of organic materials.
Process 1 Hydrothermal defatted: this consists in the partial removal of fat and protein fractions from the bone powder, using only water as a solvent. First, the bone powder US mesh 100 (147 µm) was heated at 154 °C and 4 atm for 30 min using a 100 g/1 L of deionized water. This process was carried out three times in order to avoid the use of petroleum ether. Finally, the sample was washed twice with boiling water (92 °C), and was dried in a vacuum furnace at 90 °C for 8 h. A sample obtained using petroleum ether was also used in order to compare the processes.
Processes 2 and 3 in fact, are the calcination process that includes the cooling process, as shown in Fig. 2a. Process 2 First, all the samples were calcined at 5 °C/min until reaching 600 °C, in order to produce a decomposition of the organic phase. The inset in Fig. 2b shows the SEM image of BIO-HAp-600, which shows no porosity due to the presence of some remnants of fat and proteins. After this process, two different calcination temperatures were used in this study for 2.5 and 5 °C/min, and five different final calcination temperatures at 700, 800, 900, 1000, and 1100 °C.
Process 3: the cooling process in furnace air. It has been shown that the cooling process affects the structural and microstructural (morphological) properties of the BIO-HAp (Ramirez-Gutierrez et al. [12]). For this reason, in this study, the cooling process was carried out in furnace air, which means a low cooling rate. The calcination process was carried out as follows:
Calcined: Fig. 2b shows the thermal profile used to obtain the calcined sample called BIO-HAp-5-1100 that corresponds to a sample obtained at 5 °C/min and calcined at 1100 °C. The calcination process (Thermal profile) for 2.5 and 5 °C/min was divided into five regions (see Fig. 2b): For Region I, the hydrothermal defatted bone powder sample was heated from room temperature to 600 °C at a heating rate of 5 °C/min. For Region II, after the sample reaches 600 °C, it is kept at this temperature for 3 h (isothermal conditions), in order to remove some fractions of fat and protein. For Region III, two different heating rates of 2.5 and 5.0 °C/min were used until the sample reached the following temperatures: 700, 800, 900, 1000, and 1100 °C. For Region IV, the sample was kept at each one of these temperatures for 3 h (isothermal conditions). For Region V, the furnace is turned off, and the samples are cooled in furnace air (inside of it). The first ramp (Region I) was used to eliminate the organic materials and the second part of the ramp was used to study the influence of the heating rate and final temperature on the physicochemical properties of BIO-HAp [8]. Calcination process was carried out in a Furnace Felisa, Mexico.
Surface microstructure: SEM
Morphologic analysis of all Bio-HAp-2.5 and BIO-HAp-5.0 samples obtained at different heating rates as well as the commercial sample was carried out in a Jeol JSM 6060LV Scanning Electron Microscope. The analysis was performed using 20 kV electron acceleration voltages. Before the analysis, the samples were fixed on a copper specimen holder with carbon tape and covered with gold thin film in order to make them conductive before testing.
Raman spectroscopy
Crystalline BIO-HAp 1000, as well as sponges, was studied using Raman spectroscopy. Vibrational studies of bone sponges were carried out using a Labram model microspectrometer (Senterra, Bruker). A notch filter separated the Raman signal from laser excitation. The Raman signal was processed by a spectrograph equipped with an air-cooled CCD detector. The Raman spectra were excited by a helium–neon laser (785 nm) with an output of 25 mW reaching the sample. The long working distance of the 20× microscope objective gave a spot size of the order of a few micrometers. The overall spectral resolution was 2 Δ/cm. For bone studies, spectral acquisitions were performed in the range of 300–1800 and 2700–3100 Δ/cm.
Bio-HAp structural characterization: XRD
X-ray diffraction technique was used to determine the crystalline phases in the BIO-HAp obtained at 2.5 and 5 °C/min as well as a BIO-HAp from NIST (National-REF: USA) and defatted samples obtained by hydrothermal called HTP-HAp and the soxhlet process called SP-HAp that were used as references. BIO-HAp powder samples (mesh 100) were densely packed in an Al holder. X-ray diffraction patterns of the samples were carried out on a Rigaku Ultima IV diffraction instrument operating at 35 kV, 15 mA with CuKα radiation wavelength of λ = 1.5406 Å. Diffractograms were obtained from 5° to 80° on a 2θ scale with a step size of 0.02.
Elemental composition: ICP-OES
This technique was used to analyze the elemental composition of both the commercial and biosamples. Thermo iCAP 6500 Duo View equipment was used. 0.1 g of each sample was digested with nitric acid (Baker 69e70 %) and it was made in triplicate. Upon return to the ground state, the elements excited by the argon plasma were then identified by their characteristic emission spectra. Emission intensity was then converted to elemental concentration by comparing to a standard curve.
Results and discussion
ICP composition analysis
Table 1 shows the chemical composition of Bone (bone powder), powder bone with the thermal process, BIO-HAp obtained at 600 °C, and BIO-HAps obtained at 700, 800, 900, 1000, and 1100 °C. The increase in the composition of the calcined samples up to 700 °C is due to the extraction of fat and protein from the bone matrix. This result confirmed that hydrothermal process proposed in this study does not remove these important ions. Giraldo-Betancur et al. [4] studied the chemical composition of calcined samples at 900 °C, additional to elements shown in Table 1 and found Ba (265.58 ± 13.15 mg/kg). Also, Fe was found in almost twice the amount that could be originated by the milling process.
Morphological analysis
Figure 3a, c, e, g, and i shows the SEM images of BIO-HAp samples obtained at 2.5 °C/min, and Fig. 3b, d, f, and h shows the SEM images of BIO-HAp obtained at 5 °C/min and calcined at 600, 700, 800, 900, 1000, and 1100 °C. Sample calcined at 600 °C still exhibits the presence of fat and protein, and the interconnected porousness that constitutes the organic phase (as shown in Fig. 2b). For samples calcined at 700 °C (Fig. 3a, b) and according to DSC analysis, almost all fat and protein were removed, originating the apparition of microholes. Particles that form BIO-HAp are in the range of submicron and present a non-regular shape. When the treatment temperature is 800 °C, particles become semi-spherical, and there is an apparent porosity increase, these semi-spherical particles are interconnected (Fig. 3c, d). When the temperature is between 800 and 900 °C (Fig. 3e, f), the formation of cumulus due to the coalescence of the surface interfaces is evident for the sample heated at 2.5 °C/min and in the case of samples heated at 5 °C, this event occurs before 900 °C. Here, it is important to recall that the porosity at this temperature does not correspond to the original porosity of the bone; this porosity appears due to the formation of cumulus. In the case of the sample heated at 5 °C/min and 900 °C (Fig. 3f), there exists a “poly crystal coalescence,” which originated the apparition of single crystals of BIO-HAp. At this point, the concept of porosity does not have any physics sense because the porosity here corresponds to the space between submicron HAp crystals.
When the temperature increases up to 1000 °C (Fig. 3g, h), the cumuli formed by HAp heated at 2.5 °C/min coalesces to form single particles that exhibit some growing habits; these particles exhibit hexagonal structure characteristic of HAp. For samples heated at 5 °C/min, the apparition of large single crystal with a preferential orientation (exe c) governs the HAp formation. For 1100 °C (Fig. 3i, j), it is clear that the coalescence of the HAp particles produces single crystal of HAp. Together with the single crystals, also appear other structures which will be identified in the following sections. According to these results, it is evident that the heating rate plays an important role in the morphology changes of the BIO-HAp. Here, it is also evident that due to the coalescence of the poly-crystalline BIO-HAp, the porosity corresponds to the inter-crystal spaces.
Crystalline phases
The structural transformation that takes place in the Bio-HAp as a function of the heating rate and for different calcination temperatures is studied using X-ray diffraction. Figure 4a shows the XRD patterns of a calcined sample at 600 °C at 5 °C/min, bone powder with soxhlet process (SP-HAp, Fig. 4b), bone powder with the hydrothermal process (HTP-HAp, Fig. 4c), and bone powder (Fig. 4d). The SP and HTP processes do not affect the structure of the HAp, but a very important point here is that by using the HTP, it is possible to produce defatted samples without the use of solvents such as hexane or petroleum ether. The sample obtained by calcination at 600 °C exhibits more relative crystallinity due to the increases in the sample concentration, because according to the TG analysis, around 18 % of fat and protein had been degraded and removed from the sample. No structural changes occur in this case. The followings crystalline structures are in these samples: In Fig. 4a, vertical lines correspond to the diffraction peaks for pure HAp (PDF-84-1998), and in Fig. 4b vertical lines correspond to the diffraction peaks for Calcite (CaCO3, PDF 01-0837). In order to have a good identification of each peak, Lanthanum hexaboride powder from the National Institute Standards and Technology (NIST), (Standard Reference Material 660a) was used as an internal standard in all of the X-ray diffraction patterns.
Figure 5a shows the X-ray diffraction patterns of Bio-HAp obtained at 2.5 °C/min and Fig. 5b for samples obtained at 5 °C/min (heating rate) for 700, 800, 900, 1000, and 1100 °C. HAp with PDF 84-1998 is identified in all cases for temperatures up to 700 °C; Calcium carbonate disappears for samples heated at 2.5 and 5 °C/min. All samples in Fig. 5 consist of HAp phase. The XRD patterns in Fig. 5 show the most intense peaks corresponding to (002), (211), (112), (300), and (310) planes. A detailed inspection of these patterns showed the existence of three peaks located at (300), (1 1 12), and (1 2 11), that are identified with the PDF-09-0169 as Whitlockite [Ca3(PO4)2], that corresponds to HAp dehydroxylate only for T > 800 °C. This fact can be confirmed by the analysis of Fig. 1a in which for T < 800 °C it was found a loss of around 1.54 % that corresponds to the dehydroxylate HAp.
In order to determine the effect of the temperature and the heating rate, the changes in the crystallinity were established using the full-width at half-maximum (FWHM) value of the main XRD peaks (211). This parameter is inversely proportional to the average crystalline size according to the Scherrer equation [1, 14]; indicating that for FWHM small values, the crystallite size increases as well as the crystallinity. From XRD patterns for samples obtained at two different heating rates, it is possible to obtain the FWHM values. Figure 6 shows the FWHM for the [121] peak located at 31.79 in 2θ scale as a function of the temperature for samples heated at 2.5 and 5 °C/m. According to this data, it is clear that the FWHM does not exhibit a lineal relationship as a function of temperature for samples obtained at 2.5 °C/min and in the case of samples obtained at 5 °C/min it behaves as a logistic curve. At 900 °C for samples obtained at 2.5 °C/min, the coalescence phenomenon is present, and it involves the loss of crystallinity. Above this temperature all samples correspond to the crystalline phase of BIO-HAp. For samples obtained at 5 °C/min, the crystalline phase appears around 900 °C. As mentioned before, the changes in the FWHM behave as a logistic curve, characterized by a Gaussian curve for its first derivative. The maximum value in this curve (inset in Fig. 6) corresponds to the separation between polycrystalline and crystalline phases. Here it is very important to clarify that due to the use of powder technique, sometimes there is a misinterpretation because the pattern exhibits the whole set of peaks. However, by a detailed examination of the SEM images and the behaviors of the FWHM, the existence of a crystalline phase for samples obtained at 5 °C/min and temperatures up to 938 °C is clear. Kusrini and Sontang [6] and Ooi et al. [8] found the X-ray patterns of BIO-HAp were dependent on the sintering temperature. As the temperature increases from 500 to 800 °C, the FWHM decreases; but for sintering temperatures up to 800 °C the FWHM exhibits an opposite trend. This result is in disagreement with our findings. In the study above, no data about the temperature process and time uses were reported, and it is also possible the calculation does not take into account the disorder–order transition in the case of samples heated at 5 °C/min.
Raman characterization
The bands in Raman spectra of bone powder with the hydrothermal process and HAp obtained at 600 °C with a heating rate of 5 °C/min (BIO-600-5) are shown in Fig. 7 a and b, respectively [10, 11, 15]. The phosphate group \( \left( {{\text{PO}}_{4}^{3 - } } \right) \) has four internal vibration modes: ν1 (960 cm−1), ν2 (430 cm−1), ν3 located at 1030 and 1045 cm−1 and a broad peak located at 1076 cm−1, and ν4 (∼587–604 cm−1) amide I, amide III. Phe (phenylalanine), CH stretching, and bending (fat) were identified. The internal modes of the carbonate group \( \left( {{\text{CO}}_{3}^{2 - } } \right) \) located at 1068 cm−1 (ν1 mode of B-type carbonate) and 1102 cm−1 (mode of A-type carbonate) were detected in these samples. Figure 7c and d corresponds to the Raman spectra of samples calcined at 700, 800, 900, 1000, and 1100 °C/min for 2.5 and 5 °C/min heating rate. In these figures, letter A corresponds to the O–H stretching, B through E corresponds to ν3, ν1, ν4, and ν2, of the phosphate group \( \left( {{\text{PO}}_{4}^{3 - } } \right) \). No calcium carbonate was identified for T > 800 °C for both heating rates. In Fig. 5, the existence of Whitlockite that occurs due to the dehydroxylation of HAp was identified. In Fig. 7e and f for the O–H stretching in the Raman peak located at 3572 cm−1 for samples heated at 2.5 °C/min, no change was observed. However, for samples heated up to 1000 °C, this peak undergoes a strong change evidencing this phenomenon.
Conclusions
According to the experiments mentioned above for samples cooled in furnace air, and using low heating rates, the three-step process allows the obtainment of BIO-HAp with different physicochemical properties. The hydrothermal process can remove fat and protein from the bone. The first part of the calcination process allows the complete removal of fat and almost all protein from the bone. During the first isotherm of the process, more protein is removed from the bone. From the SEM images for both heating rates 2.5 and 5 °C/min and for temperatures from 700 to 1100 °C, it is possible to determine that the heating rate affects the final morphology of the BIO-HAp. A coalesce phenomenon due to the increase of temperature appears for samples heated between 800 and 900 °C for both heating rates, which changes the porosity of the BIO-HAp. Here it is important to recall that the primary porosity corresponds to the space occupied by fat and protein, and the secondary porosity is related to the coalescence phenomenon. For temperature around 1000 °C gives as a result, the origin of single crystals of HAp. According to X-ray diffraction patterns, the calcination samples obtained at 600 °C are composed mainly of HAp and calcium carbonate that disappear after 700 °C. Whitlockite [Ca3(PO4)2] that corresponds to HAp dehydroxylate appears for T > 800 °C. FWHM for the most intense peak showed that the heating rate affects the crystalline quality of the BIO-HAp and that the increases in this parameter in the case of samples heated at 2.5 °C/min are reflecting the carbonate decomposition. The analysis of the FWHM behaviors showed that for T > 938 °C for samples heated at 5 °C/min, the crystallinity of BIO-HAp has a disorder–order (polycrystal–crystal) transition. Raman analysis showed that the calcination process removes the organic compound from the bone matrix and also it was able to determine the dehydroxylation of HAp for some fraction of the samples heated at 5 °C/min. Finally, it is crucial to bear in mind that the thermal histories are necessary in order to determine the physicochemical changes that take place during a calcination process.
References
Tadic D, Epple M (2004) A thorough physicochemical characterization of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 25:987–994
Figueiredo M, Fernando A, Martins G, Freitas J, Judas F, Figueiredo H (2010) Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram Int 36(8):2383–2393
Figueiredo M, Henriques J, Martins G, Guerra F, Judas F, Figueiredo H (2010) Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes: comparison with human bone. J Biomed Mater Res Part B 92(2):409–419
Giraldo-Betancur AL, Espinoza-Arbeláez DG, del Real-López A, Millán-Malo BM, Rivera-Muñoz E, Gutiérrez-Cortez E, Pineda-Gómez P, Jiménez-Sandoval SJ, Rodriguez-Garcia ME (2013) Comparison of physicochemical properties of bio and commercial hydroxyapatite. Curr Appl Phys 13:1383–1390
Akram M, Ahmed R, Shakir I, Wan Ibrahim WA, Hussain R (2014) Extracting hydroxyapatite and its precursors from natural resources. J Mater Sci 49:1461–1475. doi:10.1007/s10853-013-7864-x
Ooi CY, Hamdi M, Ramesh S (2007) Properties of hydroxyapatite produced by annealing of bovine bone. Ceram Int 33:1171–1177
Sofronia AM, Baies R, Anghel EM, Marinescu CA, Tanasesc S (2014) Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater Sci Eng C 43:153–163
Kusrini E, Sontang M (2012) Characterization of X-ray diffraction and electron spin resonance: effects of sintering time and temperature on bovine hydroxyapatite. Radiat Phys Chem 81:118–125
Penel G, Delfosse C, Descamps M, Leroy G (2005) Composition of bone and apatite biomaterials as revealed by intravital Raman microspectroscopy. Bone 36(5):893–901
Gamsjaeger S, Hofstetter B, Fratzl-Zelman N, Roschger P, Roschger A, Fratzl P, Brozek W, Masic A, Misof BM, Glorieux FH, Klaushofer K, Rauch F, Paschalis EP (2014) Pediatric reference Raman data for material characteristics of iliac trabecular bone. Bone 69:89–97
Kozielski M, Buchwald T, Szybowicz M, Błaszczak Z, Piotrowski A, Ciesielczyk B (2011) Determination of composition and structure of spongy bone tissue in human head of femur by Raman spectral mapping. J Mater Sci Mater Med 22:1653–1661
Ramirez-Gutierrez CF, Palechor-Ocampo AF, Londoño-Restrepo SM, Millàn-Malo BM, Rodriguez-García ME (2015) Cooling rate effects on thermal, structural, and microstructural properties of bio-hydroxyapatite obtained from bovine bone. J Biomed Res Part B. doi:10.1002/jbm.b.33401
Yoganand CP, Selvarajan V, Goudouri OM, Paraskevopoulos KM, Junshu W, Dongfeng X (2011) Preparation of bovine hydroxyapatite by transferred arc plasma. Curr Appl Phys 11:702–709
Ruksudjarit A, Pengpat K, Rujijanagul G, Tunkasiri T (2008) Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr Appl Phys 8:270–272
Guangyong Z, Xian Z, Qi F, Xueliang W (2011) Raman spectra of amino acids and their aqueous solutions. Spectrochim Acta A 78:1187–1195
Acknowledgements
Sandra M. Londoño-Restrepo and Cristian F. Ramirez-Gutierrez thank the Consejo Nacional de Ciencia y Tecnología (CONACYT-Mexico) for the financial support of their Ph.D. thesis. The authors would like to thank Dr. Genoveva Hernandez-Padrón for her technical support for the Raman experiments and Carmen Pesa for SEM technical support. Authors thank Lindsay Larson for the technical English revision of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Londoño-Restrepo, S.M., Ramirez-Gutierrez, C.F., del Real, A. et al. Study of bovine hydroxyapatite obtained by calcination at low heating rates and cooled in furnace air. J Mater Sci 51, 4431–4441 (2016). https://doi.org/10.1007/s10853-016-9755-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-9755-4